Abstract
We report that angiotensin-converting enzyme (ACE), a critical physiologic regulator of blood pressure, angiogenesis, and inflammation, is a novel marker for identifying hemangioblasts differentiating from human embryonic stem cells (hESC). We demonstrate that ACE+CD45−CD34+/− hemangioblasts are common yolk sac (YS)–like progenitors for not only endothelium but also both primitive and definitive human lymphohematopoietic cells. Thrombopoietin and basic fibroblast growth factor are identified as critical factors for the proliferation of human hemangioblasts. The developmental sequence of human embryoid body hematopoiesis is remarkably congruent to the timeline of normal human YS development, which occurs during weeks 2 to 6 of human gestation. Furthermore, ACE and the renin-angiotensin system (RAS) directly regulate hemangioblast expansion and differentiation via signaling through the angiotensin II receptors AGTR1 and AGTR2. ACE enzymatic activity is required for hemangioblast expansion, and differentiation toward either endothelium or multipotent hematopoietic progenitors is dramatically augmented after manipulation of angiotensin II signaling with either AGTR1- or AGTR2-specific inhibitors. The RAS can therefore be exploited to direct the hematopoietic or endothelial fate of hESC-derived hemangioblasts, thus providing novel opportunities for human tissue engineering. Moreover, the initial events of human hematoendotheliogenesis can be delineated in a manner previously impossible because of inaccessibility to early human embryonic tissues.
Introduction
Human hematopoiesis initiates during the third week of development with formation of yolk sac (YS) blood islands derived from extraembryonic mesoderm.1,2 The YS generates primarily nucleated primitive erythroblasts that express predominantly embryonic hemoglobins (eg, ε2ζ2, Gower I; α2ε2, Gower II; and ζ2γ2, Portland), and primitive macrophages.3 After the onset of circulation at approximately 21 days of embryonic development, YS blood cells continue to circulate in embryonic blood, but the fetal liver (FL) eventually replaces the YS as the main hematopoietic organ beginning at 5 to 6 weeks. FL hematopoiesis is dominated by adult-type definitive erythrocytes (enucleated, macrocytic) expressing abundant fetal (HbF; α2γ2), adult (HbA; α2β2) hemoglobins, but limited embryonic hemoglobins.4–6 The FL also produces rare megakaryocytes, granulocytes, macrophages, lymphocytes, and blast cells.7 Definitive erythroid, myeloid, megakaryocytic, natural killer-B (NK-B) lymphoid, and multipotent progenitors, with limited long-term engraftment potential, are briefly detected during the late YS (eg, 4-5 weeks) stage, before fetal liver hematopoiesis, in both mice and humans.2,8–9 The ephemeral nature of definitive-type YS hematopoiesis suggests a gradual yolk sac-fetal liver transition.1 Indeed, the classic embryonic → fetal → adult globin switch in early erythrocytes occurs sequentially over time and probably in the same clonal lineage. For example, late YS erythrocytes (ie, 6 weeks), produce strictly embryonic globins in vivo but, after in vitro culture, produce erythroid bursts that simultaneously coexpress the entire spectrum of embryonic, fetal, and adult hemoglobins.5,10 In contrast to the limited nature of YS hematopoiesis, the intraembryonic truncal mesoderm of the aorta-gonad-mesonephros (AGM) region generates self-renewing hematopoietic stem cells (HSCs) that ultimately colonize the FL, thymus, and bone marrow11–15 and provide the lifetime supply of adult blood cells.
In both mice and humans, there is compelling evidence that both YS and AGM-derived HSCs arise from a bipotential hemangioblast that generates hematopoietic and endothelial cells.16–22 The nature of this progenitor remains elusive, however, in part because reliable markers for this cell are lacking, and early human embryonic tissue is difficult to obtain. The efficient derivation and expansion of such hemangioblasts from human pluripotent stem cells has potentially great impact, because it may lead to strategies for treating a multitude of hematologic and vasculodestructive disorders.
We and others have described methods for the hematopoietic differentiation of human embryonic stem cells (hESCs), including the derivation of human embryoid body (hEB) progenitors with hemangioblastic potential.23–29 Our group first described a hESC model that recapitulates the yolk sac stages of human embryonic hematoendothelial development.25 Hematopoietic activity was shown to arise in hEB cells from CD45− mesodermal-hemato-endothelial (MHE) progenitors that give rise to hematopoietic blast colonies, and 2 sequential YS-like primitive and definitive hematopoietic waves. Although we delineated the kinetics of hEB differentiation for the emergence of a putative common hemangioblastic progenitor responsible for these 2 waves, we did not validate this stem-progenitor cell in a clonal manner. In contrast to murine experimental systems, clonal characterization of a human hemangioblast has been hindered by inefficient hematopoietic differentiation of hESC, lack of an accurate prospective hemangioblast surface marker, and absence of a quantitative hemangioblast assay (blast colony-forming cell: BL-CFC assay), although one group recently described such an assay.28 Furthermore, although KDR/flk-1 (VEGFR2) is a cell surface marker of populations containing hemangioblasts in the mouse,30–32 it has proven less reliable in specifying rare hemangioblasts in human tissues, because it is widely expressed (∼10%-40% hEB cells) during hESC differentiation and normal embryonic development.2,19,28
Using a monoclonal antibody (BB9) specific for the somatic isoform of surface angiotensin-converting enzyme (ACE/CD143), a primitive subset of CD34+ multilineage, NOD-SCID mouse-engrafting HSC was identified in adult bone marrow, mobilized peripheral blood, and umbilical cord blood.33,34 Moreover, BB9/ACE expression identified emerging hematopoietic cells from both CD34− and CD34+ areas of human YS, intraembryonic splanchnopleura, and hemogenic endothelium of the AGM region and FL. The embryonic pattern of human ACE expression is consistent with a dorsal emigration of ACE+CD34− hemangioblasts from the para-aortic splanchnopleura, and subsequent colonization of the ventral aspect of the dorsal aorta to give rise to CD34+ hemogenic endothelial cells. The identification of surface ACE on these primitive progenitors raises the possibility that the protean renin-angiotensin system (RAS) plays an unrecognized role in regulating the earliest stages of human hemato-endothelial differentiation, as it does in avian embryos.35
In the present studies, we tested the hypothesis that ACE expression in differentiating hESCs typifies emerging human embryonic hemangioblasts. We present serum-free (SF) culture methods for the generation and prospective isolation of ACE+ hemangioblasts and demonstrate that these cells represent common clonogenic progenitors for YS-like primitive and definitive hematopoieses as well as endothelium. More importantly, we demonstrate for the first time that the RAS axis (which is known to regulate blood pressure, inflammation, and angiogenesis in adults) is a pivotal regulator of human hemangioblast differentiation. We show that the 2 main angiotensin II–binding receptors, AGTR1 and AGTR2, are expressed on human hemangioblasts, and importantly, that differentiation into either hematopoietic or endothelial progenitors can be influenced by modulating the signaling through these receptors. These data advance our understanding of the earliest steps in human developmental hematopoiesis and may also assist the design of regenerative medicine approaches that aim to use hESC-derived hemangioblasts.
Methods
SF hEB differentiation
hESC lines H1, H9, and ES03 (National Institutes of Health [NIH] Human Embryonic Stem Cell Registry codes WA01, WA09, and ES03) were maintained on irradiated primary murine embryonic feeders, and hEBs were formed from hESC clumps in semisolid, serum-supplemented medium, exactly as previously described.25 Twenty-four hours after hESC plating (day 2), simple hEBs were harvested, washed, resuspended in PBS in 50-mL conical tubes, and collected by gentle gravity settling for 2 to 3 minutes. hEBs were resuspended into 6-well ultranonadherent plates (Corning Life Sciences, Acton, MA) at approximately 100-200 hEB/mL in SF liquid differentiation medium (SF LDM), consisting of serum-free expansion medium (SFEM; StemCell Technologies, Vancouver, BC) supplemented with 50 μg/mL ascorbic acid (Sigma-Aldrich, St Louis, MO), 1% EX-CYTE (Millipore, Billerica, MA), 0.5% insulin/transferrin/selenium (Invitrogen, Carlsbad, CA), 3% PFHM-II (Invitrogen), and penicillin/streptomycin (Sigma-Aldrich). Various combinations of human growth factors (all from R&D Systems [Minneapolis, MN], Peprotech [Rocky Hill, NJ], or Invitrogen) were added directly into SF LDM at 2 to 20 days of hEB development: BMP4 (50 ng/mL), vascular endothelial growth factor (VEGF; 50 ng/mL), FGF1 (50 ng/mL + 5 μg/mL heparan sulfate; Sigma), FGF2 (50 ng/mL + 5 μg/mL heparan sulfate). hEB cultures were fed by hemidepletion and replacement of medium every 2-3 days with fresh SF LDM and GFs. In general, optimal, robust hematopoietic differentiation relied on (1) a uniform size of hEBs initially formed from hESC clumps and (2) passage number of hESC less than 50.
To expand hEB-derived hematopoietic progenitors, enzymatically digested (Accutase; Sigma-Aldrich) day 9-10 hEB clumps, differentiated as described above, were transferred into SF LDM and allowed to adhere to gelatinized tissue culture plates. SF cultures were supplemented with various hematopoietic inductive factors including: BMP4 (50 ng/mL), stem cell factor (SCF; 50 ng/mL), Flt3L (50 ng/mL), thrombopoietin (TPO; 50 ng/mL), granulocyte macrophage–colony-stimulating factor (GM-CSF; 50 ng/mL), granulocyte–colony-stimulating factor (G-CSF; 50 ng/mL), interleukin-3 (IL-3; 20 ng/mL), IL-6 (20 ng/mL), VEGF (20 ng/mL), erythropoietin (EPO; 2 units/mL), FGF1 (10 ng/mL), and FGF2 (10 ng/mL).
SF clonogenic assays for hematopoietic and hemangioblastic colony-forming cells
hEBs were harvested from SF LDM at various time points, enzymatically disaggregated (Accutase; Sigma-Aldrich) into single-cell suspensions, and passed through a 70-μm strainer. Aliquots of viable hEB cells (which ranged between 40% and 75%) were enumerated by fluorescence-activated cell sorting (FACS) analysis before plating. hEB cells were plated (1.0-2.0 × 105 viable cells/mL) in duplicate/triplicate CFC dishes (NUNC A/S, Roskilde, Denmark), and assayed in humidified chambers for the presence of hematopoietic colony-forming cell (CFC) progenitors in 2 mL SF methylcellulose-based medium (StemCell Technologies) containing SCF (50 ng/mL), EPO (3 U/mL), GM-CSF (50 ng/mL), G-CSF (50 ng/mL), IL-3 (20 ng/mL), and IL-6 (20 ng/mL), which was supplemented with 0.5% EX-CYTE (Millipore Bioscience Research Reagents, Temecula, CA). Fourteen to 21 days later, colonies were scored, and plucked for cytospin or quantitative reverse transcription polymerase chain reaction (qRT-PCR) analysis. Hematopoietic colony morphology was scored by criteria as previously described.25 Positive control sibling CFC assays for generating adult definitive-type colonies were also prepared with approximately 1000 CD34+ FACS-enriched cord blood (CB) cells, and plated into 2 mL semisolid H4436 medium.
