Abstract
This review will focus on the interaction between multipotent stromal cells (MSCs) and carcinoma and the possible use of MSCs in cell-based anti-cancer therapies. MSCs are present in multiple tissues and are defined as cells displaying the ability to differentiate in multiple lineages including chondrocytes, osteoblasts and adipocytes. Recent evidence suggests also that they could play a role in the progression of carcinogenesis and that MSCs could migrate towards primary tumors and metastatic sites. It is possible that MSCs could be also involved in the early stages of carcinogenesis through spontaneous transformation. In addition, it is thought that MSCs can modulate tumor growth and metastasis, although this issue remains controversial and not well understood. The immuno-suppressive properties and pro-angiogenic properties of MSCs account, at least in part, for their effects on cancer development. On the other hand, cancer cells also have the ability to enhance MSC migration. This complex dialog between MSCs and cancer cells is certainly critical for the outcome of tumor development. Interestingly, several studies have shown that MSCs engineered to express anti-tumor factors could be an innovative choice as a cell-mediated gene therapy to counteract tumor growth. More evidence will be needed to understand how MSCs positively or negatively modulate carcinogenesis and to evaluate the safety of MSCs use in cell-mediated gene strategies.
Keywords: Adult, Animals, Cell Transformation, Neoplastic, Humans, Immunosuppression, Models, Animal, Neoplasms, epidemiology, Neovascularization, Pathologic, Pluripotent Stem Cells, transplantation, Risk Assessment, Stem Cell Transplantation, adverse effects, Stromal Cells, transplantation
Keywords: Multipotent stromal cells; Mesenchymal stem cells; Cellular therapy, cancer; cellular proliferation; angiogenesis; chemokines
I. General properties of multipotent stromal cells
How to define a multipotent stromal cell?
According to the International Society for Cellular Therapy (ISCT), a Multipotent Stromal Cells (MSC) is defined by the following criteria 1: 1) its property of adherence to plastic, 2) its phenotype: CD14− or CD11b−, CD19− or CD79α−, CD34−, CD45−, HLA-DR−, CD73+, CD90+, CD105+ and 3) its capacity to be differentiated into three lineages: chondrocyte, osteoblast and adipocyte. Although the MSCs are defined by their capacity to be differentiated towards these three cell lineages, they display a broader differentiation potential. Thus, the MSCs are also described according to their potential to differentiate into myocytes, tendinocytes, ligamentocytes 2, cardiomyocytes 3, neuronal cells 4, 5 and other cell types 6.
MSCs derive from mesodermal progenitors as well as from mesoepithelial cells expressing sox1 in the embryo 7. MSCs have been isolated from bone marrow (BM), adipose tissue, peripheral blood, fetal liver, lung, amniotic fluid, chorionic villi of the placenta and umbilical cord blood 8–15. Concerning the particular case of MSC isolated from adipose tissue (AT-MSCs), like BM-MSCs, they express CD13, CD29, CD44, CD90, CD105, SH-3, and STRO-1 markers, but lack CD106 9, 10. On the other hand, AT-MSCs express markers such as CD49d (α4-integrin), CD34, CD54, which are not present on BM MSCs 9. So, in conclusion, although AT-MSCs have the capacity to differentiate along the adipogenic, osteogenic, chondrogenic, and myogenic lineages, they should be considered as MSC-like cells.
MSCs possess numerous properties including immune effects, proliferative and invasive effects and osteogenic potential, making them an attractive choice as a cell-mediated gene therapy for several diseases, including bone diseases and in the treatment of human malignancies. Recent studies suggest that MSCs could home to sites of active tumorigenesis, paving the way towards the potential use of MSCs as cellular vehicles for the delivery of anticancer agents within the tumor.
