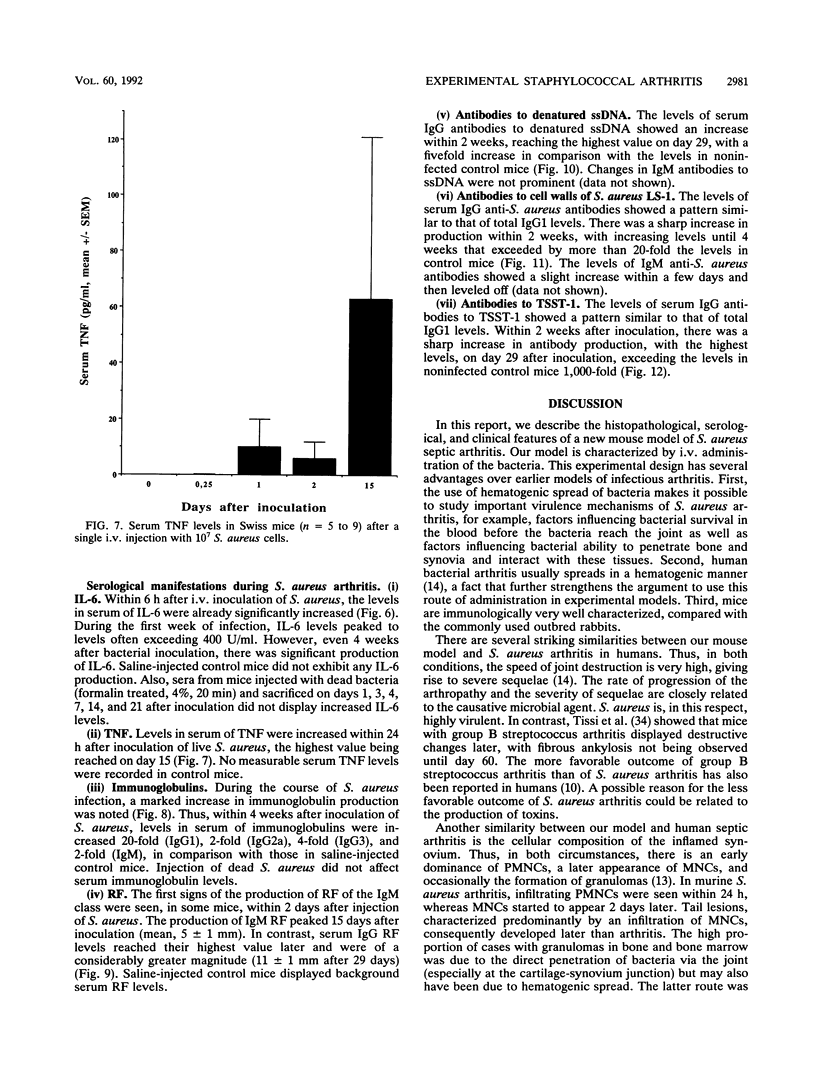
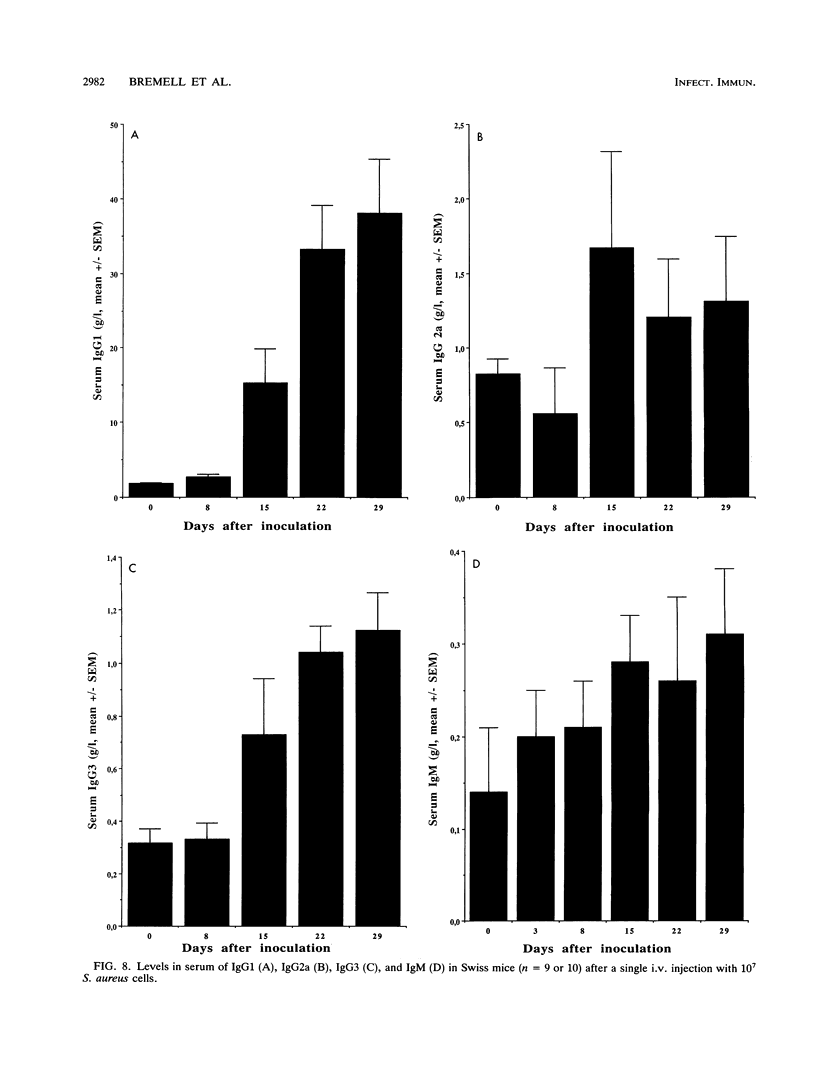
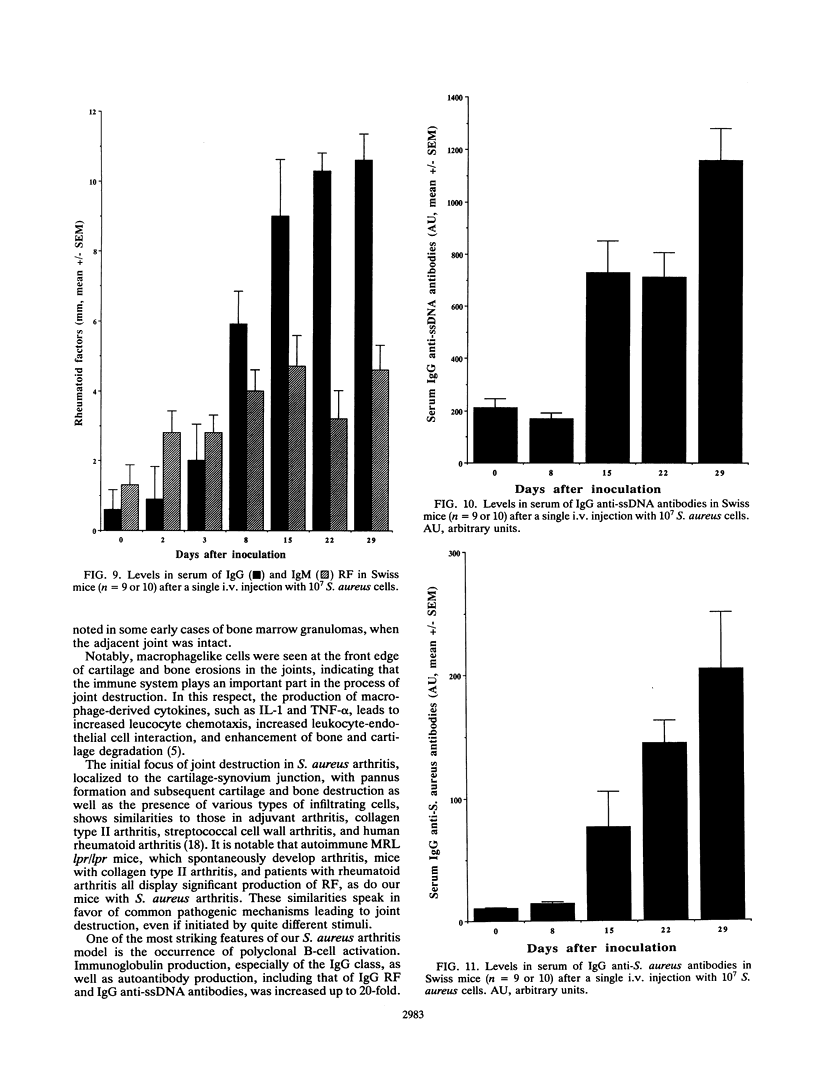
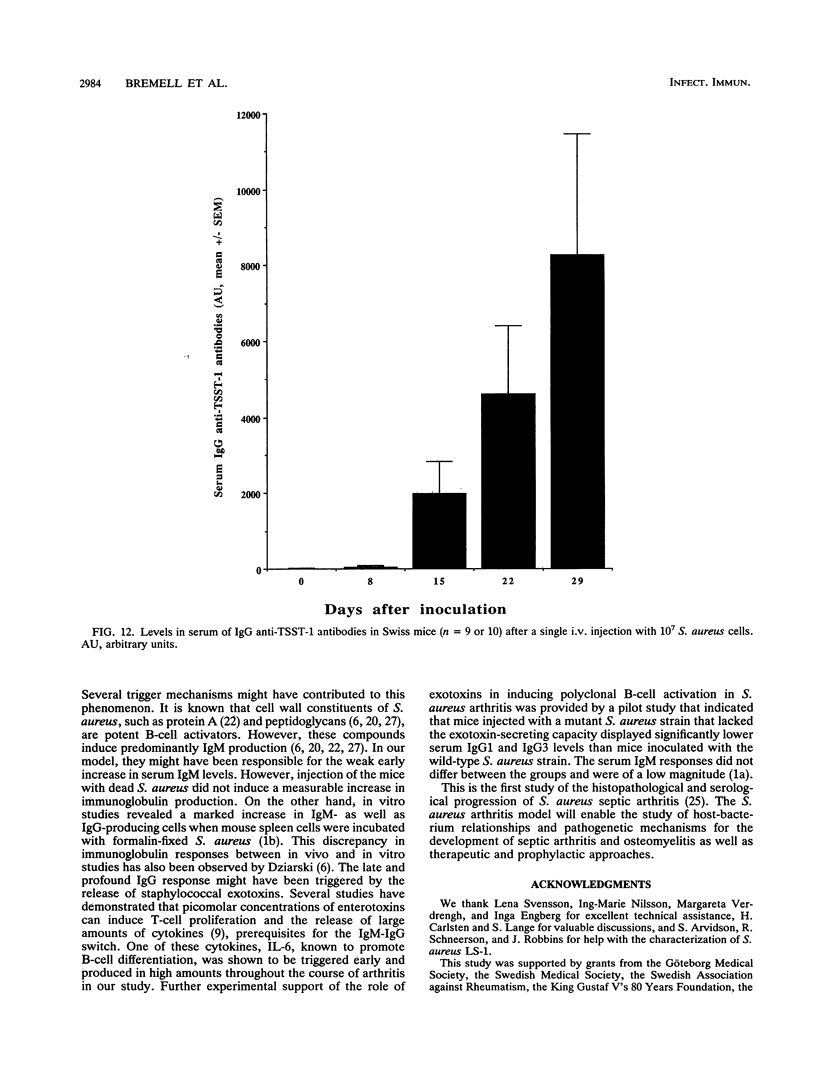
Abstract
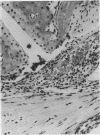
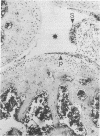
In a newly developed mouse model of Staphylococcus aureus arthritis the kinetics of joint destruction and serological manifestations as well as the clinical course of arthritis and osteitis were studied. Almost all mice developed histopathological signs of arthritis upon a single intravenous injection of 10(7) S. aureus LS-1 cells. There was rapid joint destruction, with synovial hypertrophy already visible, within 24 h after injection of the bacteria. Cartilage and/or bone erosions were seen in a majority of the mice within 72 h. Extra-articular manifestations, especially signs of bone infection, were also found soon after inoculation of the bacteria. Tail osteitis was frequent (50% of the mice) but appeared later than arthritis. Polymorphonuclear cells prevailed in the early joint lesions and were also common in the extra-articular manifestations. Within 3 days, mononuclear cells were also seen in the inflamed synovium, gaining a dominant position 3 weeks after the start of the disease. Serum interleukin-6 levels were already increased within 6 h after bacterial injection and remained elevated throughout the course of arthritis. Serum tumor necrosis factor levels were increased within 24 h. There was a tremendous induction of immunoglobulin production, especially of the immunoglobulin G1 isotype. This was paralleled by the production of specific antibodies to S. aureus (cell walls and toxin), as well as autoantibodies (rheumatoid factors and anti-single-stranded DNA antibodies), all predominantly of the immunoglobulin G isotype. The type and magnitude of the immunoglobulin G response together with the elevated interleukin-6 levels speak