BL-CFC (hemangioblast) cultures were grown at 37°C in humidified, plastic-wrapped (hypoxic), ultranonadherent plates (Corning Life Science), in 2 mL SF methylcellulose base medium (StemCell Technologies), supplemented with 50 μg/mL ascorbic acid (Sigma-Aldrich), 1% EX-CYTE, 0.5% insulin/transferrin/selenium (Invitrogen), and 3% PFHM-II (Invitrogen). Suspensions of single hEB cells at different time points of differentiation were prepared with Accutase digestion, and passaged through a 70-μm strainer. Viability of enzyme-digested hEB cell suspensions was determined by flow cytometry using ViaProbe (BD Biosciences, San Jose, CA) staining, and 1 to 1.5 × 105/mL viable hEB cells were plated in 2 mL SF methylcellulose supplemented with BMP4 (50 ng/mL), VEGF (50 ng/mL), FGF2 (50 ng/mL), heparan sulfate (5 μg/mL; Sigma-Aldrich), TPO (50 ng/mL), IL-6 (50 ng/mL), with or without additional growth factors (GFs; eg, 2 U/mL EPO and 50 ng/mL SCF), as described in the text. Blast colonies formed (per 106 viable hEB cells originally plated) were enumerated 5 to 6 days after plating.
Hemangioblastic CFC potential of single FACS-sorted hEB cells was directly determined on fibronectin-coated (10 μg/mL; Invitrogen) CFC dishes placed in humidified chambers. Purified cells were evaluated and enumerated for purity and viability by postsort FACS analysis; the corrected percentage viability was determined before plating. Five to 20 ×103 single, sorted viable cells in 2 mL of SF H4436 methylcellulose medium (StemCell Technologies) already containing SCF (50 ng/mL), EPO (3 U/mL), GM-CSF (50 ng/mL), G-CSF (50 ng/mL), IL-3 (20 ng/mL), and IL-6 (20 ng/mL). This semisolid medium was further supplemented with BMP4 (50 ng/mL), VEGF (50 ng/mL), Flt3 ligand (50 ng/mL), TPO (50 ng/mL), IGF-I (50 ng/mL), FGF2 (25 ng/mL) plus 5 μg/mL heparan sulfate, 0.5% EX-CYTE, and 1% PFHM-II. Under these conditions, primitive-type hematoendothelial colonies expanded robustly from single adherent cells. Multilineage colonies were enumerated after 10 to 14 days and were composed of nonadherent erythromyeloid cells, intermixed with adherent Dil-acetylated low-density lipoprotein (LDL)–avid, CD31+, vascular endothelial (VE)–cadherin plus endothelial cells.
Additional details are available on the Blood website; see the Supplemental Materials link at the top of the online article.
Hematopoietic differentiation cultures
Secondary plating of blast colonies was done after expansion for 5 to 6 days after plating from various time points of hEB differentiation. Individual blast colonies were plucked from methylcellulose medium with a pipet, using inverted microscopic guidance. Blast colony cells were disaggregated and directly replated for secondary hemangioblastic differentiation on fibronectin-coated 24-well dishes in 0.5 mL SF H4436 methylcellulose supplemented with the full complement of hematoendothelial GFs, exactly as described above. Primitive-type, nonadherent secondary hematopoietic colonies were enumerated, collected for FACS or qRT-PCR analysis, or gently aspirated away after 7-10 days for analysis of adherent cells by in situ immunofluorescence.
To evaluate definitive hematopoietic potential, FACS-purified hEB populations, or singly picked blast colonies were directly replated onto irradiated (2000 cGy) OP9 stromal layers in 12- or 24-well gelatinized plates. OP9 cells (ATCC, Manassas, VA) were maintained as described previously.36 OP9 differentiation medium consisted of SFEM (StemCell Technologies), supplemented with 15% fetal calf serum (FCS; StemCell Technologies), 50 μg/mL ascorbic acid (Sigma-Aldrich), 1% EX-CYTE, 0.5% insulin/transferrin/selenium (Invitrogen), and penicillin/streptomycin (Sigma-Aldrich). OP9 media were supplemented with human GFs. For erythromyeloid differentiation, we included Flt3 ligand (20 ng/mL), TPO (20 ng/mL), SCF (20 ng/mL), IL-3 (20 ng/mL), IL-6 (20 ng/mL), G-CSF (20 ng/mL), GM-CSF (20 ng/mL), and EPO (2U/mL). For B-NK lymphoid differentiation, we included Flt3 ligand (20 ng/mL), TPO (20 ng/mL), SCF (20 ng/mL), IL-3 (20 ng/mL), IL-6 (20 ng/mL), IL-7 (20 ng/mL), IL-15 (10 ng/mL), and IL-2 (5 ng/mL) for the first week, followed by Flt3L (50 ng/mL) IL-4 (20 ng/mL), IL-6 (20 ng/mL), IL-7 (20 ng/mL), IGF-I (20 ng/mL), IL-15 (20 ng/mL), and IL-2 (5 ng/mL) in subsequent weeks. All OP9 differentiation cultures were fed by hemidepletion with fresh medium and GFs twice per week for 3 weeks. Aliquots of expanded cell populations were enumerated and evaluated by FACS analysis at weekly intervals. RAS peptides or specific inhibitors were added directly into BL-CFC or hEB differentiation media, as described in the text, and included angiotensin II peptide (Sigma-Aldrich), Captopril (Calbiochem), losartan (AztraZeneca), and PD 123-319 (Sigma-Aldrich). Enzymatic ACE activity was determined from cell culture supernatants using a colorimetric assay kit (ALPCO Diagnostics, Salem, NH), according to the manufacturer's instructions.
Results
Primitive and definitive erythromyelopoiesis from hEB cells differentiated in SF conditions resembles that of the human yolk sac
In preliminary experiments, we found that the minimal GF combination of BMP4, VEGF, and FGF2/heparan sulfate (mentioned hereafter as BVF2H) was both necessary and sufficient for inducing YS-like primitive and definitive hematopoiesis from hEB differentiated in SF conditions (see Figure S1A-E for extensive details). This minimal combination replaced the need for FCS to induce hematopoietic differentiation and was used in subsequent experiments aimed at expanding hEB-derived hemangioblasts. We evaluated the frequency of hematopoietic progenitors generated from SF BVF2H-supplemented hEB cultures by measuring the hematopoietic CFC activity of disaggregated day 3 to day 15 hEBs in SF methylcellulose cultures. These conditions produced dramatically improved frequencies of primitive and definitive hematopoietic colonies (Figure S1A), yet with morphologies identical to those previously obtained in the presence of serum24 (Figure S1B,C).
It is noteworthy that although hEB-derived erythroid colonies scored as “definitive” expressed adult HbA chains, the amount was considerably less than that from control CB-derived erythroid colonies (∼30% vs ∼90% β-globin expression, Figure S1B). In addition, whereas CB-derived colonies expressed few embryonic ε-globin chains, hEB-derived definitive colonies continued to express significant amounts of ε chains (∼30%), suggesting that they are developmentally more primitive than those derived from CB. Analysis of mixed erythro-myeloid colonies from early (day 9) or later (day 14) hEB (Figure S1C), similarly revealed an ε and β globin expression pattern that was developmentally more primitive than that of CB-derived CFU-GEMM colonies. Taken together, the coexpression of embryonic, fetal, and adult hemoglobin chains in hEB-derived erythroid or mixed erythromyeloid colonies suggested that hEB colonies scored as “definitive” are more similar to the late yolk sac-early fetal liver erythropoietic developmental stage (ie, 6-8 weeks) than to adult-type definitive progenitors, which would be characterized by minimal embryonic hemoglobin synthesis, and predominant HbF and HbA expressions.5,6 Moreover, these results are consistent with a previously proposed model of hemoglobin switching in which embryonic → fetal → adult globin transition is a time-dependent process that occurs clonally rather than in separate erythroid cell lineages.10
Thrombopoietin synergizes with mesodermal morphogens to stimulate the clonal development of multilineage ACE+ blast colonies
Our original studies on hESC differentiation in the presence of serum predicted the emergence of a common hemangioblastic progenitor preceding 2 waves of primitive and definitive hematopoieses between days 4 and 10 of hEB differentiation.25 To elucidate the identity of this progenitor in a clonal manner, and to characterize the factors and regulatory mechanisms required for its expansion, we devised a completely serum-free, quantitative blast colony-forming cell (BL-CFC) assay. In designing this assay, we sought to avoid the use of FCS and conditioned media (which contain undefined factors), as has commonly been used in classic murine as well as in recently described human hemangioblast assays.28,37
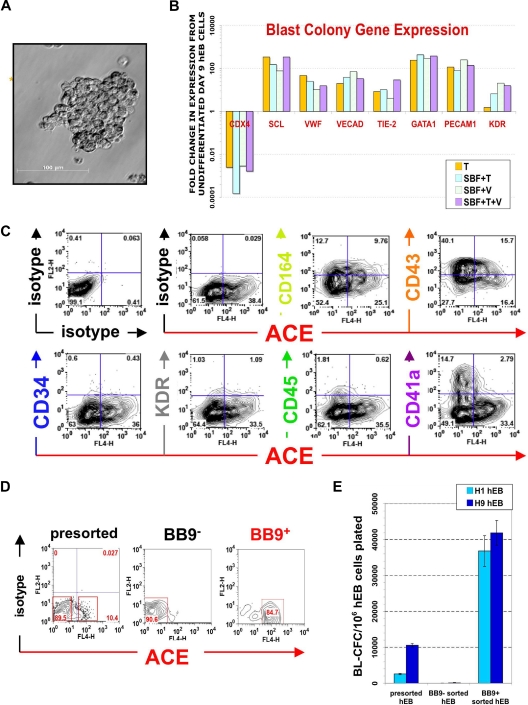
We tested a broad array of GFs known to stimulate expansion of murine BL-CFC (eg, BMP4, VEGF, FGF2/heparin, SCF, TPO, EPO, and IL-6) for their ability to generate human blast colonies. Single-cell suspensions from day 2 to 14 hEB cells (first differentiated in SF medium supplemented with BVF2H) were tested for clonogenic blast colony expansion in SF methylcellulose medium, in the presence of these GFs. With the same minimal combination of GFs used to differentiate hEB (BVF2H), loosely packed blast colonies of cells with large nuclei proliferated rapidly in SF methylcellulose, and reached their full size (∼100 cells/colony) in 5 to 6 days (Figure 1A). Their morphologies were easily distinguished from that of secondary hEB colonies that had tighter, more heterogeneous arrangements (not shown). Addition of SCF, IL-6, Flt-3 ligand, and EPO to the baseline GF combination of BVF2H did not significantly increase the frequency of blast colonies formed during days 4 to 10 of hEB development, although EPO induced more robust erythroid differentiation (data not shown). The addition of TPO, however, had a dramatic effect on blast colony generation from single hEB cells, and almost doubled the BL-CFC frequency when added along with BVF2H or even when added alone (Figure S1D). This GF combination was potent in generating blast colonies from at least three hESC lines tested; day 8 hEB contained 2578.7 (± 205; H1; WA01), 10 570 (± 495.3; H9; WA09), and 5823.9 (± 1520; ES03) BL-CFC per million cells plated (Figure 1E and data not shown).
