Abstract
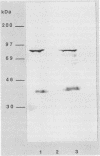
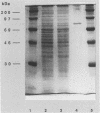
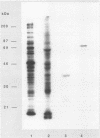
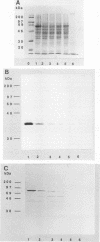
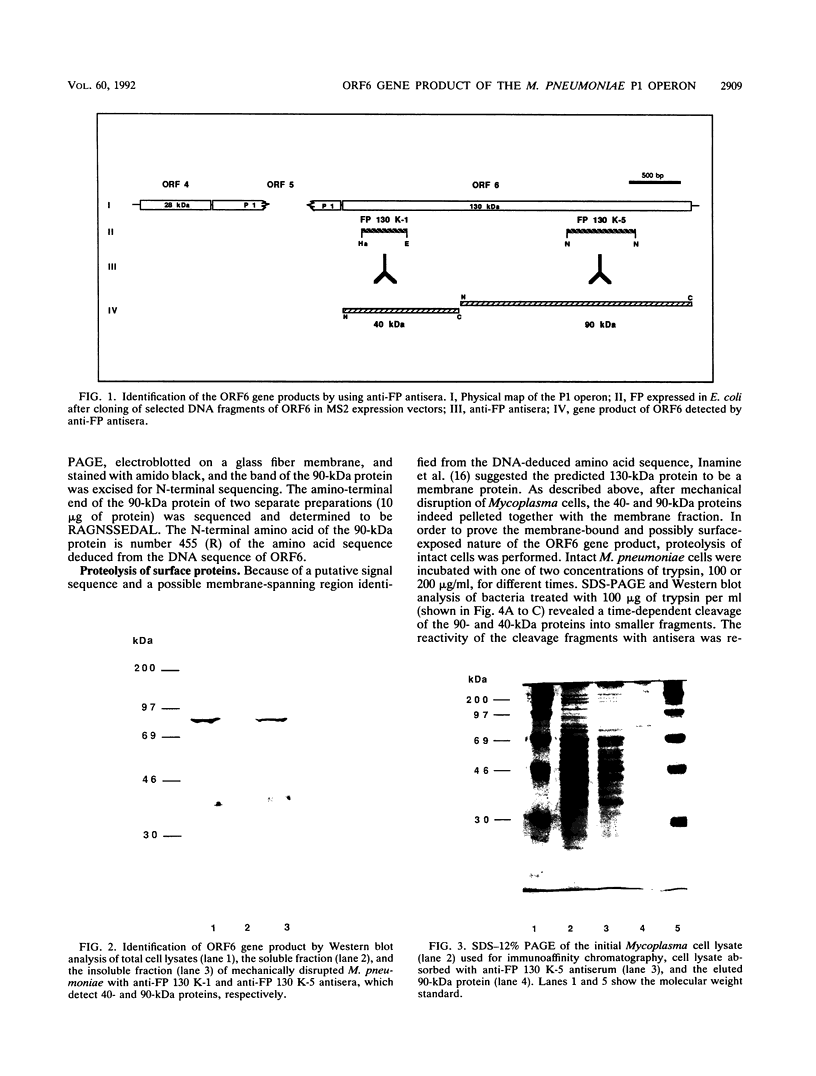
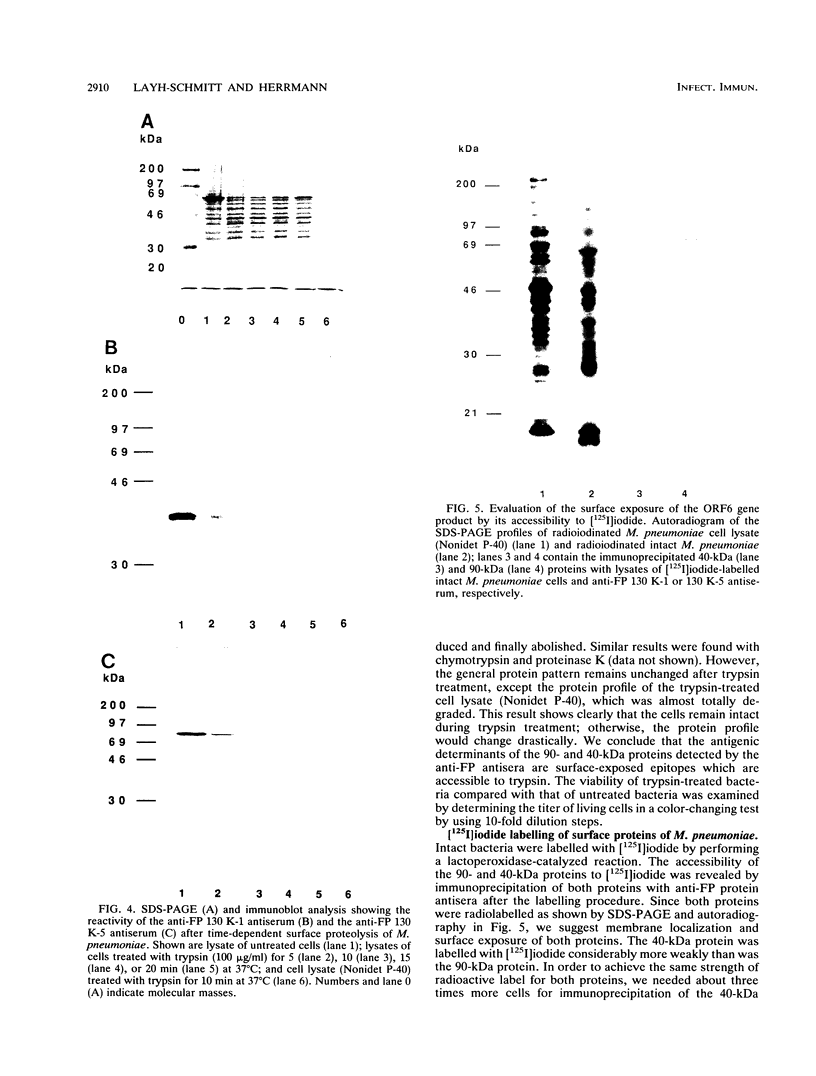
ORF6 represents one of the two open reading frames flanking the P1 attachment protein gene of Mycoplasma pneumoniae in the order ORF4-P1-ORF6 (J.M. Inamine, T.P. Denny, S. Loechel, U. Schaper, C.H. Huang, K.F. Bott, and P.C. Hu, Gene 64:217-219, 1988; J.M. Inamine, S. Loechel, and P.C. Hu, Gene 73:175-183, 1988; C.J. Su, V.V. Tryon, and J.B. Baseman, Infect. Immun. 55:3023-3029, 1987), indicating an operonlike organization. As described previously, we identified two proteins with molecular masses of 40 and 90 kDa (B. Sperker, P.C. Hu, and R. Herrmann, Mol. Microbiol. 5:299-306, 1991) which might represent two cotranslational cleavage fragments of the ORF6 gene product. To determine the site of the putative cotranslational cleavage, the first 10 amino acids of the N terminus of the isolated 90-kDa protein were sequenced. The data are consistent with the DNA-deduced amino acid sequence between amino acid positions 455 and 465 (RAGNSSETDAL). Thus, the cleavage site was identified at amino acid position 455 (R). In this study, the two proteins were localized and biochemically characterized. Both proteins are part of the insoluble fraction of M. pneumoniae as shown by immunoblots of supernatants and pellets of mechanically disrupted cells subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Surface proteolysis followed by SDS-PAGE and Western blot (immunoblot) analysis, covalent labelling of surface-exposed proteins with [125I]iodide and subsequent immunoprecipitation of both radiolabelled proteins, immunofluorescence studies with formalized and living M. pneumoniae, and immunoadsorption experiments provided strong evidence that the 40- and 90-kDa proteins are membrane-associated proteins expressing surface-exposed regions.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. J., Blobel G. Immunoprecipitation of proteins from cell-free translations. Methods Enzymol. 1983;96:111–120. doi: 10.1016/s0076-6879(83)96012-3. [DOI] [PubMed] [Google Scholar]
