Abstract
Study Objectives:
To test whether instrumental conditioning of sensorimotor rhythm (SMR; 12–15 Hz) has an impact on sleep parameters as well as declarative memory performance in humans.
Design:
Randomized, parallel group design
Setting:
10 instrumental conditioning sessions, pre- and posttreatment investigation including sleep evaluations
Participants:
27 healthy subjects (13 male)
Interventions:
SMR-conditioning (experimental group) or randomized-frequency conditioning (control group); declarative memory task before and after a 90-min nap
Measurement and Results:
The experimental group was trained to enhance the amplitude of their SMR-frequency range, whereas the control group participated in a randomized-frequency conditioning program (i.e., every session a different 3-Hz frequency bin between 7 and 20 Hz). During pre- and posttreatment the subjects had to attend the sleep laboratory to take a 90-min nap (2:00–3:30 pm) and to perform a declarative memory task before and after sleep. The experimental design was successful in conditioning an increase in relative 12–15 Hz amplitude within 10 sessions (d = 0.7). Increased SMR activity was also expressed during subsequent sleep by eliciting positive changes in different sleep parameters (sleep spindle number [d = 0.6], sleep onset latency [d = 0.7]); additionally, this increased 12–15 Hz amplitude was associated with enhancement in retrieval score computed at immediate cued recall (d = 0.9).
Conclusion:
Relative SMR amplitude increased over 10 instrumental conditioning sessions (in the experimental group only) and this “shaping of one's own brain activity” improved subsequent declarative learning and facilitated the expression of 12–15 Hz spindle oscillations during sleep. Most interestingly, these electrophysiological changes were accompanied by a shortened sleep onset latency.
Citation:
Hoedlmoser K; Pecherstorfer T; Gruber G; Anderer P; Doppelmayr M; Klimesch W; Schabus M. Instrumental conditioning of human sensorimotor rhythm (12–15 Hz) and its impact on sleep as well as declarative learning. SLEEP 2008;31(10):1401–1408.
Keywords: Instrumental conditioning, SMR, sleep spindle, sleep quality, declarative memory
ELECTROENCEPHALOGRAPHIC (EEG) RECORDINGS OVER THE SENSORIMOTOR CORTEX SHOW A VERY DISTINCTIVE OSCILLATORY PATTERN IN A FREQUENCY range between 12 and 15 Hz termed sensorimotor rhythm (SMR).1–3 These brain activities—also known as “rolandic mu” or “wicket rhythms”4,5 —appear to be dominant during quiet but alert wakefulness6 and desynchronize during planning, execution, and imagination of body movements.7,8 Active inhibition of motor behavior, conversely, results in SMR synchronization.3 Activity in this frequency range is known to be abundant during light NREM sleep and represents the classical sleep spindle band. Spindles emerge at sleep onset, have a waxing-and-waning appearance, and are known to be generated in thalamocortical circuits.9 Interestingly, recent data indicate that fast spindles (>13 Hz) also recruit a set of cortical regions usually involved in sensorimotor as well as memory processing.10
The present study tried to enhance this frequency range by using classical instrumental conditioning, a learning paradigm based on the long known “law of effect,”11,12 which postulates that rewarding certain behavior increases the likelihood of its reoccurrence. Pulling a lever or pressing a button are overt behavioral actions traditionally increased by that kind of learning paradigm in animals.13,14 Our investigation follows this logic by trying to directly modify neural activity by instrumental conditioning of certain EEG activities.
Instrumental conditioning of different EEG parameters has long been used as therapeutic tool to treat different types of disorders, all above epilepsy15 and attention deficit hyperactivity disorder (ADHD).16 Since the pioneering work of Nicolelis17 in the field of brain-computer interface also “locked-in” and partly paralyzed patients benefit of this specific method by voluntarily learning to control neuronal activity and thereby machines.18
Early findings by Sterman et al.19 could already demonstrate that facilitation of SMR-rhythm by conditioning of EEG activity during wakefulness in cats (i) selectively enhances spindle activity during subsequent sleep and (ii) produces even longer epochs of undisturbed sleep. Sterman and colleagues were thereby the first to show that SMR conditioning (in cats) can actually be transferred into sleep, inducing a facilitation of spindle bursts as well as decreasing sleep fragmentation (i.e., reduced waking and movements during NREM sleep).
In a more recent study Amzica et al.20 successfully conditioned cats to generate fast (20–50 Hz) oscillation bursts within the motor (area 4) as well as the visual cortex (area 17). Additionally the increased burst-generation was associated with an enhanced synchrony of fast oscillations at different levels of the thalamocortical network. Most interestingly, the increased thalamocortical synchrony acquired during the conditioning sessions was also expressed during subsequent quiet waking, NREM as well as REM sleep. This again indicates that the facilitation of specific oscillations during wakefulness selectively enhances similar brain patterns during subsequent sleep periods.
Sterman and Shouse21 further applied instrumental SMR conditioning (ISC) to epileptic patients and succeeded in increasing SMR activity during sleep, accompanied by a reduction in seizure rate. It has also been shown that ISC can be effective in treating psychophysiologic insomnia.22,23 Hauri and coworkers22,23 revealed that tense and anxious insomnia patients respond more to EMG-theta than ISC, while those who were more relaxed at study intake preferentially responded to ISC. These authors were the first to show that appropriate instrumental conditioning can have long-lasting effects on insomnia and that instrumental conditioning tailored to specific patient needs appears to be most efficacious.
Recent research on EEG conditioning focused more on healthy individuals with the purpose of increasing cognitive performance.24 Reports have indicated that conditioning protocols can be successfully used to improve attentional processing,25,26 increase accuracy in working memory tasks,27 or enhance performance in mental rotation.28
In a pilot study done in our laboratory,29 we showed that ISC can have a direct influence on subsequent sleep spindle frequency band power in a healthy human population. We also examined whether increased spindle activity has an impact on memory consolidation during sleep, as indicated by recent findings demonstrating a significant positive correlation between overnight change in declarative memory performance and spindle intensity change from control to learning nights.30 However, no effects on memory performance were evident in that study, probably reflecting the fact that the amount of training was limited to 4 × 10 min immediately preceding sleep. Therefore, the study was not comparable to the much more intense training protocols used in other conditioning paradigms.