Figure 1.
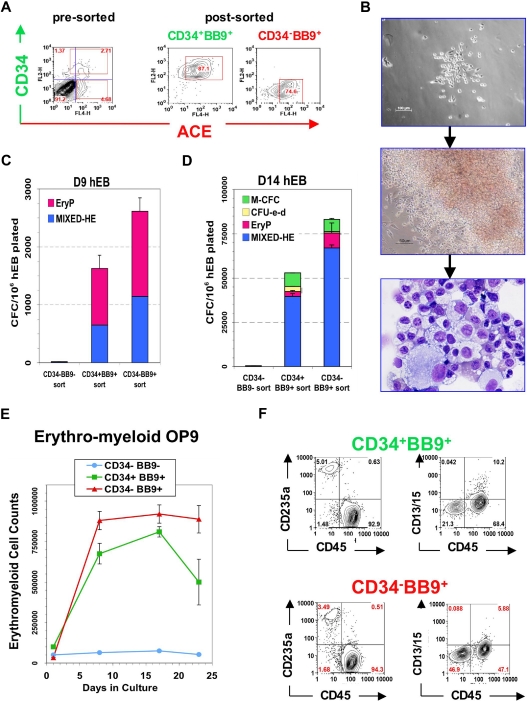
Phenotype of ACE + blast colonies generated in SF conditions. (A) Typical morphology of a blast colony with loosely packed cells generated from day 7 (line H1) hEB differentiated in BVF2H; 5-6 days post-hEB cell plating. (B) Quantitative RT-PCR expression profiles: day 9 hEB blasts were expanded with 50 ng/mL TPO alone (T), 50 ng/mL each of SCF, BMP4, FGF2/heparin, TPO (SBF + T), 50 ng/mL each of SCF, BMP4, FGF2/heparin, VEGF (SBF + V), or 50 ng/mL each of SCF, BMP4, FGF2/heparin, TPO, and VEGF (SBF + T + V). Blast colonies (∼10-15) from each GF condition were plucked from methylcellulose, pooled for RNA harvest, and analyzed for expression of indicated transcripts by qRT-PCR, using the 2−ΔΔT method25 (see Document S1). Shown are the relative, normalized expressions of CDX4, SCL, VWF, VE-cadherin, TIE-2, GATA1, PECAM1, and KDR/flk1 compared with expressions in control, undifferentiated day 9 hEB cells. qRT-PCR products were verified to be specifically amplified by agarose gel electrophoresis at linear ranges of amplification for the indicated conditions (data not shown). (C) FACS analysis of pooled (∼8-10) blast colonies from day 7 hEB cells revealed abundant expression of ACE/BB9, CD164, CD41a, and CD43, and minor amounts of CD34, KDR, and CD45. BL-CFC activity is contained entirely within BB9+ hEB cell fractions. (D) Single cell suspensions from a representative sorting experiment: day 8 hEB (presorted hEB; hESC lines H1 and H9) were FACS-purified into BB9+ or BB9− sorted populations as described under “Methods.” (E) Eight to 15 × 104 viable, purified day 8 hEB cells, or 15 × 104 viable, presorted total day 8 hEB cells from lines H1 or H9 (differentiated as described in Document S1) were recultured in duplicate in SF methylcellulose containing 50 ng/mL each of BMP4, FGF2/heparin, TPO, VEGF, and IL-6. Blast colonies generated per viable, sorted hEB cells plated were enumerated after 4 to 5 days.
Blast cell colonies that had fully matured (> 6 days old) from single (days 4-10) hEB cells were pooled and analyzed by FACS and qRT-PCR for expression of markers of both hematopoietic and endothelial cell lineages (Figure 1B). Regardless of the GF combination used (GFs with or without TPO or TPO alone), blast colonies all exhibited comparable expression profiles of key hematoendothelial transcripts (eg, CDX4, SCL, von Willebrand factor [VWF], VE-cadherin, TIE-2, GATA1, CD31 [PECAM1], and KDR). In general, these transcripts were all overexpressed in blasts, relative to undifferentiated hEB (except for CDX4). Furthermore, FACS analysis of mature blast colonies demonstrated robust surface expressions of CD164 (hematoendothelial cell lineage), CD43 (embryonic panhematopoietic cell marker),38,39 and CD41 (embryonic hematopoietic-megakaryocytic cell marker) and weaker expressions of CD34, KDR (hemato-endothelial progenitor markers), and CD45 (adult-type panhematopoietic cell marker).
Most importantly, we tested blast colonies for expression of the BB9/ACE antigen, which we recently described as uniquely typifying the emergence of human embryonic/fetal hematopoietic progenitors. Indeed, mature blast colonies expressed abundant amounts of surface ACE (Figure 1C). This observation prompted the questions as to whether (1) surface ACE also typifies undifferentiated hemangioblast colony-forming hEB cells (BL-CFC) that presumptively give rise to blast colonies directly, and (2) whether ACE-catalyzed angiotensin II peptide production plays a regulatory role in blast differentiation and/or expansion.
A single ACE + hemangioblast is a common progenitor for 2 waves of hEB-derived YS-like primitive and definitive hematopoieses
In murine embryoid bodies, KDR/flk-1+ cells include all BL-CFC,40 whereas in hEB, BL-CFC were described in both KDR+ and KDR− fractions.28 Furthermore, KDR+ cells represent a large percentage of cells (∼10-40%) during days 5-10 of hEB differentiation (Figure S2A,B). To test the hypothesis that ACE expression serves as a more reliable marker for BL-CFC than KDR/flk-1, we sorted hEB cells into BB9− and BB9+ fractions (Figure 1D). Purified cells were recultured in SF methylcellulose along with our optimized hemangioblast GFs (BVF2H + TPO + IL-6) and blast colonies enumerated as above. This experiment revealed that BL-CFC activity resides entirely within the BB9/ACE+ hEB cell population (at a frequency of ∼3% for day 9 hEB cells; Figure 1E).
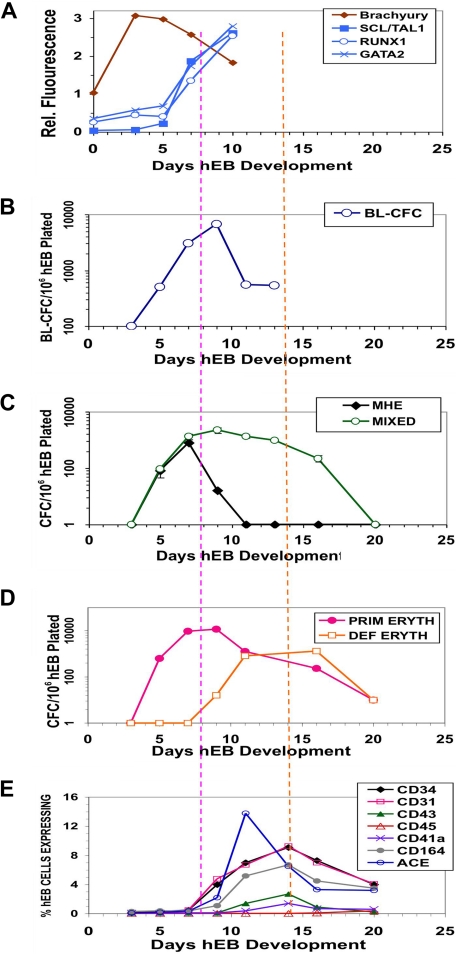
To link expression of ACE, blast colony emergence, and onset of primitive and definitive hematopoieses during hEB differentiation, surface expressions of the hematoendothelial cell markers ACE, CD34, CD31, CD43, CD45, CD41, and CD164 were simultaneously evaluated in the same hEB cells used for CFC assays between 3 and 20 days of differentiation (Figure 2). This analysis revealed a unique developmental sequence that began with initially low expression of ACE on days 5 to 7 hEB (Figures 2E, S2A) and correlated exactly with the expression (assessed by gene microarray analysis) of transcription factors associated with hematopoietic cell genesis in differentiating hEB. All microarray data have been deposited at Gene Expression Omnibus (GEO) under accession number GSE12531. These events all directly followed mesoderm-specific brachyury expression (Figures 2, S5). MHE clusters (Figures 2C, S1C), which give rise to mesenchymal cells, endothelium, and multipotent hematopoietic blasts,25 arose at 3 to 9 days of hEB differentiation. The emergence of these MHE clusters roughly preceded ACE expression by hEB cells, BL-CFC activity (Figure 2B), and mixed erythromyeloid CFC waves (Figure 2C), which peaked during days 5 to 10 of hEB differentiation. Furthermore, BL-CFC activity was correlated exactly with the onset of expression of factors associated with hemangioblast genesis including SCL/TAL1, RUNX1, and GATA2 (Figure 2A). These waves of activity were followed by 2 waves of primitive (days 6-12) and definitive (days 10-20) erythropoieses (Figure 2D). It is noteworthy that although ACE expression by hEB cells rose in parallel with primitive hematopoiesis, it peaked earlier than that of the hematoendothelial cell marker CD34 (Figure 2E), which was better correlated with expressions of CD31, CD164, CD43, CD41, and CD45 (Figure 2E). Peak expression of all these latter markers was also more closely correlated with definitive-type erythromyelopoiesis during days 10 to 20 of hEB differentiation (Figure 2D).
Figure 2.