- Barile M. F., Chandler D. K., Yoshida H., Grabowski M. W., Harasawa R., Razin S. Parameters of Mycoplasma pneumoniae infection in Syrian hamsters. Infect Immun. 1988 Sep;56(9):2443–2449. doi: 10.1128/iai.56.9.2443-2449.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baseman J. B., Cole R. M., Krause D. C., Leith D. K. Molecular basis for cytadsorption of Mycoplasma pneumoniae. J Bacteriol. 1982 Sep;151(3):1514–1522. doi: 10.1128/jb.151.3.1514-1522.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Empirical predictions of protein conformation. Annu Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- Dallo S. F., Chavoya A., Baseman J. B. Characterization of the gene for a 30-kilodalton adhesion-related protein of Mycoplasma pneumoniae. Infect Immun. 1990 Dec;58(12):4163–4165. doi: 10.1128/iai.58.12.4163-4165.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckerskorn C., Mewes W., Goretzki H., Lottspeich F. A new siliconized-glass fiber as support for protein-chemical analysis of electroblotted proteins. Eur J Biochem. 1988 Oct 1;176(3):509–519. doi: 10.1111/j.1432-1033.1988.tb14308.x. [DOI] [PubMed] [Google Scholar]
- Engleberg N. C., Pearlman E., Eisenstein B. I. Legionella pneumophila surface antigens cloned and expressed in Escherichia coli are translocated to the host cell surface and interact with specific anti-Legionella antibodies. J Bacteriol. 1984 Oct;160(1):199–203. doi: 10.1128/jb.160.1.199-203.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldner J., Göbel U., Bredt W. Mycoplasma pneumoniae adhesin localized to tip structure by monoclonal antibody. Nature. 1982 Aug 19;298(5876):765–767. doi: 10.1038/298765a0. [DOI] [PubMed] [Google Scholar]
- Goding J. W. Conjugation of antibodies with fluorochromes: modifications to the standard methods. J Immunol Methods. 1976;13(3-4):215–226. doi: 10.1016/0022-1759(76)90068-5. [DOI] [PubMed] [Google Scholar]
- Hayflick L. Tissue cultures and mycoplasmas. Tex Rep Biol Med. 1965 Jun;23(Suppl):285+–285+. [PubMed] [Google Scholar]
- Hu P. C., Cole R. M., Huang Y. S., Graham J. A., Gardner D. E., Collier A. M., Clyde W. A., Jr Mycoplasma pneumoniae infection: role of a surface protein in the attachment organelle. Science. 1982 Apr 16;216(4543):313–315. doi: 10.1126/science.6801766. [DOI] [PubMed] [Google Scholar]