in favor of both antigen-specific and polyclonal B-cell activation during S. aureus arthritis. This study points out important similarities between our new model of S. aureus arthritis and human S. aureus arthritis. This resemblance will enable controlled studies of pathogenetic mechanisms of septic arthritis as well as therapeutic and prophylactic approaches.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aarden L. A., De Groot E. R., Schaap O. L., Lansdorp P. M. Production of hybridoma growth factor by human monocytes. Eur J Immunol. 1987 Oct;17(10):1411–1416. doi: 10.1002/eji.1830171004. [DOI] [PubMed] [Google Scholar]
- Brakenhoff J. P., de Groot E. R., Evers R. F., Pannekoek H., Aarden L. A. Molecular cloning and expression of hybridoma growth factor in Escherichia coli. J Immunol. 1987 Dec 15;139(12):4116–4121. [PubMed] [Google Scholar]
- Bremell T., Lange S., Svensson L., Jennische E., Gröndahl K., Carlsten H., Tarkowski A. Outbreak of spontaneous staphylococcal arthritis and osteitis in mice. Arthritis Rheum. 1990 Nov;33(11):1739–1744. doi: 10.1002/art.1780331120. [DOI] [PubMed] [Google Scholar]
- Bremell T., Lange S., Yacoub A., Rydén C., Tarkowski A. Experimental Staphylococcus aureus arthritis in mice. Infect Immun. 1991 Aug;59(8):2615–2623. doi: 10.1128/iai.59.8.2615-2623.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung A. L., Koomey J. M., Lee S., Jaffe E. A., Fischetti V. A. Recombinant human tumor necrosis factor alpha promotes adherence of Staphylococcus aureus to cultured human endothelial cells. Infect Immun. 1991 Oct;59(10):3827–3831. doi: 10.1128/iai.59.10.3827-3831.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziarski R. Comparison of in vitro and in vivo mitogenic and polyclonal antibody and autoantibody responses to peptidoglycan, LPS, protein A, PWM, PHA and Con A in normal and autoimmune mice. J Clin Lab Immunol. 1985 Feb;16(2):93–109. [PubMed] [Google Scholar]