Differentiation kinetics and surface phenotypes of clonogenic progenitors of hEB-derived primitive and definitive hematopoieses. Line H1 hEB were differentiated in SF medium and BVF2H for 3-20 days, as described under “Methods.” hEB cells at different time points were evaluated for global gene expressions (A) by DNA microarray (Agilent) analysis (see Table S2 for full list), surface marker expression (E), or recultured at indicated time points in CFC assays (B-D). Replicate CFC assay results represent the average, normalized (per 106 single, viable hEB cells plated), for independent experiments done at least 3 times, with all standard deviations less than 5%-10%). (A) Mesoderm marker Brachyury and hemangioblast-associated (SCL/TAL1, RUNX1, GATA2) genes during days 0-10 of hEB differentiation are selectively shown. (B) The kinetics of formation of BL-CFC, (C) MHE clusters, mixed primitive + definitive erythro-myelopoietic colonies (MIXED) colonies, (D) primitive erythropoietic (PRIM ERYTH), and definitive erythropoietic (DEF ERYTH) CFU are shown during days 3-20 of hEB differentiation. Purple vertical dashed line emphasizes that the initiation and pattern of blast colony formation (starting at day 5 of hEB differentiation) which correlates simultaneously with the onset of ACE/BB9 and CD34 hEB expression and also the emergence of primitive multipotent (MIXED), also primitive erythroid (PRIM ERYTH) CFU (E). In contrast, CD34, CD164, CD31, CD43, and CD41a hEB cell peak cell surface expressions patterns correlated more with the emergence of definitive-type CFU from hEB at later time points (orange dashed line).
To confirm the full differentiation potential of ACE+ blast colonies, individual ones derived from day 4 to 10 hEB cells were plucked from methylcellulose, disaggregated, and (secondarily) recultured onto fibronectin-coated wells containing SF methylcellulose medium supplemented with a broad mixture of hemato-endothelial GFs. The hemato-endothelial cell replating potential of blast colonies was notably considerably improved when IL-6 was included during blast colony expansion (Figure 3A). Under these conditions, individual colony cells readily adhered to fibronectin, and rapidly differentiated into multiple (2-10) secondary colonies of round, hemoglobinizing, hematopoietic cells intermixed with adherent, elongated endothelioid cells (Figure 3B). Cytospin stains of these hematopoietic cells revealed a mixed, primitive, erythromyelopoietic phenotype, dominated by large nucleated erythroblasts. FACS analysis of these cells demonstrated an abundance of glycophorin A (CD235a)-positive erythroid cells and the absence of adult-type CD45+ hematopoietic cells. Elongated, adherent cells intermixed with hematopoietic cells were CD31+, VE-cadherin+, Ulex europaeus agglutinin-binding, and readily took up acetylated Dil-LDL, hence were likely endothelial cells (Figures 3B, S3B). Thus, blast colonies recultured under SF clonogenic conditions gave rise to YS-like hematoendothelial progeny. In addition, blasts from early (eg, day 5) hEB cells generated an approximately 50:50 mixture of hematopoietic and endothelial cells (Figure 3B), whereas those from later stages (day 9-10 hEB) produced less robust endothelial differentiation (∼10%-20% of secondary colonies), but more robust hematopoiesis (80%-90% of secondary colonies). Thus, blasts gradually lost their bipotentiality between days 5 and 10 of hEB differentiation, in a manner similar to that described for murine blast colonies.41
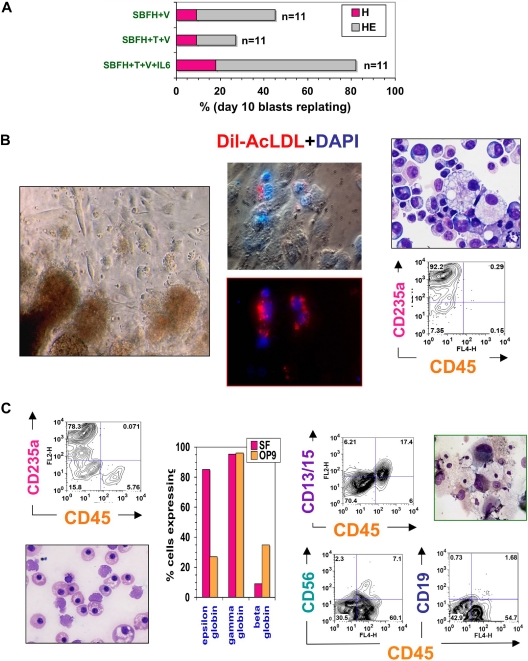
Figure 3.
Hematopoietic potential of ACE+ blast colonies. (A) Secondary colony replating efficiencies of individually picked blast colonies. Blast colonies from hESC Line H1 day 9-10 hEB cells were generated, as described in “Methods,” in the presence of 50 ng/mL each of SCF, BMP4, FGF2/heparin + VEGF (SBFH + V), 50 ng/mL each of SCF, BMP4, FGF2/heparin + TPO + VEGF (SBFH + T + V), or 50 ng/mL each of SCF, BMP4, FGF2/heparin + TPO + VEGF + IL-6 (SBFH + T + V + IL-6). The percentage of individually picked blast colonies that formed secondary hematopoietic-only (H), or hematopoietic and endothelial (HE) colonies, for each condition is shown. The addition of IL-6 did not increase the frequency of blast colony generation (data not shown) but greatly enhanced their secondary replating potential to aproximately 80%. Shown is a representative experiment (done at least twice), with the number of single blast colonies evaluated per condition (n = 11). Day 5-9 hEB-derived blast colonies were individually plucked, and secondarily recultured (5-6 days after single hEB cell plating) into single wells on either (B) fibronectin-coated dishes containing SF methylcellulose medium and GFs (SCF, BMP4, Flt3L, TPO, IGF-1, GCSF, GMCSF, IL-3, IL-6, EPO, VEGF), or (C) OP9 bone marrow stromal layers containing the same GFs, for definitive hematopoietic differentiation. Day 5 hEB blast colonies secondarily recultured into SF conditions consistently resulted in simultaneous differentiation into primitive, YS-like CD235a/CD45- erythro-myelopoietic (B, left panel and far right panels), and adherent endothelial cells that take up acetylated Dil-LDL (B, middle panel). Nuclei are stained blue with DAPI. These erythroblasts express only ε and γ, but not β hemoglobin chains (C; “SF,” middle panel). In contrast, single blast colonies recultured onto OP9 layers with human lymphohematopoietic growth factors (EPO, SCF, Flt3L, TPO, IL-3, IL-6, G-CSF, GM-CSF, IL-2, IL-7, IL-15) produced definitive-type CD235a/CD45+ erythromyelopoietic differentiation (C, top left panel), with enucleating β hemoglobin-expressing erythrocytes (C, bottom left panel), definitive-type myeloid cells (C, top right panel, CD13 + CD15 + CD45+), and also NK-lymphoid (CD56 + CD45+) but scarce amounts of phenotypic B-lymphoid cells (CD19+ CD45+; C, bottom right panel).
To test definitive hematopoietic potential, individual blast colonies were plucked and recultured onto OP9 stromal layers36,38,42 supplemented with erythromyeloid or NK-B lymphoid human GFs. Singly picked blast colonies differentiated robustly into glycophorin A+ erythroid and adult-type CD45+ myeloid cells but poorly into CD56+CD45+and CD19+CD45+ B-NK lymphoid progeny (Figure 3C). CD45+ myeloid cells also coexpressed CD13 and CD15 (myeloid cell markers), and CD41 (megakaryocyte cell marker). Cytospin stains of these differentiated cells revealed adult-type erythroid cells with occasional enucleation, as well as granulocytes, macrophages, and rare polyploid megakaryocytes. Hemoglobin analysis of CD71+ cells from these OP9 cocultures demonstrated that, compared with blasts differentiated in SF conditions, these erythroid cells expressed less embryonic (ε) globin and more adult (β) globin (Figure 3C). Nonetheless, the coexpression of embryonic ε combined with low adult β-globin synthesis demonstrated that blast-derived erythroid cells from OP9 cocultures were less mature developmentally than CB-derived erythroid cells (which express high levels of β-globin, and little ε-globin).
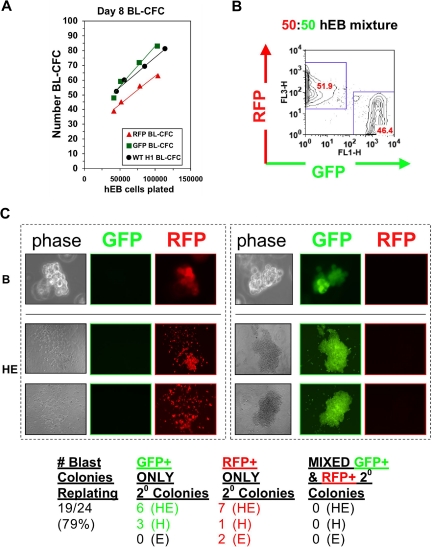
The clonal origin of blast colonies was evaluated more rigorously with dose-response and “mixing” experiments, using hESC lines constitutively expressing lentiviral constructs encoding enhanced green fluorescent protein (GFP) or red fluorescent protein (RFP). For dose response analysis, day 5 hEB cells from normal (wild-type [WT]), GFP+, or RFP+ H1 hESC lines were cultured in the presence of hemangioblast GFs, as above at increasing numbers, and assayed for BL-CFC activity. A linear correlation with a slope approximating 1 was observed between the number of blast colonies developing and the number of hEB cells plated (Figure 4A), which suggested a single hEB cell-single blast colony relationship. Likewise, in mixing experiments with 50:50 mixtures of GFP+ and RFP+ hEB cells, blasts and their hematoendothelial secondary progeny were always either only GFP+ or only RFP+, but never both GFP+ and RFP+ (Figure 5B). These analyses strongly suggest that multipotent blast colonies arise from single stem-progenitor cells, and are not derived from 2 lineage-distinct hEB cells sticking together, or possibly even joined by fusion events.
Figure 4.
A single hEB cell generates one blast colony with bipotential hematoendothelial capacity. (A) hEB cell dose response. To demonstrate that a single hEB cell has hemangioblastic capacity, the linear correlation of BL-CFC to the number of unfractionated Line H1 day 8 hEB plated was evaluated. Shown is the direct linear relationship observed with a slope ∼1, which is highly suggestive that each colony is derived from a single colony-forming cell. hEB cells from H1 hESC subclones transduced with lentiviral constructs expressing GFP or RFP demonstrated robust BL-CFC formation comparable with the H1 parental line (WT), with a similar linear correlation to numbers of cells plated. (B,C) GFP-RFP hEB Mixing Experiments. To further rule out the possibility that BL-CFC have apparent bipotentiality from the possibility that 2 separately derived progenitors are stuck or fused together, a 50:50 single-cell suspension mixture of GFP-expressing and RFP-expressing day 9 hEB cells was prepared (B), and blast colonies (phase image, “B” were expanded from this GFP-RFP mixture in BVF2H + TPO + IL-6, as before. (C) Secondary colonies from individually replated blast colonies (“B”) produced secondary endothelial colonies (“E”), hematopoietic colonies (“H”), or mixed hematoendothelial (“HE”) colonies. These secondary colonies were always GFP or RFP-expressing under fluorescent microscopy, and there were never colonies with both GFP and RFP expression mixed together. This experiment (with n = 24 picked blast colonies replated) was repeated with similar results, for a total of n = 50 colonies evaluated.