- Hu P. C., Collier A. M., Baseman J. B. Surface parasitism by Mycoplasma pneumoniae of respiratory epithelium. J Exp Med. 1977 May 1;145(5):1328–1343. doi: 10.1084/jem.145.5.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P. C., Collier A. M., Clyde W. A., Jr Serological comparison of virulent and avirulent Mycoplasma pneumoniae by monoclonal antibodies. Isr J Med Sci. 1984 Sep;20(9):870–873. [PubMed] [Google Scholar]
- Inamine J. M., Denny T. P., Loechel S., Schaper U., Huang C. H., Bott K. F., Hu P. C. Nucleotide sequence of the P1 attachment-protein gene of Mycoplasma pneumoniae. Gene. 1988 Apr 29;64(2):217–229. doi: 10.1016/0378-1119(88)90337-x. [DOI] [PubMed] [Google Scholar]
- Inamine J. M., Loechel S., Hu P. C. Analysis of the nucleotide sequence of the P1 operon of Mycoplasma pneumoniae. Gene. 1988 Dec 15;73(1):175–183. doi: 10.1016/0378-1119(88)90323-x. [DOI] [PubMed] [Google Scholar]
- Izumikawa K., Chandler D. K., Barile M. F. Mycoplasma pneumoniae attachment to glutaraldehyde-treated human WiDr cell cultures. Proc Soc Exp Biol Med. 1986 Apr;181(4):507–511. doi: 10.3181/00379727-181-42284. [DOI] [PubMed] [Google Scholar]
- Krause D. C., Baseman J. B. Mycoplasma pneumoniae proteins that selectively bind to host cells. Infect Immun. 1982 Jul;37(1):382–386. doi: 10.1128/iai.37.1.382-386.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause D. C., Leith D. K., Baseman J. B. Reacquisition of specific proteins confers virulence in Mycoplasma pneumoniae. Infect Immun. 1983 Feb;39(2):830–836. doi: 10.1128/iai.39.2.830-836.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause D. C., Leith D. K., Wilson R. M., Baseman J. B. Identification of Mycoplasma pneumoniae proteins associated with hemadsorption and virulence. Infect Immun. 1982 Mar;35(3):809–817. doi: 10.1128/iai.35.3.809-817.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küpper H., Keller W., Kurz C., Forss S., Schaller H., Franze R., Strohmaier K., Marquardt O., Zaslavsky V. G., Hofschneider P. H. Cloning of cDNA of major antigen of foot and mouth disease virus and expression in E. coli. Nature. 1981 Feb 12;289(5798):555–559. doi: 10.1038/289555a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marchalonis J. J., Cone R. E., Santer V. Enzymic iodination. A probe for accessible surface proteins of normal and neoplastic lymphocytes. Biochem J. 1971 Oct;124(5):921–927. doi: 10.1042/bj1240921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin S. Mycoplasmas: the smallest pathogenic procaryotes. Isr J Med Sci. 1981 Jul;17(7):510–515. [PubMed] [Google Scholar]