- Elwing H., Nygren H. Diffusion in gel-enzyme linked immunosorbent assay (DIG-ELISA): a simple method for quantitation of class-specific antibodies. J Immunol Methods. 1979;31(1-2):101–107. doi: 10.1016/0022-1759(79)90290-4. [DOI] [PubMed] [Google Scholar]
- Espevik T., Nissen-Meyer J. A highly sensitive cell line, WEHI 164 clone 13, for measuring cytotoxic factor/tumor necrosis factor from human monocytes. J Immunol Methods. 1986 Dec 4;95(1):99–105. doi: 10.1016/0022-1759(86)90322-4. [DOI] [PubMed] [Google Scholar]
- Fischer H., Dohlsten M., Andersson U., Hedlund G., Ericsson P., Hansson J., Sjögren H. O. Production of TNF-alpha and TNF-beta by staphylococcal enterotoxin A activated human T cells. J Immunol. 1990 Jun 15;144(12):4663–4669. [PubMed] [Google Scholar]
- Goldenberg D. L., Chisholm P. L., Rice P. A. Experimental models of bacterial arthritis: a microbiologic and histopathologic characterization of the arthritis after the intraarticular injections of Neisseria gonorrhoeae, Staphylococcus aureus, group A streptococci, and Escherichia coli. J Rheumatol. 1983 Feb;10(1):5–11. [PubMed] [Google Scholar]
- Goldenberg D. L., Cohen A. S. Synovial membrane histopathology in the differential diagnosis of rheumatoid arthritis, gout, pseudogout, systemic lupus erythematosus, infectious arthritis and degenerative joint disease. Medicine (Baltimore) 1978 May;57(3):239–252. doi: 10.1097/00005792-197805000-00004. [DOI] [PubMed] [Google Scholar]
- Goldenberg D. L. Infectious arthritis complicating rheumatoid arthritis and other chronic rheumatic disorders. Arthritis Rheum. 1989 Apr;32(4):496–502. doi: 10.1002/anr.1780320422. [DOI] [PubMed] [Google Scholar]
- Goldenberg D. L., Reed J. I. Bacterial arthritis. N Engl J Med. 1985 Mar 21;312(12):764–771. doi: 10.1056/NEJM198503213121206. [DOI] [PubMed] [Google Scholar]