There is a growing body of evidence suggesting the feasibility of learning to regulate specific brain oscillations to counteract maladaptive brain activity associated with various disorders such as epilepsy, ADHD, or insomnia. Unfortunately, much of previous ISC research (often termed “SMR neurofeedback”) has suffered from missing standardized measures of target symptoms, neglected the assessment of EEG changes after training, and often lacked appropriate control groups. Therefore well-controlled investigations are needed before ISC can be considered a reliable nonpharmacological treatment for various disorders such as epilepsy, ADHD, or insomnia.
In the present investigation we explore whether ISC as compared to a randomized-frequency conditioning protocol can significantly increase SMR amplitude over the course of 10 sessions. Furthermore, we were interested in whether ISC might transfer into sleep and change classical sleep parameters such as sleep onset latency in a positive manner. Finally, we wanted to see if these changes would lead to positive impact on declarative memory performance.
EXPERIMENTAL DESIGN AND METHODOLOGY
Twenty-seven healthy subjects (13 male, 14 female; mean = 23.63 years, SD = 2.69) were randomly assigned (parallel group design) to either (i) an SMR-conditioning (ISC) protocol (experimental group; N = 16) or to (ii) a randomized-frequency conditioning protocol (control group; N = 11).
Subjects attended the laboratory on 13 occasions (Figure 1). First subjects had to pass an entrance examination consisting of several parts: clinical evaluation of sleep quality (Pittsburgh Sleep Quality Index31); depression (self-rated depression scale32); anxiety (self-rated anxiety scale33); memory (Wechsler Memory Scale–Revised34), and intelligence (Advanced Progressive Matrices35).
Figure 1.
Study design. Subjects had to attend the laboratory 13 times. The first visit (3 days prior to pretreatment) served as entrance examination. Pretreatment included a declarative memory task (encoding [ENCpre], retrieval before nap [RET1pre], retrieval after nap [RET2pre]) as well as a 90-min nap (NAPpre) and was followed by 10 instrumental conditioning sessions on 10 consecutive days (except weekends). Posttreatment (same procedure like pretreatment: ENCpost, RET1post, NAPpost and RET2post) —one day after the last conditioning session—completed the study protocol.
Throughout participation, subjects had to complete a sleep diary every day in the evening and in the morning (Self-rating scale for Sleep and Awakening quality36) to control sleep-wake-cycle and to prevent sleep deprivation prior to laboratory examination. During the pre- and posttreatment session and preceding a 90-min nap the subjects performed a declarative word-pair association task.37 Subjects had to learn a first list of 80 word-pairs during pretreatment (ENCpre), and a second different list during posttreatment (ENCpost). Word-pairs in each encoding session were presented twice in randomized order on a computer screen. Both presentations were separated by a short break of approximately 2 min. In the encoding sessions each word-pair was presented for 1500 ms, immediately followed by a (white) centered fixation cross flipping to grey (after 5 sec), resulting in a total learning time of 2 × 13.5 min. To better control for the mnemonic strategies used, subjects were instructed to visually imagine a relation between the 2 otherwise unrelated words of each pair. After presentation of all pairs and following a 10-min break, subjects performed a (cued) recall task (RET1pre/post). Cued recall was repeated after the nap (RET2 pre/post). The correct response score consisted of (i) the number of correct responses and (ii) the number of (unambiguous) semantically correct answers (e.g., “flow” or “stream” instead of “river”) which were weighted by 0.5 to account for the weaker but still present memory trace. The obtained values were expressed as percentages ([correct response score/80]*100) and are subsequently referred to as “retrieval score.”
Polygraphic sleep recordings using Synamps EEG amplifiers (NeuroScan Inc. Singen, Germany) started at 2:00 pm and ended at 3:30 pm. All signals were filtered (0.10 Hz high-pass filter; 70 Hz low-pass filter; 50-Hz notch filter) and digitized online with 500-Hz sampling rate. Fifteen gold-plated silver electrodes were attached according to the international 10/20 system (F3, Fz, F4, C3, Cz, C4, P3, Pz, P4, O1, Oz, O2, as well as A1 and A2 for later re-referencing) and were referenced to FCz. In addition, 4 electrooculogram (EOG) channels, one submental electromyogram channel, one electrocardiogram channel (ECG), and one respiratory channel (chest wall movements) were recorded.
Instrumental Conditioning Procedure
Between pre- and posttreatment, the subjects were trained to enhance band amplitude within specific frequency bins during 10 conditioning sessions on 10 consecutive days. Visual online feedback was provided using the eldith THERA PRAX (neuroConn GmbH, Llmenau, Germany) system. Each session was conducted in a standardized procedure and lasted for 1 hour. After electrode adjustment subjects were instructed to relax during a 2-min eyes-closed resting condition followed by a 2-min eyes open resting condition in which a fixation cross was presented on the computer screen. Immediately afterwards, subjects had to perform eight 3-min blocks of instrumental conditioning. Another 2 resting conditions (eyes-open, eyes-closed) completed each session. EEG was recorded using a sampling rate of 500 Hz from C3 with reference on the right earlobe and ground electrode placed on the left earlobe. For offline artifact rejection, a bipolar vertical EOG channel was recorded. The ongoing EEG at site C3 was band-pass filtered to continuously extract amplitude values (μV) within the frequency of interest. Band amplitude of interest was calculated online and used as relevant conditioning parameter.