- Schmitt S., Layh G., Buchanan T. M. Purification of protein IA from Neisseria gonorrhoeae using monoclonal antibody affinity chromatography. Microb Pathog. 1987 Sep;3(3):221–225. doi: 10.1016/0882-4010(87)90099-4. [DOI] [PubMed] [Google Scholar]
- Schneider C., Newman R. A., Sutherland D. R., Asser U., Greaves M. F. A one-step purification of membrane proteins using a high efficiency immunomatrix. J Biol Chem. 1982 Sep 25;257(18):10766–10769. [PubMed] [Google Scholar]
- Sorensen K., Brodbeck U. Assessment of coating-efficiency in ELISA plates by direct protein determination. J Immunol Methods. 1986 Dec 24;95(2):291–293. doi: 10.1016/0022-1759(86)90419-9. [DOI] [PubMed] [Google Scholar]
- Sperker B., Hu P., Herrmann R. Identification of gene products of the P1 operon of Mycoplasma pneumoniae. Mol Microbiol. 1991 Feb;5(2):299–306. doi: 10.1111/j.1365-2958.1991.tb02110.x. [DOI] [PubMed] [Google Scholar]
- Strebel K., Beck E., Strohmaier K., Schaller H. Characterization of foot-and-mouth disease virus gene products with antisera against bacterially synthesized fusion proteins. J Virol. 1986 Mar;57(3):983–991. doi: 10.1128/jvi.57.3.983-991.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su C. J., Tryon V. V., Baseman J. B. Cloning and sequence analysis of cytadhesin P1 gene from Mycoplasma pneumoniae. Infect Immun. 1987 Dec;55(12):3023–3029. doi: 10.1128/iai.55.12.3023-3029.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu A. C., Foy H. M., Cartwright F. D., Kenny G. E. The principal protein antigens of isolates of Mycoplasma pneumoniae as measured by levels of immunoglobulin G in human serum are stable in strains collected over a 10-year period. Infect Immun. 1987 Aug;55(8):1830–1836. doi: 10.1128/iai.55.8.1830-1836.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yayoshi M., Hayatsu E., Yoshioka M. [Protective effects of Mycoplasma pneumoniae live vaccine or its hyperimmune serum on the experimental infection in mice]. Kansenshogaku Zasshi. 1989 Jul;63(7):684–691. doi: 10.11150/kansenshogakuzasshi1970.63.684. [DOI] [PubMed] [Google Scholar]