- Helle M., Boeije L., Aarden L. A. Functional discrimination between interleukin 6 and interleukin 1. Eur J Immunol. 1988 Oct;18(10):1535–1540. doi: 10.1002/eji.1830181010. [DOI] [PubMed] [Google Scholar]
- Johnson A. H., Campbell W. G., Jr, Callahan B. C. Infection of rabbit knee joints after intra-articular injection of Staphylococcus aureus. Comparison with joints injected with Staphylococcus albus. Am J Pathol. 1970 Aug;60(2):165–202. [PMC free article] [PubMed] [Google Scholar]
- Klareskog L. What can we learn about rheumatoid arthritis from animal models? Springer Semin Immunopathol. 1989;11(3):315–333. doi: 10.1007/BF00197310. [DOI] [PubMed] [Google Scholar]
- LEWIS G. W., CLUFF L. E. SYNOVITIS IN RABBITS DURING BACTEREMIA AND VACCINATION. Bull Johns Hopkins Hosp. 1965 Mar;116:175–190. [PubMed] [Google Scholar]
- Lansdorp P. M., Aarden L. A., Calafat J., Zeiljemaker W. P. A growth-factor dependent B-cell hybridoma. Curr Top Microbiol Immunol. 1986;132:105–113. doi: 10.1007/978-3-642-71562-4_14. [DOI] [PubMed] [Google Scholar]
- Levy R. J., Haidar M., Park H., Tar L., Levinson A. I. Bacterial peptidoglycan induces in vitro rheumatoid factor production by lymphocytes of healthy subjects. Clin Exp Immunol. 1986 May;64(2):311–317. [PMC free article] [PubMed] [Google Scholar]
- Lipsky P. E. Staphylococcal protein A, a T cell-regulated polyclonal activator of human B cells. J Immunol. 1980 Jul;125(1):155–162. [PubMed] [Google Scholar]
- Lue C., Tarkowski A., Mestecky J. Systemic immunization with pneumococcal polysaccharide vaccine induces a predominant IgA2 response of peripheral blood lymphocytes and increases of both serum and secretory anti-pneumococcal antibodies. J Immunol. 1988 Jun 1;140(11):3793–3800. [PubMed] [Google Scholar]
- Mahowald M. L. Animal models of infectious arthritis. Clin Rheum Dis. 1986 Aug;12(2):403–421. [PubMed] [Google Scholar]