MSCs and hematopoiesis
MSCs play a role of hematopoiesis support through their adhesion/interaction with the hematopoietic stem cells (HSC) and the secretion of cytokines and growth factors that are necessary to the HSC differentiation 16. Indeed, MSCs secrete a number of growth factors such as stem cell factor (SCF), interleukin (IL)-6, lymphocyte inhibitory factor (LIF), granulocyte macrophage-colony stimulating factor (GM-CSF), granulocyte colony-stimulating factor (G-CSF), macrophage colony-stimulating factor (M-CSF), suggesting a possible growth effect on hematopoiesis 17. They also produce negative regulators of hematopoiesis such as interleukin-8 (IL-8/CXCL8), macrophage inflammatory protein-1 (MIP-1α/CCL3), transforming growth factor (TGF-β), and cytokines that induce the synthesis of other cytokines by the macrophages (in particular, the pro-inflammatory cytokines, IL-1 and tumor necrosis factor (TNF-α)) 18. These cytokines can act at various levels of hematopoiesis, being at the same time negative regulators or growth factors (TGF-β, MIP-1α) according to the targeted cells and acting not only on the hematopoietic cells, but also on the stromal cells to control their proliferation (M-CSF, IL-6, TGF-β, IL-1, TNF-α) 17.
MSCs express adhesion molecules which are mediators involved in the migration and homing of the cells to the bone marrow. They include the integrin family (α1β1, α5β1), the immunoglobulin superfamily (inter-cellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), HCA), CD44, the ligand of hyaluronic acid, and other molecules of the extracellular matrix (ECM) 19, 20. In addition, the stromal cells synthesize and assemble many molecules of the ECM: fibronectins, laminins, collagens, tenascins and syndecans and other glycosaminoglycans. Thus, molecules of the ECM are part of the architecture of the adherent layer allowing the anchoring of HSC and the site of accumulation of a great number of cytokines (Stem Cell Factor (SCF), IL-3, GM-CSF, M-CSF, TGF-β, basic fibroblast growth factor (bFGF), MIP-1α) secreted by stromal cells 17.
Immunosuppressive properties of MSCs
At least part of the effects of MSCs on tumor growth could arise from their immunosuppressive properties. MSCs have been shown to suppress the lymphocyte proliferative response to allogenic or xenogenic antigens 21–23. MSCs modulate the function of the major immune cell populations when stimulated by a mitogenic signal 24. The inhibitory effect of MSCs on B lymphocytes was recently shown to occur through an arrest in the G0/G1 phase of the cell cycle and not through the induction of apoptosis 25. MSCs are not sensitive to CD8+ cytotoxic T lymphocytes (CTL)-mediated lysis and are able to inhibit CTL cytotoxicity in a dose-dependent manner when present at CTL priming 26, 27. Although MSCs were reported to be unable to activate natural killers (NK) cells 27, they inhibit interferon-γ (IFN-γ) production by IL-2 stimulated NK cells 28 and are lysed by IL-2-activated NK cells 29, 30.
This suppressive effect of MSCs is dose-dependent, decreasing with lower amounts of MSCs in the mixed lymphocyte reaction (MLR), but a weak concentration of MSCs has been shown to have a stimulating effect on T cell proliferation 31, 32. The suppression of the immune response is mediated by soluble factors after MSCs activation by culture in presence of immune cells, but their identity is still the object of controversy.
MSCs are a source of soluble factors involved in angiogenesis
Multipotent stromal cells express several pro-angiogenic factors including angiopoietin-1 (Ang-1), vascular endothelial growth factor (VEGF), growth factors such as Platelet-derived growth factor (PDGF), fibroblast growth factor (FGF)- 2 and FGF-7, but also cytokines (IL-6, TNF-α), as well as plasminogen activator 33, 34. All these molecules act synergistically on endothelial cells to promote vasculogenesis and angiogenesis. Moreover, MSCs express chemokines such as IL-8, which is involved in the recruitment of endothelial progenitors 33. Multipotent stromal cells have been shown to activate endothelial cells, through soluble factors as well as cell contacts between the two types of cells. Indeed, MSCs induce vascular endothelial growth factor receptor 2 (Flk-1) and Tie2 expression on the target cells, and cell coculture resulted in a high expression of VEGF and Ang1, the corresponding ligand 35. Beside the promotion of angiogenesis, MSCs induce the expression of junction proteins such as occludin and an increase in microvascular integrity 35.
II. Roles of multipotent stromal cells in carcinogenesis
Homing of Multipotent stromal cells to tumors (primary and metastases)
Several studies have demonstrated that MSCs have the ability into home to the primary tumor site and, eventually, to metastasis locations. It is worthwhile to mention at this stage that the route of administration, the nature of the tumor cells, the location of the primary tumor and the type of MSCs injected appear to be the main determinants in the homing of MSCs.