Figure 5.
Hematopoietic potential of purified BB9+ and CD34+ hEB populations. Line H1 day 9 hEB cell suspensions (presorted) were FACS-purified into (A) CD34+BB9+, CD34−BB9+, and CD34−BB9− fractions (postsort analysis of purified positive fractions shown in middle and right panels). (B-D) Sorted cells were cultured onto duplicate fibronectin-coated dishes with SF methylcellulose and hemato-endothelial GFs, as described under “SF clonogenic assays for hematopoietic and hemangioblastic colony-forming cells.” (B,C) single day 9 CD34+BB9+ and CD34−BB9+ (but not CD34−BB9−) hEB cells differentiated into hemangioblastic colonies of mixed primitive erythromyeloid cells and adherent endothelioid cells (MIXED-HE), as well as primitive erythroid (EryP) colonies. Day 14 hEB similarly FACS-purified into these same populations (D), generated more varied CFU including mixed hematoendothelial (MIXED-HE), primitive erythroid (EryP), definitive erythroid (CFU-e-d), and macrophage (M-CFC) colonies. At both days 9 and 14, hematopoietic CFU progenitor activity was greater in CD34-BB9+ than in CD34+BB9+ populations; at Day 14 this represented approximately 6% of CD34+BB9+ cells, and approximately 10% of CD34−BB9+ cells. (E,F) Definitive hematopoietic differentiation of these same populations on OP9 stroma with erythromyeloid GFs. Both CD34+BB9+ and CD34-BB9+ (but not CD34−BB9−) fractions produced robust definitive hematopoietic potential, but CD235a+/CD45+CD13+CD15+ definitive-type erythro-myeloid cells had a more robust proliferation in the CD34−BB9+ fraction.
Taken together, these data demonstrate that hESC-derived blood cells emerge from a clonogenic, ACE+, common hemangioblastic stem-progenitor of 2 waves of YS-like primitive and definitive hematopoiesis. They also predict that these hemangioblasts are likely derived from an earlier ACE− mesodermal progenitor. Finally, these data collectively underscore critical and synergistic roles for the RAS axis, TPO/MPL, FGF, and BMP signaling pathways in regulating the emergence of human hematopoiesis.
ACE expression prospectively identifies all multipotent progenitors of embryonic hematopoiesis
In the adult, ACE is expressed on the surface of a wide variety of nonrenal cells, including differentiated endothelial and stromal cells. Because hemangioblastic activity in our system was contained entirely within a small subset (∼3%) of days 5 to 10 ACE+ hEB cells, we further characterized the surface and molecular phenotype of this hEB population. Because a significant portion of ACE/BB9+ cells also coexpress KDR/flk-1 and CD34 (Figure S2C), we delineated the hemangioblastic potential of ACE+ cells in the context of these better-characterized hematoendothelial cell markers.
hEB cells were FACS-purified into BB9+ and BB9− fractions, and coexpression of various hematoendothelial cell surface markers was evaluated (Figure S4A). The majority of BB9+ hEB cells at this stage coexpressed multiple hemato-endothelial cell markers such as CD34, CD31, KDR/flk-1, and CD164 but scarcely expressed the strictly hematopoietic cell markers CD43, CD41, and CD45. Thus, BB9+ cell populations display a “prehematopoietic” phenotype at this stage. A quantitative qRT-PCR analysis revealed that, in contrast to BB9− populations, BB9+ hEB cells express higher levels of factors associated with embryonic hematopoiesis, including AML1 isoforms, C-MYB, SCL, GATA1, PU.1, and MPL (TPO receptor). Furthermore, expression of genes associated with embryonic hematopoiesis including CDX2, EpoR, FLT3, MLL, HOXB4, LMO2, GATA2, AML1 isoforms, C-MYB, SCL, GATA1, PU.1, and MPL was dramatically up-regulated in blast colonies derived from BB9 + hEB cells (Figure S4B).
Various studies have demonstrated that CD34 and KDR/flk-1 may not comprehensively mark all primitive hematopoietic stem-progenitor populations. Primitive hematopoietic progenitors, for example, exist within both fetal and adult CD34+ and CD34− populations in several species.43,44 Because significant portions of purified BB9+ hEB cells did not coexpress CD34 (Figure S4A), we next tested the hematopoietic potential of both BB9+CD34− and BB9+CD34+ hEB cell fractions. Single, FACS-purified cells were assayed for either primitive hematopoietic potential in SF hemangioblast CFC assays, (Figure 5A-D), or definitive-type hematopoietic potential on OP9 stromal layers (Figure 5E,F). CFC assays at day 9 and day 14 of hEB development revealed that clonogenic multipotent hematopoietic progenitors are present in both CD34+BB9+ and CD34−BB9+ fractions, but not within CD34−BB9− cells (Figures 1D and 5C). Interestingly, the majority of multilineage colonies were present in CD34−BB9+, rather than in CD34+BB9+ cell populations. Thus, BB9/ACE surface expression was more comprehensive in identifying hemangioblastic and multipotent hematopoietic progenitors among hEB cells than was CD34 expression alone. Similar experiments conducted with FACS-purified KDR+BB9− and KDR−BB9+/KDR+BB9+ day 5 hEB cells revealed that BB9/ACE is a more comprehensive marker for identifying clonogenic hemangioblastic progenitors in hEB cells than KDR expression alone (Figure S2C).
To test for the definitive hematopoietic potential of BB9+ hEB cells, these sorted hEB populations were seeded onto OP9 stroma under erythromyeloid or NK-B lymphoid GF conditions. Once again, CD34−BB9+ hEB cells exhibited more robust multilineage definitive lymphohematopoietic potential than CD34+BB9+ hEB cells (Figure 5E,F). Both populations, however, exhibited relatively poor B-lymphoid differentiation potential (data not shown) with CD45+CD19lo putative B-lymphoid lineage cells expressing RAG1 but scant surrogate light chains (VpreB, λ5) and Cμ heavy chains, consistent with a pro-B developmental stage. Nonetheless, BB9/ACE was the sole marker necessary and sufficient for identifying the entire range of YS-like primitive/definitive hematoendothelial progenitors during human EB differentiation.
The renin-angiotensin axis regulates the expansion and differentiation of human embryonic hemangioblasts
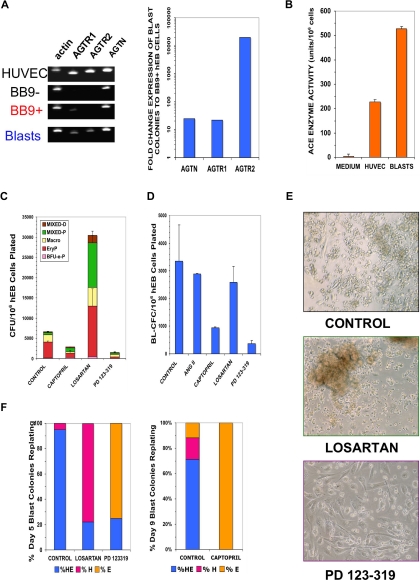
The validation of BB9/ACE as a positive marker of hESC-derived hemangioblasts and multipotent progenitors strongly predicted that the RAS regulates human embryonic hematopoiesis. We examined this possibility by first probing the expression of critical components of the RAS axis on ACE− hEB cells, ACE+ hEB cells, and ACE+ blast colonies (Figure 6A,B). Blast colonies with surface BB9/ACE expression had not only robust enzymatic ACE activity (Figure 6B) but also expressed transcripts for angiotensinogen. This essential precursor is catalyzed by renin into angiotensin I (Ang I), which is subsequently catalyzed by ACE into the bioactive peptide angiotensin II (Ang II). It is noteworthy that BB9+ but not BB9− hEB cells expressed angiotensin II type 1 receptor transcripts (AGTR1; Figure 6A,B), but poor amounts of angiotensin II type 2 receptor (AGTR2). Furthermore, AGTR2, which is normally rarely expressed in adult tissues, but abundant in the developing fetus,45–47 was dramatically up-regulated (> 104-fold) during blast colony expansion (Figures 6A, S5B). Thus, all the components necessary for bioactive Ang II synthesis (angiotensinogen, ACE), and Ang II signaling (AGTR1, AGTR2) were synthesized by blast colonies (locally), apparently in an autocrine or paracrine manner. Accordingly, addition of saturating concentrations of exogenous Ang II peptide to BL-CFC assays did not further influence the frequency of blast colony formation from hEB cells (Figure 6D).
Figure 6.
Blast colonies express RAS components that regulate their expansion and differentiation. (A) RNA was harvested from sorted hESC Line H1 day 9 BB9 + hEB (BB9+), day 9 BB9− hEB (BB9−), day 9 blast colonies (Blasts), or control human umbilical vein endothelial cells (HUVECs). Expression was assayed for actin, AGTR1, AGTR2, or angiotensinogen (AGTN) by qRT-PCR, as described in Document S1. Left panel demonstrates agarose gels of samples of PCR products (1/5 sample loaded) at linear amplification ranges for assayed transcripts. In right panel are relative, actin-normalized quantitative comparisons; fold change expression differences indicate levels of transcripts (calculated using the 2−ΔΔT method) of pooled day 9 blast colonies, compared with expression levels from sorted day 9 BB9+ hEB cells. (B) ACE enzymatic activity (units per number cultured cells assayed; mean ± SEM) was determined by colorimetric assay (in triplicate) from supernatants of pooled day 9 hEB blast colonies (BLASTS), or control supernatants from HUVEC (which express high levels of surface BB9/ACE (data not shown). (C) hEB were differentiated for hematopoietic progenitors (Figure S1A) in the presence or absence of specific RAS inhibitors. Captopril (100 μM; ACE inhibitor), 100 μM losartan (AGTR1 inhibitor), or 100 μM PD 123-319 (AGTR2 inhibitor) were included starting at day 4 of hEB culture. Day 14 hEB cell suspensions were assayed in duplicate in CFC assays, as before. (D) BL-CFC assays from days 5 to 9 hEB were conducted in the presence of supplemental angiotensin II peptide (ANG II; 100 μg/mL), captopril (100 μM), losartan (100 μM), or PD 123-319 (100 μM). Shown is the average of 2 independent experiments from day 9 hEB, with a similar pattern of results obtained with inhibitors in days 5 and 7 hEB BL-CFC assays (data not shown). (E,F) Blast colonies generated from day 5 or day 9 hEB cells were expanded in the presence of No inhibitor (control), 100 μM losartan, or 100 μM PD 123-319, and replated for secondary hematoendothelial CFC, as before. Although control blasts generated both hematopoietic and endothelial (HE) secondary progeny, the majority of blast colonies from losartan-treated blasts regenerated (robust) hematopoietic-only (H) progeny, whereas the majority of PD 123-319–treated blasts generated primarily endothelial-only (E) progeny. Shown panel F results are from blasts individually picked and replated blasts (n = 20 per condition), with typical secondary colony morphologies shown in panel E.