- Mahowald M. L., Peterson L., Raskind J., Raddatz D. A., Shafer R., Gerding D. Antigen-induced experimental septic arthritis in rabbits after intraarticular injection of Staphylococcus aureus. J Infect Dis. 1986 Aug;154(2):273–282. doi: 10.1093/infdis/154.2.273. [DOI] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Russell M. W., Brown T. A., Radl J., Haaijman J. J., Mestecky J. Assay of human IgA subclass antibodies in serum and secretions by means of monoclonal antibodies. J Immunol Methods. 1986 Feb 27;87(1):87–93. doi: 10.1016/0022-1759(86)90347-9. [DOI] [PubMed] [Google Scholar]
- Räsänen L., Lehto M., Jokinen I., Leinikki P. Polyclonal antibody formation of human lymphocytes to bacterial components. Immunology. 1986 Aug;58(4):577–581. [PMC free article] [PubMed] [Google Scholar]
- Schurman D. J., Johnson B. L., Jr, Amstutz H. C. Knee joint infections with Staphylococcus aureus and Micrococcus species. J Bone Joint Surg Am. 1975 Jan;57(1):40–49. [PubMed] [Google Scholar]
- Schurman D. J., Mirra J., Ding A., Nagel D. A. Experimental E. coli arthritis in the rabbit. A model of infectious and post-infectious inflammatory synovitis. J Rheumatol. 1977 Summer;4(2):118–128. [PubMed] [Google Scholar]
- Tarkowski A., Czerkinsky C., Nilsson L. A. Detection of IgG rheumatoid factor secreting cells in autoimmune MRL/1 mice: a kinetic study. Clin Exp Immunol. 1984 Oct;58(1):7–12. [PMC free article] [PubMed] [Google Scholar]
- Tissi L., Marconi P., Mosci P., Merletti L., Cornacchione P., Rosati E., Recchia S., von Hunolstein C., Orefici G. Experimental model of type IV Streptococcus agalactiae (group B streptococcus) infection in mice with early development of septic arthritis. Infect Immun. 1990 Sep;58(9):3093–3100. doi: 10.1128/iai.58.9.3093-3100.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. Z., Xu J. C., Xue D. M. Experimental study of acute suppurative bone and joint infection. II. Suppurative arthritis. Chin Med J (Engl) 1983 Dec;96(12):907–912. [PubMed] [Google Scholar]