The homing of MSCs in whole animals has first been investigated in the non-tumoral context. It was shown that allogeneic and autologous MSCs distributed to a wide range of tissues in baboons, including the lung, the thymus, the bone, the skin, the cerebellum and gastrointestinal tract 36. In rats, injection of MSCs led to an engraftment in the lung and thereafter in the liver 37. In mouse, MSCs home to many organs including the lung, marrow, bone, skin, brain and spleen 38. In breast cancer patients, after i.v. infusion, human MSCs were detected in the circulation of some patients, only within the first hour of injection 39. Patients with severe osteogenesis imperfecta, injected with MSCs, displayed MSC engraftment in the marrow, the bone and the skin 40, whereas MSC DNA was detected in the colon and lymph node of a patient treated with MSCs for steroid-resistant graft-versus-host-disease (GVHD) 41. In summary, based on these different studies, MSCs have the ability to home to a wide range of organs, without specificity. In case of local inflammation, like in EAE (experimental autoimmune encephalomyelitis), Ucelli and coworkers showed that MSCs injected i.p. migrated to the subarachnoïdal space in close contact with the immune cells 42. This suggests that chronic inflammation might alter the homing of MSCs.
In the context of cancer, Houghton et al were the first to report, in a model of gastric cancer induced by helicobacter, that the transplantation of MSCs led to their engraftment into gastric glands 43.
A number of groups have been interested in the homing of MSCs towards glioblastoma. Using bone marrow multipotent stromal cells (BM-MSCs), it was shown that injection of MSCs in the contralateral hemisphere, into the carotid vein or the tail vein led to the homing of MSCs to the hemisphere bearing the tumor, suggesting that MSCs could cross the blood-brain barrier 44, 45.
MSCs are also able to target tumors which have been implanted subcutaneously (s.c.). Indeed, in a model of Kaposi’s sarcoma (KS), human BM-MSCs injected intravenously (i.v.) home to sites of tumorigenesis 46. When administered i.v., human adipose tissue-derived MSCs (AT-MSCs) and colon cancer cells implanted s.c., AT-MSCs are also able to home early after injection primarily to tumor sites, the lungs and the liver 47. Other routes of administration of MSCs have been used, in particular intra-peritoneal injections. Komarova et al. showed that BM-MSCs homed primarily to tumor sites, in a model of ovarian cancer, in which intra-peritoneally established xenografts were subsequently injected with MSCs 48.
A recurrent question was the ability of MSCs to migrate to the metastatic sites and, in particular, to the lung. BM-MSCs injected into the tail vein of nude mice migrate to the lung metastases sites of mice bearing melanoma metastases 49, 50. However, one might argue that in this type of setting, the ability of MSCs to migrate to metastatic sites is questionable as many types of cells home to the lung when injected iv.
Collectively, these studies suggest that MSCs can migrate towards the primary tumor and metastatic sites, although the homing is not completely specific of tumor cell locations. Indeed, in most cases, MSCs were also able to colonize organs which did not bear tumor cells, such as lung, kidney, liver or spleen. So, one might question the fact that MSC tropism is clearly dictated by the presence of tumor cells. The efficiency of MSCs homing to tumors is also questionable as there is no quantification of the percentage of MSCs, which really migrate to the carcinoma. One important point will be also to assess possible side effects of MSCs on other organs, as the homing of MSCs does not appear completely selective. Indeed, the engraftment sites of MSCs do not seem very different in the absence and the presence of carcinoma. Furthermore, it is also important to mention that the issue of the homing of MSCs has been raised by using MSCs, which were injected in the animals. So far, there is no demonstration that, in the pathologic situation, MSCs detected in the primary tumor site, originate from the local mesenchyme or from bone-marrow. This will be a key question for future developments of anti-cancer therapies based on MSCs.
Potential effects of MSCs on tumor growth and development
In addition to the homing ability of MSCs, the main controversial issue remains their ability to modulate tumor growth. The results arise both from in vitro studies and in vivo studies, either by co-injection in the same sites of tumor cells with MSCs or, by the injection of MSCs at distance from the tumor. These are summarized in figure 1.