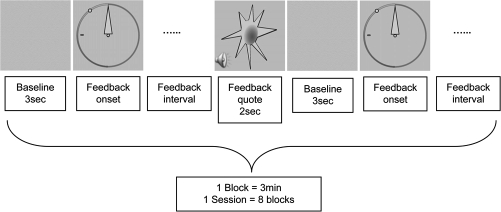
The instrumental conditioning design of the experimental group was performed as follows (Figure 2): One trial consisted of a 3-sec baseline interval followed by a continuous feedback interval lasting until the EEG signal exceeded the predefined reward threshold measured during the baseline for ≥ 250 ms. The aim was to move the needle as far to the left as possible reaching the previously fixed threshold represented by a green dot. Any time this SMR amplitude threshold was exceeded for ≥ 250 ms, subjects got an audiovisual reward (appearance of a sun for 2 sec accompanied by a 200 ms lasting sound of 800 Hz). Subjects were encouraged to look themselves for appropriate strategies like physiological relaxation combined with positive mental activity. After the appearance of the reward and a randomized interval lasting for 1–3 sec the baseline value was refreshed followed by a new continuous feedback interval until the next reward. After a 3-min block, including a varying number of rewards, instrumental conditioning was paused for about 1 min and thereafter continued. To prevent rewards elicited by movements, eye or muscle artifacts, trials with amplitudes exceeding ± 200 μV were abandoned by starting a new trial (without rewarding the prior trial).
Figure 2.
Schematic representation of one block within an instrumental conditioning session. A 3-sec “Baseline” before visual feedback onset was used to calculate the mean amplitude within the frequency of interest which served as reference during “Feedback interval.” An audiovisual “Feedback quote” was triggered by an EEG signal containing ≥ 250 ms of the frequency of interest at an amplitude exceeding a certain reward threshold measured during the 3-sec baseline.
The instrumental conditioning design of the control group was identical and differed only in frequency adjustments. Whereas the experimental group had to enhance SMR amplitude throughout all 10 instrumental conditioning sessions, the controls were dedicated to a randomized frequency paradigm. Therefore the band amplitude of randomized 3-Hz frequency bins between 7 and 20 Hz (except 12–15 Hz) was used as relevant conditioning parameter. At each of the 10 sessions the subjects of the control group had to enhance the amplitude of a different frequency range to avoid enhancement within any specific frequency bin. All participants remained blind to their group assignment and were not debriefed until the end of the investigation.
EEG Data Analyses
EEG Data Analyses during Instrumental Conditioning
For offline processing of EEG the Brain Vision Analyzer software (Version 1.05.0002; Brain Products GmbH) was employed. In a first step, ocular artifacts were automatically minimized and visually controlled. To obtain amplitude values in the frequency domain fast Fourier transformation was applied. By averaging in the frequency domain, amplitude spectra were calculated in steps of 1 Hz for each of the feedback intervals (“Feedback onset” to “Feedback quote”; Figure 2) during a 3-min trial. In order to reduce the huge and nonspecific effects of intersubject variation in absolute amplitude values, we obtained normalized amplitude values by dividing the mean amplitude during the feedback interval by the mean amplitude during rest with eyes open preceding the instrumental conditioning sessions. These derived relative amplitude values were then averaged across all blocks within one session. In order to compare EEG during early vs. late instrumental conditioning, EEG data were further averaged across session 2 to 4 (early conditioning) and session 8 to 10 (late conditioning) for each subject. The first instrumental conditioning session served as training session and was excluded from analyses.
EEG Data Analyses during Nap
Sleep was scored and staged automatically by The Siesta Group (Somnolyzer 24 × 7) according to classical criteria from Rechtschaffen and Kales.38 Spindle detection was based on a new automatic algorithm, which is a further development of the bandpass filtering method developed by Schimicek et al.39 In a first step, the EEG signal was filtered with a phase linear fourth order Butterworth band-pass filter in the frequency range of 10–18 Hz and the envelope of the filtered signal was determined by Hilbert transformation. In a second step, sleep spindles were automatically identified based on the following criteria: (i) minimal amplitude of 12 μV, (ii) spindle duration 0.3–2.0 sec, and (iii) a frequency range of 11–16 Hz. These thresholds were determined from the distribution of these variables in a polysomnographic pattern database including several thousand visually identified spindles from 189 healthy controls and 90 patients (see Anderer40). The algorithm identifies first “possible” spindles and then determines “real” spindle episodes by means of a linear discrimination analysis using 5 log-transformed features (spindle duration and mean amplitudes in 4 frequency bands: spindle, theta, alpha, and fast beta) of the “possible” spindles. Rather than measuring the mean number of sleep spindles per time (spindle density), the applied algorithm provides sleep spindle features such as the duration, amplitude, and frequency, and therefore reflects the activity or intensity of the spindle process. The measure “spindle intensity” is composed of the mean amplitude and mean duration of sleep spindle events. Additionally, the overall number of spindle detections and traditional spindle density were computed. In order to detect all possible spindle events, the spindle algorithm was set to a high sensitivity level (discrimination value = 0.8).
Statistical Analyses
Statistical analyses were performed using SPSS 15.0.0 software (SPSS Inc., Chicago, Illinois). Kolmogorov-Smirnov tests were applied to test for the normality of the distribution of the data, which was given in all cases. The significance level was set to P < 0.05 and for post hoc comparisons paired-sample t-tests were performed. Within-group effect sizes (pooled SD) were calculated using the Cohen d formula41 (Cohen 0.2 is indicative of a small effect size, 0.5 a medium effect size, and 0.8 a large effect size).
Only data of good signal quality were included in statistical analyses. Outliers (values >3 SD) were excluded from analyses. Therefore number of valid subjects varies throughout the different ANOVAs between 25 and 27. Because of technical problems regarding the declarative memory performance task, retrieval scores of only 20 subjects (7 missing) could be analyzed.
To estimate relative amplitude changes after instrumental conditioning, a 2-way-analysis of variance (ANOVA) for repeated measures was performed with the within-subject factor CONDITIONING (early conditioning [session 2–4] vs. late conditioning [session 8–10]) and the between-subject factor GROUP (experimental group vs. control group). Relative SMR amplitude was the dependent measure. For sake of clarity and ruling out the possibility that these changes might be caused by baseline modifications, the same ANOVA was also calculated with relative SMR amplitude during baseline (mean amplitude values during the baseline divided by mean amplitude values during rest with eyes open preceding the instrumental conditioning). Additionally, to control absolute amplitude changes, this ANOVA was computed with the dependent measures absolute SMR amplitude during (i) baseline, (ii) feedback interval, and (iii) rest with eyes open.