To ascertain whether either ACE function or AGTR1/AGTR2 signaling regulates hEB-derived hematopoiesis, we conducted differentiation and BL-CFC assays in the presence of specific inhibitors that block either synthesis or signaling of ANG II (Figure 6C-F). Differentiating hEB were treated with either an ACE inhibitor (captopril), AGTR1 inhibitor (Losartan), or an AGTR2 inhibitor (PD 123-319; 1-[[4-(dimethylamino)-3-methylphenyl]-methyl]-5-(diphenyl lacetyl)-4,5,6,7-tetrahydro-1H-imidazo[4,5-c]pyridine-6-carboxylic acid ditrifluoroacetate). It is noteworthy that inhibition of both AGTR2 and ACE severely reduced the ability of day 14 hEB to generate hematopoietic CFC, thus demonstrating a requirement of Ang II synthesis and/or AGTR2 signaling in hematopoietic progenitor expansion from earlier hEB cells (Figure 6C). We were surprised to find that inhibition of AGTR1 signaling with losartan robustly stimulated the expansion of hematopoietic CFU from day 14 hEB, including an approximately 7- to 10-fold increase in mixed, multipotent progenitors (Figure 6C).
To determine whether ACE or AGTR inhibitors act either directly on hemangioblast expansion, or on more committed progenitors, we included these blockers in BL-CFC assays (Figure 6D). Addition of captopril or the AGTR2 inhibitor PD 123-319 to day 5-9 hEB directly inhibited the expansion of blast colonies, whereas addition of the AGTR1 inhibitor losartan did not appreciably affect the frequency of blast formation at any time point. These data strongly suggested that both the catalysis of angiotensin II peptide by ACE, as well as its subsequent signaling via AGTR2 were both critical for blast colony proliferation. Replating of individual inhibitor-treated blast colonies, however, revealed a surprising finding. Although captopril and PD 123-319 dramatically diminished the expansion of blasts, replated captopril or PD 123-319–treated blasts produced a predominantly endothelial progeny, with poor hematopoietic differentiation. Conversely, losartan-treated blasts produced a predominantly hematopoietic progeny, with limited endothelial differentiation (Figure 6E,F). Because the 2 Ang II receptors generally antagonize each other's functions,48 unopposed AGTR2 signaling consequent to losartan-AGTR1 inhibition seemed to direct and augment blast differentiation toward multilineage hematopoietic progeny. Conversely, unopposed AGTR1 signaling in PD 123-319-AGTR2-inhibited blasts, although fewer in number, produced predominantly endothelial cells in secondary cultures (Figure 6E,F).
Taken together, these data suggest that hemangioblast differentiation into either hematopoietic or endothelial progeny is differentially regulated via Ang II signaling through AGTR1 and AGTR2. These data thus reveal how human embryonic hematoendotheliogenesis from hemangioblasts may be regulated in local stem cell niches where angiotensin peptides are normally synthesized.
Discussion
Because it begins as early as the third week of gestation, the developmental biology of human blood cell emergence currently remains almost inaccessible to investigation. We now outline a surrogate experimental system that recapitulates the step-wise developmental origins of human yolk sac hematopoiesis using differentiating hESC. Scarce characterizations of YS tissue at approximately 2 to 3 weeks of human gestation2,3,49 have demonstrated the presence of mesodermal clusters (blood islands) that differentiate into comingled CD34+ endothelium and blood cells, inferring the existence of a putative hemangioblast at the origin of both. The existence of a human hemangioblast, however, has been impossible to demonstrate directly and in a clonal manner, as it has been in the mouse embryo.50
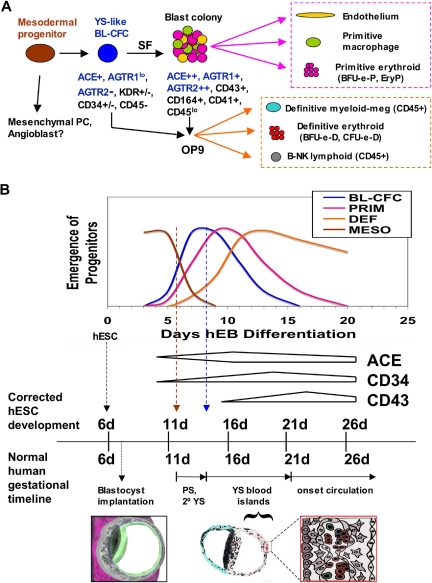
Our work herein characterizes a clonogenic, hESC-derived hemangioblast that expresses surface angiotensin-converting enzyme (CD143) and initiates both primitive and definitive YS-like hematopoieses (Figure 7A). The ontogenic pattern of development of this novel human stem-progenitor cell into primitive and definitive YS lineages seems to be almost identical to that of hemangioblasts generated from murine embryoid bodies37 and murine embryos.50 Moreover, our hEB system seems to recreate hematopoietic events in vitro that normally occur during the first weeks of human development and with a remarkably parallel timeline (Figure 7B). The hESC lines (H1;WA01, H9;WA09) primarily used in these studies were derived from the inner cell masses of an approximately 6-day-old human blastocyst51 and were maintained in a pluripotent, undifferentiated state before hematopoietic hEB differentiation. During normal gestation, human YS development directly follows formation of the primitive streak (PS; Figure 7B) and secondary YS extraembryonic mesoderm layers (2° YS; Figure 7B) at days 12-13. The formation of primordial blood islands (YS blood islands; Figure 7B) first occurs at days 14 to 16, with subsequent generation of primitive erythromyelopoietic cell lines starting after 18 to 20 days.3,49 Because in vitro hEB differentiation is an experimental surrogate for postimplantation developmental events, the normal human YS developmental milestones are indeed remarkably congruent to hematopoietic hEB differentiation. In murine embryos, hemangioblasts first appear in the posterior region of the primitive streak.50 MHE clusters and ACE+ BL-CFC similarly arise in our system at a corrected gestational timeline of approximately 12 to 13 days (6 days of blastocyst age + days 6-7 of hEB differentiation), which is when the human primitive streak first develops. Subsequently, primitive erythropoiesis arises at a corrected timeline of approximately 14 to 16 days (6 days of blastocyst age + 8-10 days of hEB differentiation), which is the milestone for YS blood island generation. Finally, definitive erythromyelopoiesis arises in our system at a corrected timeline of more than 18 to 20 days (6 days of blastocyst age + 12-20 days of hEB differentiation), when similar events occur in the human YS, before the onset of fetal circulation at approximately 21 days.
Figure 7.
Scheme for the ontogeny of in vitro, hESC-derived hemangioblasts (A), and its congruence with the developmental timeline of normal human YS development (B). PC, progenitor cell; MESO, mesoderm progenitor; BL-CFC, hemangioblast progenitor; PRIM, primitive hematopoiesis; DEF, definitive (YS-like) hematopoiesis; PS, primitive streak; 2° YS, secondary yolk sac.
The most compelling aspect of our results is that BB9/ACE+ expression prospectively identifies hemangioblasts derived from hEB. ACE+ hemangioblasts are found within not only CD34+, but also (and even more robustly) among CD34− hEB cells. This result is in agreement with our immunohistochemical analysis of the human AGM, which shows the migration of ACE+CD34−CD45− mesoderm cells from the splanchnopleura toward the dorsal aorta before the onset of intraembryonic hematopoiesis.34 Although it is well known that HSC exist in CD34− fetal and adult blood in both mouse and humans,43,44,52 it remains to be seen whether ACE expression will represent a more comprehensive prospective marker than CD34 alone for identifying primitive human HSC.
Most importantly, our studies suggest a role for the renin-angiotensin axis in directly regulating the genesis of human embryonic angio-hematopoiesis. Captopril inhibition of ACE enzymatic activity, severely inhibited human hemangioblast colony expansion, similar to effects described on progenitors in murine bone marrow cultures.53 In addition, the decision to differentiate into either endothelial or hematopoietic progenitors was directly determined via differential signaling through the 2 main angiotensin II receptors, which are expressed on human hemangioblasts. Although the RAS was originally thought to be restricted to regulating cardiovascular functions, ACE and its main enzymatic product, the angiotensin II peptide (Ang II), have recently emerged as important determinants of angiogenesis, inflammation, tumor progression, and hematopoiesis.54–56 The effects of the bioactive Ang II peptide, are mediated via specific binding and signaling through its 2 main receptors, AGTR1 and AGTR2. ACE inhibitors and angiotensin receptor blockers are widely used clinically and well tolerated, although with sporadic reports of decreases in hematocrit or even bone marrow aplasia. Regulatory effects of the RAS on hematopoiesis have amply been reported. ACE, angiotensinogen, and renin are all expressed during blood island differentiation in the chick YS, and ACE inhibitors dramatically reduce chick primitive erythroid cell development. Our studies are the first, to our knowledge, that demonstrate and extend similar effects on human primitive hematopoiesis. Moreover, ACE and Ang II receptor inhibition are associated with improved HSC recovery after chemotherapy induction, an effect that was originally interpreted to be due to the tetrapeptide AcSKDP, a negative regulator of hematopoietic proliferation, that is cleaved into its inactive form by ACE.57,58 Because the Ang II peptide is a direct mitogen for both human erythroid and CD34+ hematopoietic cell progenitors59,60 and specific AGTR1 inhibition has been reported to favor hematopoietic progenitor expansion through an unknown mechanism,61 AcSDKP is unlikely to be an important mediator of RAS function in hematopoietic progenitors. The mechanism of ACE regulation on hemangioblast differentiation, although currently unknown, is more likely related to direct Ang II receptor signaling but may be complicated by involvement of bradykinin, another peptide cleaved by ACE into an inactive form that also has important proangiogenic effects.56
Our studies further suggest that manipulation of AGTR2 signaling is an important strategy for expanding multipotent hematopoietic progenitors in general. In humans and rodents, AGTR2 is transiently expressed during fetal development but scarcely expressed in adults.45,46 Components of the renin-angiotensin axis are locally expressed in the YS, liver, kidney, embryonic aorta, and retina/choroid regions, which are all important sites of emerging angiohematopoiesis. In adults, the effects of Ang II (eg in the cardiovascular system) are primarily mediated by the G-protein–coupled AGTR1 complex. In our studies, AGTR2 function was necessary for expansion of hemangioblast colonies into multipotent hematopoietic progenitors, and its inhibition with PD 123-319 completely abolished hematopoietic differentiation, while imposing an almost exclusive endothelial fate on hemangioblast colonies. Conversely, specific inhibition of AGTR1 signaling with losartan dramatically augmented differentiation into multipotent hematopoietic progenitors, perhaps by allowing unopposed AGTR2 signaling by Ang II. Because the AGTR2 receptor is known to antagonize AGTR1 directly,48,61 or compete for Ang II binding, these results may implicate a mechanism by which emerging hemangioblasts in the YS or AGM are directed by the stem cell niche in patterning the generation of hematopoietic stem cells or alternatively the construction of vascular-endothelial networks. Although the mechanism involved is currently unknown, AGTR1/AGTR2-mediated stem cell proliferation may be a generalized phenomenon, since AGTR1 inhibition by losartan is reported to increase skeletal muscle regeneration via TGF-β signaling.62 Indeed, the importance of autocrine and paracrine regulation by the RAS axis is well recognized in the development of multiple fetal organs, and both ACE and AGTR1 receptor blockers are known teratogens. AGTR2 signaling, for example, plays central roles in regulating cellular growth or apoptosis during vascular and neural development.47,63 In addition, mutations of AGTR2 exist in patients with severe mental retardation and autism,64 confirming a critical role for neural development. Pathologic effects on other organ systems (eg, the hematopoietic system) in these patients were not described.