Fig. 1. A complex network of communication between MSCs and bone resident cells.
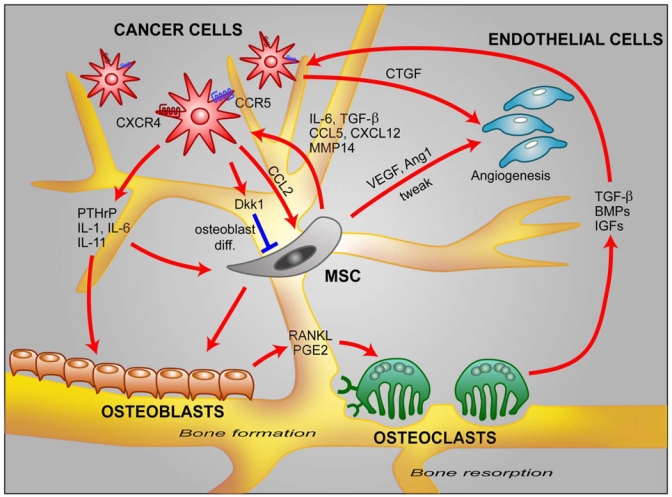
In the bone, cancer cells reduce the ability of MSCs to differentiate into osteoblasts by secreting Dkk1. Cancer cells secrete also a number of factors involved in bone metastasis including CCL2, CTGF, PTHrP, IL-1, IL-6, IL-11 91. MSCs secrete chemokines such as CCL5 and CXCL12 which increase cancer cell invasion. MSCs produce also IL-6, TGF-β, MMP-14 and increase angiogenesis by acting on endothelial cells through VEGF, Ang1 and tweak secretion.
Inhibition of tumor growth
The inhibition of tumor growth by MSCs has been observed in different types of animal models. Maestroni and colleagues first reported in experimental models of Lewis lung carcinoma and B16 melanoma that the co-injection of mouse MSCs with tumor cells inhibited primary tumor growth. Although the factors mediating the anti-tumor activity of MSCs were not identified by the authors, data from this study suggested that they were distinct from inflammatory cytokines 51. The anti-proliferative action of MSCs was also reported in a model of colon carcinogenesis in rats, in which the co-injection of MSCs with tumor cells in a gelatin matrix implanted s.c. led to growth inhibition 52. In addition, the coinjection of both cells triggered a more pronounced infiltration of monocytes and granulocytes 52.
Rat MSCs have the ability to migrate towards glioma cells and to inhibit their proliferation and, when implanted into the contralateral hemisphere, to migrate to the hemisphere bearing the tumor 44. When injected directly into the tumor, hSDSCs also reduce brain tumor size 53. hSDSCs were also able to reduce tumor progression in Tyrp1-Tag mice 53.
In an experimental model of KS, the authors demonstrated that systematically injected primary human MSCs exerted a potent tumor-suppressive effect on KS in vivo, through direct cell contact 46. The in vivo tumor-suppressive effects of MSCs correlate with their ability to inhibit KS cell Akt activity, as KS cells expressing a constitutively activated form of Akt are no longer sensitive to i.v. MSCs administration 46. Collectively, these findings suggest that human malignancies characterized by a deregulated Akt may be specific targets of the antitumorigenic properties of MSCs.
Enhancement of tumor growth and development
Several studies show that MSCs can increase tumor growth. Fetal or adult MSCs injected s.c. together with tumor cells can favor tumor growth 54. This is accompanied by extensive necrosis and angiogenesis compared to mice injected only with tumor cells 54. Similar results have been obtained with Raji cells which were injected s.c. 55. MSCs can also favor the growth of tumor cells within the bone. Indeed, multiple myeloma malignancy (MM) leads to the formation of osteolytic lesions in the bone, which is enhanced by the interaction of MM with MSCs 56.
Immunosuppression could be also one explanation for the enhancement of tumor growth by MSCs. We have shown that, when injected s.c. into an allogenic recipient, melanoma cells led to tumor formation only in the presence of MSCs 22. Interestingly, the action of MSCs could take place when MSCs were coinjected at the same site as tumor cells or when MSCs were injected at distance 57.