In order to determine instrumental conditioning effects on sleep parameters (during NAPpre vs. NAPpost) as well as on memory retrieval (RET1pre/post, RET2pre/post), 2-way-ANOVAs (PREPOST × GROUP) were performed. With respect to sleep parameters we analyzed spindle intensity, spindle number, total sleep period, wake after sleep onset, sleep onset latency, duration of stage 1, stage 2, SWS, and REM sleep.
RESULTS
Instrumental Conditioning of SMR
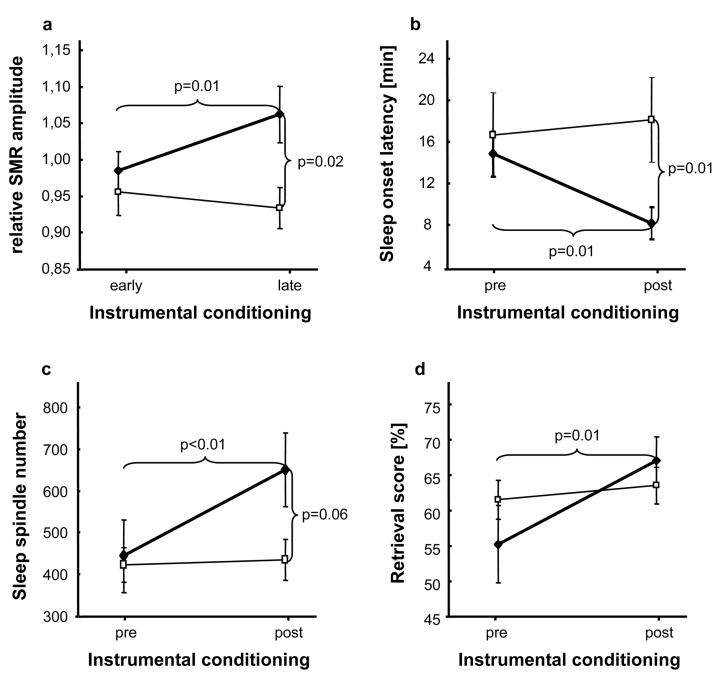
On average (N = 25) relative SMR amplitude was 0.97 (SD = 0.10) during early conditioning (training session 2–4) and 1.01 (SD = 1.39) during late conditioning (training session 8–10). Results of the 2-way ANOVA (Table 1) showed a significant interaction between CONDITIONING × GROUP (F1,23 = 7.814; P = 0.010). Pairwise comparisons revealed a significant increase of relative SMR amplitude between early and late instrumental conditioning only in the experimental group (t13 = −2.832; P = 0.014; d = 0.7; Figure 3a). There were no systematic changes over the course of training concerning neither relative SMR amplitude during baseline nor absolute SMR amplitude (measured during baseline, feedback, and rest with eyes open).
Table 1.
Relative SMR amplitude values during early (training session 2–4) and late (training session 8–10) conditioning measured during baseline and feedback interval; absolute SMR amplitude values (μV) during early (training session 2–4) and late (training session 8–10) conditioning measured during baseline, feedback interval as well as rest with eyes open. Time in bed, time spent in sleep stage 1, sleep stage 2, SWS, REM, sleep spindle number, sleep spindle intensity, sleep onset latency, total sleep period, and wake after sleep onset for NAPpre and NAPpost. Retrieval scores before (RET1) and after (RET2) NAPpre/post in experimental and control group.
| Early Conditioning | Late Conditioning | Paired-samples t test | ANOVA CONDITIONING* GROUP | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Experimental | Control | Experimental | Control | Experimental | Control | |||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | t | P | T | P | F | P | |
| relative SMR amplitude feedback | 0.98 | 0.10 | 0.96 | 0.11 | 1.06 | 0.14 | 0.93 | 0.09 | −2.83 | 0.01 | 1.11 | 0.30 | 7.81 | 0.01 |
| relative SMR amplitude baseline | 1.09 | 0.16 | 0.98 | 0.13 | 1.07 | 0.13 | 0.94 | 0.13 | 0.36 | 0.73 | 1.03 | 0.33 | 0.07 | 0.80 |
| absoluteSMR amplitude feedback | 0.59 | 0.19 | 0.60 | 0.28 | 0.66 | 0.20 | 0.62 | 0.31 | −2.75 | 0.02 | −1.22 | 0.25 | 2.45 | 0.13 |
| absolute SMR amplitude baseline | 0.64 | 0.20 | 0.62 | 0.29 | 0.67 | 0.26 | 0.64 | 0.35 | −0.99 | 0.34 | −0.90 | 0.39 | 0.05 | 0.82 |
| absoluteSMR amplitude rest | 0.60 | 0.20 | 0.63 | 0.26 | 0.63 | 0.21 | 0.67 | 0.31 | −1.28 | 0.22 | −1.75 | 0.11 | 0.11 | 0.74 |
| Pretreatment | Posttreatment | Paired-samples t test | ANOVA PREPOST* GROUP | |||||||||||
| Experimental | Control | Experimental | Control | Experimental | Control | |||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | t | P | T | P | F | P | |
| Time in bed, min | 87.72 | 5.81 | 89.73 | 0.34 | 88.34 | 4.66 | 90.18 | 0.72 | −0.52 | 0.61 | −1.84 | 0.10 | 0.01 | 0.91 |
| Stage 1, min | 29.46 | 28.60 | 8.85 | 5.76 | 18.07 | 17.86 | 10.90 | 5.61 | 1.48 | 0.16 | −1.16 | 0.27 | 2.03 | 0.17 |
| Stage 2, min | 56.21 | 26.56 | 45.38 | 13.20 | 55.81 | 16.55 | 44.58 | 18.44 | 0.06 | 0.95 | 0.15 | 0.88 | <0.01 | 0.96 |
| SWS, min | 9.06 | 15.54 | 38.99 | 19.01 | 19.74 | 18.70 | 38.62 | 20.25 | −1.90 | 0.08 | 0.07 | 0.95 | 1.80 | 0.19 |
| REM, min | 5.28 | 8.90 | 6.80 | 8.28 | 6.41 | 7.70 | 5.92 | 6.87 | −0.52 | 0.61 | 0.41 | 0.70 | 0.40 | 0.53 |
| Sleep Spindle Number | 444.43 | 325.18 | 422.55 | 135.46 | 650.86 | 330.91 | 434.00 | 162.05 | −3.49 | 0.00 | −0.38 | 0.71 | 7.29 | 0.01 |
| Sleep Spindle Intensity | 18.30 | 4.94 | 15.88 | 2.42 | 19.07 | 4.34 | 15.94 | 2.99 | −0.56 | 0.59 | −0.13 | 0.90 | 0.19 | 0.67 |
| Sleep Onset Latency, min | 14.75 | 11.51 | 16.55 | 11.56 | 8.09 | 5.93 | 18.05 | 13.25 | 3.17 | 0.01 | −0.56 | 0.59 | 5.85 | 0.02 |
| Total Sleep Period, min | 68.31 | 20.64 | 73.32 | 11.81 | 78.66 | 8.99 | 72.82 | 14.80 | −2.14 | 0.05 | 0.12 | 0.91 | 2.56 | 0.12 |
| Wake After Sleep Onset, min | 20.30 | 16.58 | 6.27 | 7.64 | 16.28 | 17.33 | 7.91 | 9.79 | 0.59 | 0.57 | −0.53 | 0.61 | 0.49 | 0.49 |
| RET1, % | 54.83 | 16.30 | 61.09 | 9.12 | 66.61 | 10.06 | 63.14 | 8.57 | −3.28 | 0.01 | −0.86 | 0.41 | 5.46 | 0.03 |