In summary, we show that human angio-hematopoiesis initiates from an ACE+ hemangioblastic progenitor of primitive and (limited) definitive hematopoiesis. Strikingly, the developmental timeline of normal YS development appears parallel to our hEB differentiation events, suggesting an inherently programmed series of genetic steps that are invariant in both hESC and developing human embryos. Finally, we describe a previously unrecognized role of the angiotensin receptors in regulating human hemangioblast differentiation that should enlighten our understanding of HSC expansion in general, as well as guide future strategies for directing and efficiently expanding hESC-derived hemangioblasts for vascular and hematologic therapies.
Supplementary Material
Acknowledgments
We thank Curt Civin for his scientific mentorship during these studies, Leslie Meszler and the Johns Hopkins Medical Institutions Cell Imaging Core Facility for excellent graphics support, and Ian McNiece for CD34+ cord blood samples. This work is dedicated to the memory of Evnomia Zambidis.
This work was supported by NIH grant K08-HL077595 (E.T.Z.), and the Maryland Stem Cell Research Fund (E.T.Z.).
Footnotes
The online version of this article contains a data supplement.
Portions of this work were presented in oral/abstract form at the 49th Annual Meeting of the American Society of Hematology, Atlanta, GA, December 8-11, 2007.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: E.T.Z. initiated the study, designed and performed experiments, interpreted results, and wrote the manuscript; T.S.P., A.T., M.L., X.Y., M.P., and W.Y. performed experiments and interpreted results; and B.P. initiated the study, interpreted results, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Elias T. Zambidis, MD, PhD, Institute of Cell Engineering, Stem Cell Program, Johns Hopkins University School of Medicine, 733 N Broadway, BRB 755, Baltimore, MD 21205; e-mail: ezambid1@jhmi.edu.
References
- 1.Migliaccio G, Migliaccio AR, Petti S, et al. Human embryonic hemopoiesis. Kinetics of progenitors and precursors underlying the yolk sac-liver transition. J Clin Invest. 1986;78:51–60. doi: 10.1172/JCI112572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tavian M, Hallais MF, BPeault Emergence of intraembryonic hematopoietic precursors in the pre-liver human embryo. Development. 1999;126:793–803. doi: 10.1242/dev.126.4.793. [DOI] [PubMed] [Google Scholar]
- 3.Bloom W, Bartelmez GW. Hematopoiesis in young human embryos. Am J Anat. 1940;67:21–53. [Google Scholar]
- 4.Rowley RT, Ohisson-Wilhelm BM, Farley B. Erythroid colony formation from human fetal liver. Proc Natl Acad Sci U S A. 1978;75:984–988. doi: 10.1073/pnas.75.2.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peschle C, Migliaccio AR, Migliaccio G, et al. Embryonic→fetal Hb switch in humans: studies on erythroid bursts generated by embryonic progenitors from yolk sac and liver. Proc Natl Acad Sci U S A. 1984;81:2416–2420. doi: 10.1073/pnas.81.8.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peschle C, Mavilio F, Care A, et al. Haemoglobin switching in human embryos: asynchrony of ζ–α and ε–γ-globin switches in primitive and definite erythropoietic lineage. Nature. 1985;313:235–238. doi: 10.1038/313235a0. [DOI] [PubMed] [Google Scholar]
- 7.Thomas DB, Yoffey JM. Human foetal haematopoiesis. II. Hepatic haematopoiesis in the human foetus. Br J Haematol. 1964;10:193–197. doi: 10.1111/j.1365-2141.1964.tb00694.x. [DOI] [PubMed] [Google Scholar]
- 8.Moore MA, Metcalf D. Ontogeny of the haemopoietic system: yolk sac origin of in vivo and in vitro colony forming cells in the developing mouse embryo. Br J Haematol. 1970;18:279–296. doi: 10.1111/j.1365-2141.1970.tb01443.x. [DOI] [PubMed] [Google Scholar]
- 9.Palis J, Robertson S, Kennedy M, Wall C, Keller G. Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development. 1999;126:5073–5084. doi: 10.1242/dev.126.22.5073. [DOI] [PubMed] [Google Scholar]
- 10.Stamatoyannopoulos G, Constantoulakis M, Brice M, Kurachi S, Papayannopoulos T. Coexpression of embryonic, fetal, and adult globins in erythroid cells of human embryos: relevance to the cell lineage models of globin switching. Dev Biol. 1987;123:191–197. doi: 10.1016/0012-1606(87)90441-6. [DOI] [PubMed] [Google Scholar]
- 11.Medvinsky A, Dzierzak E. Definitive hematopoiesis is autonomously initiated by the AGM region. Cell. 1996;86:897–906. doi: 10.1016/s0092-8674(00)80165-8. [DOI] [PubMed] [Google Scholar]
- 12.Cumano A, Ferraz JC, Klaine M, Di Santo JP, Godin I. Intraembryonic, but not yolk sac hematopoietic precursors, isolated before circulation, provide long-term multilineage reconstitution. Immunity. 2001;15:477–485. doi: 10.1016/s1074-7613(01)00190-x. [DOI] [PubMed] [Google Scholar]
- 13.Toles JF, Chui DH, Belbeck LW, Starr E, Barker JE. Hemopoietic stem cells in murine embryonic yolk sac and peripheral blood. Proc Natl Acad Sci U S A. 1989;86:7456–7459. doi: 10.1073/pnas.86.19.7456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoder MC, Hiatt K. Engraftment of embryonic hematopoietic cells in conditioned newborn recipients. Blood. 1997;89:2176–2183. [PubMed] [Google Scholar]
- 15.Yoder MC, Hiatt K, Mukherjee P. In vivo repopulating hematopoietic stem cells are present in the murine yolk sac at day 9.0 postcoitus. Proc Natl Acad Sci U S A. 1997;94:6776–6780. doi: 10.1073/pnas.94.13.6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Samokhvalov IM, Samakhvalova NI, Nishikawa SI. Cell tracing shows the contribution of the yolk sac to adult hematopoiesis. Nature. 2007;446:1056–1061. doi: 10.1038/nature05725. [DOI] [PubMed] [Google Scholar]
- 17.Sabin FR. Studies on the origin of blood vessels and of red blood corpuscles as seen in the living blastoderm of checks during the second day of incubation. Contrib Embryol. 1920;9:214. Carnegie Inst. Wash. Pub. no. 272. [Google Scholar]
- 18.Nishikawa SI, Nishikawa S, Kawamoto H, et al. In vitro generation of lymphohematopoietic cells from endothelial cells purified from murine embryos. Immunity. 1998;8:761–769. doi: 10.1016/s1074-7613(00)80581-6. [DOI] [PubMed] [Google Scholar]
- 19.Cortés F, Debacker C, Peault B, Labastie MC. Differential expression of KDR/VEGFR-2 and CD34 during mesoderm development of the early human embryo. Mech Dev. 1999;83:161–164. doi: 10.1016/s0925-4773(99)00030-1. [DOI] [PubMed] [Google Scholar]
- 20.Tavian M, Robin C, Coulombel L, Peault B. The human embryo, but not its yolk sac, generates lymphomyeloid stem cells: mapping multipotent hematopoietic cell fate in intraembryonic mesoderm. Immunity. 2001;15:487–495. doi: 10.1016/s1074-7613(01)00193-5. [DOI] [PubMed] [Google Scholar]
- 21.Oberlin E, Tavian M, Blazsek I, Peault B. Blood-forming potential of vascular endothelium in the human embryo. Development. 2002;129:4147–4157. doi: 10.1242/dev.129.17.4147. [DOI] [PubMed] [Google Scholar]
- 22.de Bruijn MF, Ma X, Robin C, Ottersbach K, Sanchez MJ, Dzierzak E. Hematopoietic stem cells localize to the endothelial cell layer in the midgestation mouse aorta. Immunity. 2002;16:673–683. doi: 10.1016/s1074-7613(02)00313-8. [DOI] [PubMed] [Google Scholar]
- 23.Kaufman DS, Hanson ET, Lewis RL, Auerbach R, Thomson JA. Hematopoietic colony-forming cells derived from human embryonic stem cells. Proc Natl Acad Sci U S A. 2001;98:10716–10721. doi: 10.1073/pnas.191362598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang L, Li L, Shojaei F, et al. Endothelial and hematopoietic cell fate of human embryonic stem cells originates from primitive endothelium with hemangioblastic properties. Immunity. 2004;21:31–41. doi: 10.1016/j.immuni.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 25.Zambidis E, Peault B, Park TS, Bunz F, Civin CI. Hematopoietic differentiation of human embryonic stem cells progresses through sequential hemato-endothelial, primitive, and definitive stages resembling human yolk sac development. Blood. 2005;106:860–870. doi: 10.1182/blood-2004-11-4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ng E, Davis RP, Azzola L, Stanley EG, Elefanty AG. Forced aggregation of defined numbers of human embryonic stem cells into embryoid bodies fosters robust, reproducible hematopoietic differentiation. Blood. 2005;106:1601–1603. doi: 10.1182/blood-2005-03-0987. [DOI] [PubMed] [Google Scholar]