| RET2, % | 54.83 | 17.34 | 60.68 | 9.34 | 66.28 | 9.88 | 63.82 | 8.77 | −2.96 | 0.02 | −1.18 | 0.27 | 3.32 | 0.09 |
Note the significant alterations (marked bold) in relative as well as absolute SMR amplitude during feedback interval, sleep spindle number, sleep onset latency and retrieval score (RET1, RET2). Statistical trends (P < 0.10) are underlined. Paired-samples t tests (dark grey) depict changes in SMR amplitude over the course of training (top panel) as well as sleep architecture and retrieval score changes pre- to posttreatment (bottom panel) within the respective groups (all 2-tailed). The light grey shaded box depicts the ANOVA interactions of interest: CONDITIONING (early vs. late) × GROUP (experimental vs. control) at the top as well as PREPOST (pre-treatment vs. posttreatment) × GROUP (experimental vs. control) at the bottom.
Figure 3.
2-way ANOVAs depicting differences between experimental (♦) and control (□) group.
a. Significant increase of relative SMR amplitude after ISC (experimental group) vs. randomized-frequency-conditioning (control group). b. Significant reduction of sleep onset latency during NAPpost compared to NAPpre. c. Significant increase of sleep spindle number from NAPpre to NAPpost. d. Significant enhancement of retrieval score computed at immediate cued recall (RET1) after ISC (experimental group) compared to randomized-frequency-conditioning (control group). Note that only 12–15 Hz conditioning (experimental group) could increase relative SMR amplitude, sleep spindle number, and retrieval score as well as decrease sleep onset latency. Error bars indicate standard errors of mean.
Sleep Parameters
Sleep parameters for the experimental (N = 16) and control group (N = 11) are depicted in Table 1. On average time in bed (N = 27) was 88.54 min (SD = 4.53) during midday naps before instrumental conditioning (NAPpre) and 89.09 min (SD = 3.69) after the 10 conditioning sessions (NAPpost). No group (experimental vs. control) showed significant increase in duration of stage 1, 2, SWS or REM sleep after instrumental conditioning. However, there is a trend (P < 0.10) towards longer SWS duration (t15 = −1.902; P = 0.077) and increase in total sleep period (t15 = −2.143; P = 0.049) after ISC in the experimental group (Table 1).
Sleep Onset Latency
Results of the 2-way ANOVA showed a significant PREPOST × GROUP interaction (F1,25 = 5.845; P = 0.023). Pairwise comparisons revealed a significant decrease of sleep onset latency from NAPpre to NAPpost in the experimental group only (t15 = 3.169; P = 0.006; d = 0.7; Figure 3b).
Sleep Spindle Number
A significant main effect for PREPOST (F1,23 = 9.098; P = 0.006) and a significant interaction between PREPOST × GROUP (F1,23 = 7.285; P = 0.013) could be demonstrated. Pairwise comparisons revealed a significant increase of sleep spindle number from NAPpre to NAPpost for the experimental group only (t13 = −3.488; P = 0.004; d = 0.6; Figure 3c).
The PREPOST × GROUP interaction for the dependent measures sleep spindle intensity, time in bed, wake after sleep onset (WASO), total sleep period (TSP; time from the first to the last epoch in any sleep stage), duration of stage 1, stage 2, SWS, and REM were not significant (Table 1; light grey).
Declarative Memory Performance
Retrieval scores (RET1pre/post, RET2pre/post) for the experimental (N = 16) and control group (N = 11) are depicted in Table 1. On average (N = 20) “overnap” change in retrieval score was unchanged during pretreatment (before NAPpre: M = 58.28%; SD = 12.88% vs. after NAPpre: M = 58.05%; SD = 13.47%) as well as during posttreatment (before NAPpost: M = 64.70%; SD = 9.19% vs. after NAPpost: M = 64.93%; SD = 9.12%). This was also true when both study groups were analyzed separately. To test whether retrieval scores changed according to the training protocol, we calculated a 2-way ANOVA, with the within-subject factor PREPOST (retrieval score during pretreatment vs. retrieval score during posttreatment) and the between-subject factor GROUP (experimental group vs. control group). Results demonstrated an increase in retrieval score after conditioning (F1,18 = 11.02, P = 0.004) but no overall group difference (F1,18 = 0.09, P = 0.764). A significant interaction PREPOST × GROUP (F1,18 = 5.461, P = 0.031) revealed that only subjects of the experimental group had higher retrieval scores (RET1) after 10 ISC sessions (t8 = −3.281; P = 0.011; d = 0.9; Figure 3d).
Running the same analysis for memory retrieval after nap (RET2) did show similar effects. For the interaction PREPOST × GROUP a trend towards significance was found (F1,18 = 3.32, P = 0.085) with the pairwise comparisons again indicating an increase in retrieval score (RET2) after conditioning only in the experimental group (t8 = −2.956; P = 0.018; d = 0.8).
DISCUSSION
The present study examined the effects of instrumental SMR-conditioning (experimental group) as compared to randomized-frequency conditioning (control group) on sleep as well as declarative memory performance. Robust and significant SMR amplitude changes from early to late conditioning sessions confirmed the success of the ISC (Figure 3a). Furthermore, and most interestingly, these EEG changes transferred into sleep (Figure 3b, 3c) and even improved immediate memory retrieval after learning (Figure 3d). Memory consolidation (i.e., “overnap” change in memory performance after ISC) on the other hand was unchanged, indicating a more nonspecific effect of ISC. Possibly heightened attention or relaxation levels (during learning and/or retrieval) after 10 ISC sessions thus account for the observed improvement in word-pair recall. Frequencies in the 12–15 Hz range are associated with inhibition of motor activity2,3,42 and constitute the dominant “standby” frequency of the integrated thalamocortical, somatosensory, and somatomotor pathways.15 Similarly, synchronization of the related mu frequency has been suggested to be a correlate of an idling43 or “nil-working” state.44 Furthermore Hummel et al.45 presented direct evidence for the association of mu frequencies over sensorimotor areas and effective inhibition of acquired motor programs. In our theoretical perspective, the utility of SMR relates to the numerous findings that define this EEG rhythm as a thalamocortical consequence of decreased motor excitability,19,46 arising most likely both intentionally (waking SMR) and passively (sleep spindle) from the imposition of motor inhibition from functional reorganization within striatal (basal ganglia) circuits.15 Through a process of ISC (during waking), we intended to facilitate those circuits during the subsequent sleep periods. Results support our general rationale that an increase in relaxation and a decrease in muscle tension might lead to less movement during sleep and thereby augment the restorative and learning enhancing benefits of sleep. The present data widely replicate findings from Sterman and coworkers19 and demonstrate to our best knowledge for the first time successful ISC in a healthy human population (Figure 3a) leading to enhancement of sleep spindles (Figure 3c). Further, sleep onset latency (Figure 3b) was significantly shortened during the nap after only 10 ISC sessions as compared to a nap before instrumental conditioning. Importantly, we here used a much more rigorous control group than usually adopted. The control group underwent the exactly same study protocol and amount of treatment, with only the type of feedback being altered (i.e., different frequency bands to be trained at each session). Interestingly and in line with results by Sterman19 we found an increase in the number of sleep spindles, indicating that specific neural mechanism trained during wakefulness can be translated into sleep. As no change in the duration of stage 2 sleep was evident (Table 1), it can be ruled out that the spindle increase is reflecting an increase of stage 2 sleep. Furthermore, our results support Hauri's approach22,23 of using ISC as an alternative treatment for primary insomnia, since significant improvement in sleep onset latency (Figure 3b) was achieved after only 10 instrumental conditioning sessions. Future studies have to address whether this kind of training might be successfully applied to insomnia patients and whether observed changes are stable in the long term. The crucial point about this kind of “brain training” is that it directly acts on those brain oscillations which are altered in various types of disorders (for review see Cortoos 49).
As it has been proposed that sleep spindles are linked to declarative memory consolidation,30,47,48,50 we hypothesized that increasing spindle band power might also enhance declarative memory performance in a word-pair association task. Although our previous findings30 suggested an association between increased sleep spindle intensity during experimental nights and overnight improvement in declarative memory, we could not demonstrate that kind of change in our “overnap” protocol. However, comparisons of the retrieval score during pre- and posttreatment revealed significantly better learning performance after ISC but not randomized-frequency conditioning (Figure 3d). As ISC-protocols are also readily used to enhance attention in ADHD patients,16 it is possible that observed changes in retrieval scores are a result of changes in attention rather than direct influences on memory. Future studies should continue to focus on the effects of ISC on various cognitive tasks and also address the potential clinical significance of this kind of training.
In summary, we were able to demonstrate that intentional facilitation of SMR activity throughout wakefulness can directly enhance related brain oscillations during sleep (e.g., sleep spindles). Astonishingly, the successful enhancement of SMR amplitude by ISC, improved sleep quality (indicated by decreased sleep onset latency) as well as declarative learning. ISC might thus be considered a promising nonpharmacological treatment for primary insomnia and other disorders.
ABBREVIATIONS
- ADHD
attention deficit hyperactivity disorder
- ANOVA
analysis of variance
- d
Cohen's d
- EEG
Electroencephalography
- EMG
Electromyogram
- ENC
Encoding
- Hz
hertz
- ISC
instrumental SMR conditioning
- min
minute
- ms
millisecond
- NREM
non-rapid eye movement
- Pre
pretreatment
- Post
posttreatment
- REM
rapid eye movement
- RET1
Retrieval 1 (before nap)
- RET2
Retrieval 2 (after nap)
- SD
Standard deviation
- sec
second
- SMR
Sensorimotor rhythm
- SWS
Slow wave sleep
- TSP
Total sleep period
- WASO
Wake after sleep onset
- μV
microvolt
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Anderer is shareholder and Chief Scientific Officer (CSO) of The Siesta Group Schlafanalyse GmbH. Dr. Gruber is a part-time employee of the Siesta Group Schlafanalyse GmbH; both have participated in research projects on automated sleep analyses. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
We are grateful to Sonja Moser and Hermann Griessenberger (Department of Psychology, University of Salzburg, Austria) for data recording assistance. This research was supported by the “Forschungsstipendium Salzburg” and the “Stiftungs- und Foerderungsgesellschaft der Universitaet Salzburg.”