- 27.Chang KH, Nelson AM, Cao H, et al. Definitive-like erythroid cells derived from human embryonic stem cells co-express high levels of embryonic and fetal with little or no adult globin. Blood. 2006;108:1515–1523. doi: 10.1182/blood-2005-11-011874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kennedy M, D'Souza S, Lynch-Kattman M, Schwantz S, Keller G. Development of the hemangioblast defines the onset of hematopoiesis in human ES cell differentiation cultures. Blood. 2007;109:2679–2687. doi: 10.1182/blood-2006-09-047704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu SJ, Feng Q, Caballero S, et al. Generation of functional hemangioblasts from human embryonic stem cells. Nature Methods. 2007;4:501–509. doi: 10.1038/nmeth1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kabrun N, Buhring HJ, Choi K, Ullrich A, Risau W, Keller G. Flk-1 expression defines a population of early embryonic hematopoietic precursors. Development. 1997;124:2039–2048. doi: 10.1242/dev.124.10.2039. [DOI] [PubMed] [Google Scholar]
- 31.Shalaby F, Ho J, Stanford WL, et al. A requirement for Flk1 in primitive and definitive hematopoiesis and vasculogenesis. Cell. 1997;89:981–990. doi: 10.1016/s0092-8674(00)80283-4. [DOI] [PubMed] [Google Scholar]
- 32.Chung YS, Zhang WJ, Arentson E, Kingsley PD, Palis J, Choi K. Lineage analysis of the hemangioblast as defined by FLK1 and SCL expression. Development. 2002;129:5511–5520. doi: 10.1242/dev.00149. [DOI] [PubMed] [Google Scholar]
- 33.Ramshaw HS, Haylock D, Swart B, et al. Monoclonal antibody BB9 raised against bone marrow stromal cells identifies a cell-surface glycoprotein expressed by primitive human hemopoietic progenitors. Exp Hematol. 2001;29:981–992. doi: 10.1016/s0301-472x(01)00671-3. [DOI] [PubMed] [Google Scholar]
- 34.Jokubaitis V, Sinka L, Driessen R, et al. Angiotensin-converting enzyme (CD143) marks human embryonic, fetal, and adult primitive hematopoietic cells. Blood. 2008;111:4055–4063. doi: 10.1182/blood-2007-05-091710. [DOI] [PubMed] [Google Scholar]
- 35.Savary K, Michaud A, Favier J, Larger E, Corvol P, Gasc JM. Role of the renin-angiotensin system in primitive erythropoiesis in the chick embryo. Blood. 2005;105:103–110. doi: 10.1182/blood-2004-04-1570. [DOI] [PubMed] [Google Scholar]
- 36.Nakano T, Kodama H, Honjo T. In vitro development of primitive and definitive erythrocytes from different precursors. Science. 1996;272:722–724. doi: 10.1126/science.272.5262.722. [DOI] [PubMed] [Google Scholar]
- 37.Kennedy M, Firpo M, Choi K, et al. A common precursor for primitive erythropoiesis and definitive haematopoiesis. Nature. 1997;386:488–493. doi: 10.1038/386488a0. [DOI] [PubMed] [Google Scholar]
- 38.Vodyanik MA, Thomson JA, Slukvin II. Leukosialin (CD43) defines hematopoietic progenitors in human embryonic stem cell differentiation cultures. Blood. 2006;108:2095–2105. doi: 10.1182/blood-2006-02-003327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Timens W, Kamps WA. Hemopoiesis in human fetal and embryonic liver. Microsc Res Tech. 1997;39:387–397. doi: 10.1002/(SICI)1097-0029(19971201)39:5<387::AID-JEMT1>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 40.Schuh AC, Faloon P, Hu QL, Bhimani M, Choi KC. In vitro hematopoietic and endothelial potential of flk-1(−/−) embryonic stem cells and embryos. Proc Natl Acad Sci U S A. 1999;96:2159–2164. doi: 10.1073/pnas.96.5.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Choi K, Kennedy M, Kazarov A, Papadimitriou JC, Keller G. A common precursor for hematopoietic and endothelial cells. Development. 1998;125:725–732. doi: 10.1242/dev.125.4.725. [DOI] [PubMed] [Google Scholar]
- 42.Vodyanik MA, Bork JA, Thomson JA, Slukvin II. Human embryonic stem cell-derived CD34+ cells: efficient production in the co-culture with OP9 stromal cells and analysis of lymphohematopoietic potential. Blood. 2005;105:617–625. doi: 10.1182/blood-2004-04-1649. [DOI] [PubMed] [Google Scholar]
- 43.Goodell MA, Rosenzweig M, Kim H, et al. Dye efflux studies suggest that hematopoietic stem cells expressing low or undetectable levels of CD34 antigen exist in multiple species. Nature Med. 1997;3:1337–1345. doi: 10.1038/nm1297-1337. [DOI] [PubMed] [Google Scholar]
- 44.Bhatia M, Bonnet D, Murdoch B, Gan OI, Dick JE. A newly discovered class of human hematopoietic cells with SCID-repopulating activity. Nat Med. 1998;4:1038–1045. doi: 10.1038/2023. [DOI] [PubMed] [Google Scholar]
- 45.Grady EF, Sechi LA, Griffin C, Schambelan M, Kalinyak JE. Expression of AT2 Receptors in the developing rat fetus. J Clin Invest. 1991;88:921–929. doi: 10.1172/JCI115395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schütz S, Moullec JM, Corvol P, Gasc JM. Early expression of all the components of the renin-angiotensin system in human development. Am J Pathol. 1996;149:2067–2079. [PMC free article] [PubMed] [Google Scholar]
- 47.Akishita M, Ito M, Lehtonene J, Daviet L, Dzau V, Horiuchi M. Expression of the AT2 receptor developmentally programs extracellular signal-regulated kinase activity and influences fetal vascular growth. J Clin Invest. 1999;103:63–71. doi: 10.1172/JCI5182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nakajima M, Hutchinson HG, Fujinaga M, et al. The angiotensin II type 2 (AT2) receptor antagonizes the growth effects of the AT1 receptor: gain-of-function study using gene transfer. Proc Natl Acad Sci U S A. 1995;92:10663–10667. doi: 10.1073/pnas.92.23.10663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Luckett WP. Origin and differentiation of the yolk sac and extraembryonic mesoderm in presomite human and rhesus monkey embryos. Am J Anat. 1978;152:59–97. doi: 10.1002/aja.1001520106. [DOI] [PubMed] [Google Scholar]
- 50.Huber TL, Kouskoff V, Fehling HJ, Pali J, Keller G. Haemangioblast commitment is initiated in the primitive streak of the mouse embryo. Nature. 2004;432:625–630. doi: 10.1038/nature03122. [DOI] [PubMed] [Google Scholar]
- 51.Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 52.Osawa M, Hanada KI, Hamada H, Nakauchi H. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science. 1996;273:242–245. doi: 10.1126/science.273.5272.242. [DOI] [PubMed] [Google Scholar]
- 53.Chisi J, Wdzieczak-Bakala J, Thierry J, Briscoe C, Riches AC. Captopril inhibits the proliferation of hematopoietic stem and progenitor cells in murine long-term bone marrow cultures. Stem Cells. 1999;17:339–344. doi: 10.1002/stem.170339. [DOI] [PubMed] [Google Scholar]
- 54.Egami K, Murohara T, Shimada T, et al. Role of host angiotensin II type 1 receptor in tumor angiogenesis and growth. J Clin Invest. 2003;112:67–75. doi: 10.1172/JCI16645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hubert C, Savary K, Gasc JM, Corvol P. The hematopoietic system: a new niche for the renin-angiotensin system. Nat Clin Pract Cardiovasc Med. 2006;3:80–85. doi: 10.1038/ncpcardio0449. [DOI] [PubMed] [Google Scholar]
- 56.Heffelfinger SC. The renin angiotensin system in the regulation of angiogenesis. Curr Pharm Des. 2007;13:1215–1229. doi: 10.2174/138161207780618858. [DOI] [PubMed] [Google Scholar]
- 57.Rousseau A, Michaud A, Chauvet M-T, Lenfant M, Corvol P. The homoregulatory peptide N-acetyl-ser-asp-lys-pro is a natural and specific substrate of the N-terminal active site of human angiotensin-converting enzyme. J Biol Chem. 1995;270:3656–3661. doi: 10.1074/jbc.270.8.3656. [DOI] [PubMed] [Google Scholar]
- 58.Bogden A, Moreau J-P, Gamba-Vitalo C, et al. Goralatide (AcSDKP), a negative growth regulator, protects the stem cell compartment during chemotherapy enhancing the myelopoietic response to GM-CSF. Int J Cancer. 1998;76:38–46. doi: 10.1002/(sici)1097-0215(19980330)76:1<38::aid-ijc8>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 59.Mrug M, Stopka T, Julian BA, Prchal J, Prchal J. Angiotensin II stimulates proliferation of normal early erythroid progenitors. J Clin Invest. 1997;100:2310–2314. doi: 10.1172/JCI119769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rodgers KE, Xiong S, Steer R, diZerega GS. Effect of angiotensin II on hematopoietic progenitor cell proliferation. Stem Cells. 2000;18:287–294. doi: 10.1634/stemcells.18-4-287. [DOI] [PubMed] [Google Scholar]
- 61.Charrier S, Michaud A, Badaoui S, et al. Inhibition of angiotensin I-converting enzyme induces radioprotection by preserving murine hematopoietic short-term reconstituting cells (2004). Blood. 2004;104:978–985. doi: 10.1182/blood-2003-11-3828. [DOI] [PubMed] [Google Scholar]
- 62.Cohn RD, van Erp C, Habashi JP, et al. Angiotensin II type 1 receptor blockade attenuates TGF-b-induced failure of muscel regeneration in multiple myopathic states. Nat Med. 2007;13:204–210. doi: 10.1038/nm1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grammatopoulos TN, Ahmedi F, Jones SM, Fariss MW, Wehenmeyer JA, Zawada WM. Angiotensin II protects cultured midbrain dopaminergic neurons against rotenone-induced cell death. Brain Res. 2005;1045:64–71. doi: 10.1016/j.brainres.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 64.Vervoort VS, Beacham MA, Edwards PS, et al. AGTR2 mutations in X-linked mental retardation. Science. 2002;296:2401–2403. doi: 10.1126/science.1072191. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.