REFERENCES
- 1.Sterman MB, Wyrwicka W. EEG correlates of sleep: evidence for separate forebrain substrates. Brain Res. 1967;1:143–63. doi: 10.1016/0006-8993(67)90186-2. [DOI] [PubMed] [Google Scholar]
- 2.Chase MH, Harper RM. Somatomotor and visceromotor correlates of operantly conditioned 12–14 C-SEC sensorimotor cortical activity. Electroencephalogr Clin Neurophysiol. 1971;31:85–92. doi: 10.1016/0013-4694(71)90292-6. [DOI] [PubMed] [Google Scholar]
- 3.Howe RC, Sterman MB. Cortical-subcortical EEG correlates of suppressed motor behavior during sleep and waking in the cat. Electroencephalogr Clin Neurophysiol. 1972;32:681–95. doi: 10.1016/0013-4694(72)90104-6. [DOI] [PubMed] [Google Scholar]
- 4.Niedermeyer E. The normal EEG of the waking adult. In: Niedermeyer E, Lopes da Silva F, editors. Electroencephalography: basic principles, clinical applications, and related fields. Baltimore: Williams & Williams; 2005. pp. 149–73. [Google Scholar]
- 5.Gastaut H. Etude electrocorticographique de la reactivite des rhythms rolandiques. Rev Neurol (Paris) 1952;87:176–82. [PubMed] [Google Scholar]
- 6.Roth SR, Sterman MB, Clemente CD. Comparison of EEG correlates of reinforcement, internal inhibition and sleep. Electroencephalogr Clin Neurophysiol. 1967;23:509–20. doi: 10.1016/0013-4694(67)90017-x. [DOI] [PubMed] [Google Scholar]
- 7.Pfurtscheller G, Brunner C, Schlögl A, Lopes da Silva FH. Mu rhythm (de)synchronization and EEG single-trial classification of different motor imagery tasks. Neuroimage. 2006;31:153–59. doi: 10.1016/j.neuroimage.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 8.Neuper C, Wörtz M, Pfurtscheller G. ERD/ERS patterns reflecting sensorimotor activation and deactivation. Prog Brain Res. 2006;159:211–22. doi: 10.1016/S0079-6123(06)59014-4. [DOI] [PubMed] [Google Scholar]
- 9.Steriade M. Coherent oscillations and short-term plasticity in corticothalamic networks. Trends Neurosci. 1999;22:337–45. doi: 10.1016/s0166-2236(99)01407-1. [DOI] [PubMed] [Google Scholar]
- 10.Schabus M, Dang-Vu TT, Albouy G, et al. Hemodynamic cerebral correlates of sleep spindles during human non-REM sleep. Proc Natl Acad Sci U S A. 2007;104:13164–169. doi: 10.1073/pnas.0703084104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thorndike EL. The law of effect. Am J Psychol. 1927;39:212–22. [Google Scholar]
- 12.Thorndike EL. A proof of the law of effect. Science. 1933;77:173–75. doi: 10.1126/science.77.1989.173-a. [DOI] [PubMed] [Google Scholar]
- 13.Thorndike EL. Animal intelligence. New York: Macmillan; 1911. [Google Scholar]
- 14.Skinner BF. The behavior of organisms. New York: Appleton Century Crofts; 1938. [Google Scholar]
- 15.Sterman MB, Egner T. Foundation and practice of neurofeedback for the treatment of epilepsy. Appl Psychophysiol Biofeedback. 2006;31:21–35. doi: 10.1007/s10484-006-9002-x. [DOI] [PubMed] [Google Scholar]
- 16.Heinrich H, Gevensleben H, Strehl U. Annotation: neurofeedback - train your brain to train behaviour. J Child Psychol Psychiatry. 2007;1:3–16. doi: 10.1111/j.1469-7610.2006.01665.x. [DOI] [PubMed] [Google Scholar]
- 17.Nicolelis MA. Brain-machine interfaces to restore motor function and probe neural circuits. Nat Rev Neurosci. 2003;4:417–22. doi: 10.1038/nrn1105. [DOI] [PubMed] [Google Scholar]
- 18.Hinterberger T, Neumann N, Pham M, et al. A multimodal brain-based feedback and communication system. Exp Brain Res. 2004;154:521–26. doi: 10.1007/s00221-003-1690-3. [DOI] [PubMed] [Google Scholar]
- 19.Sterman MB, Howe RC, MacDonald LR. Facilitation of spindle-burst sleep by conditioning of electroencephalographic activity while awake. Science. 1970;167:1146–48. doi: 10.1126/science.167.3921.1146. [DOI] [PubMed] [Google Scholar]
- 20.Amzica F, Neckelmann D, Steriade M. Instrumental conditioning of fast (20- to 50-Hz) oscillations in corticothalamic networks. Proc Natl Acad Sci U S A. 1997;94:1985–89. doi: 10.1073/pnas.94.5.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sterman MB, Shouse MN. Quantitative analysis of training, sleep EEG and clinical response to EEG operant conditioning in epileptics. Electroencephalogr Clin Neurophysiol. 1980;49:558–76. doi: 10.1016/0013-4694(80)90397-1. [DOI] [PubMed] [Google Scholar]
- 22.Hauri PJ. Treating psychophysiologic insomnia with biofeedback. Arch Gen Psychiatry. 1981;38:752–58. doi: 10.1001/archpsyc.1981.01780320032002. [DOI] [PubMed] [Google Scholar]
- 23.Hauri PJ, Percy L, Hellekson C, Hartmann E, Russ D. The treatment of psychophysiologic insomnia with biofeedback: a replication study. Biofeedback Self Regul. 1982;7:223–35. doi: 10.1007/BF00998785. [DOI] [PubMed] [Google Scholar]
- 24.Gruzelier JH, Egner T, Vernon D. Validating the efficacy of neurofeedback for optimising performance. Prog Brain Res. 2006;159(421):31. doi: 10.1016/S0079-6123(06)59027-2. [DOI] [PubMed] [Google Scholar]
- 25.Egner T, Strawson E, Gruzelier JH. EEG signature and phenomenology of alpha / theta neurofeedback training versus mock feedback. Appl Psychophysiol Biofeedback. 2002;27:261–70. doi: 10.1023/a:1021063416558. [DOI] [PubMed] [Google Scholar]
- 26.Egner T, Gruzelier JH. Ecological validity of neurofeedback: modulation of slow wave EEG enhances musical performance. Neuroreport. 2003;14:1221–24. doi: 10.1097/01.wnr.0000081875.45938.d1. [DOI] [PubMed] [Google Scholar]
- 27.Vernon D, Egner T, Cooper N, et al. The effect of training distinct neurofeedback protocols on aspects of cognitive performance. Int J Psychophysiol. 2003;47:75–85. doi: 10.1016/s0167-8760(02)00091-0. [DOI] [PubMed] [Google Scholar]
- 28.Hanslmayr S, Sauseng P, Doppelmayr M, Schabus M, Klimesch W. Increasing individual upper alpha power by neurofeedback improves cognitive performance in human subjects. Appl Psychophysiol Biofeedback. 2005;30:1–10. doi: 10.1007/s10484-005-2169-8. [DOI] [PubMed] [Google Scholar]
- 29.Berner I, Schabus M, Wienerroither T, Klimesch W. The Significance of Sigma Neurofeedback Training on Sleep Spindles and Aspects of Declarative Memory. Appl Psychophysiol Biofeedback. 2006;31:97–114. doi: 10.1007/s10484-006-9013-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schabus M, Gruber G, Parapatics S, et al. Sleep spindles and their significance for declarative memory consolidation. Sleep. 2004;27:1479–85. doi: 10.1093/sleep/27.7.1479. [DOI] [PubMed] [Google Scholar]
- 31.Buysse DJ, Reynolds CH, Monks TH, Berman S, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for Psychiatric Practice and Research. Psychiatry Res. 1988;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 32.Zung WW. A self-rating depression scale. Arch Gen Psychiatry. 1965;12:63–70. doi: 10.1001/archpsyc.1965.01720310065008. [DOI] [PubMed] [Google Scholar]
- 33.Zung WW. A rating instrument for anxiety disorders. Psychosomatics. 1971;12:371–79. doi: 10.1016/S0033-3182(71)71479-0. [DOI] [PubMed] [Google Scholar]
- 34.Härting C, Markowitsch HJ, Neufeld H, Calabrese P, Deisinger K. Wechsler Gedächtnis Test-Revidierte Fassung. Bern: Verlag Hans Huber; 2000. [Google Scholar]
- 35.Raven J, Raven JC, Court JH. Advanced Progressive Matrices. Oxford: Psychologists Press; 1998. [Google Scholar]
- 36.Saletu B, Wessely P, Grünberger J, Schultes M. Erste klinische Erfahrungen mit einem neuen schlafanstoßenden Benzodiazepin, Clomazepam, mittel eines Selbstbeurteilungsbogens für Schlaf- und Aufwachqualität (SSA) Neuropsychiatr. 1987;1:169–76. [Google Scholar]
- 37.Plihal W, Born J. Effects of early and late nocturnal sleep on declarative and procedural memory. J Cogn Neurosci. 1997;9:534–47. doi: 10.1162/jocn.1997.9.4.534. [DOI] [PubMed] [Google Scholar]
- 38.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subject. Los Angeles: Brain Information Service/Brain Research Institute; 1968. [Google Scholar]
- 39.Schimicek P, Zeitlhofer J, Anderer P, Saletu B. Automatic sleep-spindle detection procedure: aspects of reliability and validity. Clin EEG Neurosci. 1994;25:26–9. doi: 10.1177/155005949402500108. [DOI] [PubMed] [Google Scholar]
- 40.Anderer P, Gruber G, Parapatics S, et al. An E-health solution for automatic sleep classification according to Rechtschaffen and Kales: validation study of the Somnolyzer 24x7 utilizing the Siesta database. Neuropsychobiology. 2005;51:115–33. doi: 10.1159/000085205. [DOI] [PubMed] [Google Scholar]
- 41.Cohen J. Statistical power analysis for the behavioral sciences. New York: Academic Press; 1988. [Google Scholar]
- 42.Sterman MB. Sensorimotor EEG operant conditioning: experimental and clinical effects. Pavlov J Biol Sci. 1977;12:63–92. doi: 10.1007/BF03004496. [DOI] [PubMed] [Google Scholar]
- 43.Pfurtscheller G. Event-related synchronization (ERS): an electrophysiological correlate of cortical areas at rest. Electroencephalogr Clin Neurophysiol. 1992;83:62–69. doi: 10.1016/0013-4694(92)90133-3. [DOI] [PubMed] [Google Scholar]
- 44.Mulholland T. Human EEG, behavioral stillness and biofeedback. Int J Psychophysiol. 1995;19:263–79. doi: 10.1016/0167-8760(95)00019-o. [DOI] [PubMed] [Google Scholar]
- 45.Hummel F, Andres F, Altenmüller E, Dichgans J, Gerloff C. Inhibitory control of acquired motor programmes in the human brain. Brain. 2002;125:404–20. doi: 10.1093/brain/awf030. [DOI] [PubMed] [Google Scholar]
- 46.Pfurtscheller G, Stancák A, Neuper C. Event-related synchronization (ERS) in the alpha band-an electrophysiological correlate of cortical idling: a review. Int J Psychophysiol. 1996;24:39–46. doi: 10.1016/s0167-8760(96)00066-9. [DOI] [PubMed] [Google Scholar]
- 47.Gais S, Mölle M, Helms K, Born J. Learning-dependent increases in sleep spindle density. J Neurosci. 2002;22:6830–34. doi: 10.1523/JNEUROSCI.22-15-06830.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clemens Z, Fabó D, Halász P. Overnight verbal memory retention correlates with the number of sleep spindles. Neuroscience. 2005;132:529–35. doi: 10.1016/j.neuroscience.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 49.Cortoos A, Verstraeten E, Cluydts R. Neurophysiological aspects of primary insomnia: implications for its treatment. Sleep Med Rev. 2006;10:255–66. doi: 10.1016/j.smrv.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 50.Schmidt C, Peigneux P, Muto V, et al. Encoding difficulty promotes postlearning changes in sleep spindle activity during napping. J Neurosci. 2006;26:8976–82. doi: 10.1523/JNEUROSCI.2464-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]