Only a few studies have begun to identify the molecules involved in the enhancement of cancer cell proliferation by MSCs. Indeed, co-culture or indirect interaction of MSCs with breast cancer cells enhance tumor cell proliferation, which suggests that soluble factors are involved in this phenomenon 58. MM secretes the Wnt inhibitor Dickkopf-1 (Dkk1), which in turn prevents MSCs from differentiating into osteoblasts. On the other hand, MSCs secrete IL-6 which stimulate MM proliferation 56. Furthermore, Sasser et al. reported that MSCs secrete high levels of IL-6, which in turn leads to the phosphorylation of signal transducer and activator of transcription 3 (STAT3) through a paracrine effect 59. Estrogen receptor alpha (ERα)-positive breast tumor cell lines display a low basal activation of STAT3 until exposed to MSCs, which induces a chronic phosphorylation of STAT3 on tyrosine-705 59. ERα-negative breast cancer cells, on the other hand, expressed constitutively phosphorylated STAT3. IL-6 exposure, either in a paracrine or autocrine manner, increases ERα-positive cell line growth. In vivo, ERα positive breast cancer cells transfected with IL-6 showed enhanced growth 59.
No apparent effect
Some studies reported no effect on tumor growth. This was the case in a model of ovarian cancer, in which intra-peritoneally established xenografts were subsequently injected with bone marrow MSCs 48, and also of human adipose tissue-derived MSCs (AT-MSCs), which did not modify colon cancer cell growth in vitro 47. Human MSCs seem also to have no effect in most cases on tumor growth of breast cancer cells implanted s.c. in athymic mice 60. The action of MSCs on cancer cell growth can be even more difficult to interpret as they can have opposite effects in vitro and in vivo. Indeed, when cocultured in vitro with hematopoietic and non hematopoietic cancer cells, MSCs display anti-proliferative properties by triggering a cell cycle arrest in G1 phase on tumor cells 61. In addition, MSCs also reduce the apoptotic rate of cancer cells. This inhibition is reversible by removing MSCs, and affects mainly cyclin D-dependent kinase levels. On the other hand, MSCs favor the in vivo tumor growth when coinjected s.c. to NOD/SCID mice 61. One other critical parameter seems also to be the ratio of MSCs over cancer cells. We observed that murine MSCs co-injected with Renca tumor cells in syngeneic immunocompetent mice displayed different effects on the kinetics of subcutaneous tumor growth, depending on the proportion of each cell type. Indeed, the growth of the tumor was not affected by the co-injection of the same amounts of MSCs, but was increased in the presence of ten-fold more MSCs, although ten-fold less MSCs completely abolished tumor formation 57.
MSCs and Metastasis
So far, very few studies have addressed the question of the effects of MSCs on metastasis. It was shown that murine MSC could reduce metastasis in a model of Lewis lung carcinoma and B16 melanoma 51. A more recent study with human MSCs showed that, in most cases, MSCs did not modify the growth of human breast cancer xenograft 60. However, the most striking result of this study was the fact that MSCs could increase the metastasis rate of breast cancer cells through secretion of Rantes (CCL5) by MSCs, suggesting that the main adverse role of MSCs was its pro-invasive potential 60. It is likely that other molecules participate to the enhancement of metastasis by MSCs and this will be the challenge of future studies.
In summary, depending on the nature of cancer cells and MSCs, on the integrity of the immune system of the mice that were injected, on the sites of injections; inhibition, enhancement or no apparent effect on tumor growth have been reported. One example of this complexity is shown in the study by the group of Weinberg, which showed that human MSCs did not affect tumor growth of MDA-MB-231 and MDA-MB-435 cells but increased the one of MCF-7/Ras cells 60, suggesting that the nature of cancer cells is critical. For these reasons, if one wants to use MSCs in anti-cancer therapies, it will be essential to identify the factors produced by MSCs cells responsible for the inhibition or the enhancement of tumor growth and those governing the response of tumor cells. It is also interesting to note that cancer cells can modify the growth or migration of MSCs, making the picture even more complex. Indeed, when co-injected s.c. to nude mice with melanoma cancer cells, MSCs display an increased proliferation 49. In addition, it was shown recently that MCP-1 (CCL2) secreted by breast cancer cells increased the migration of MSCs 62.
MSCs and their impact on tumoral angiogenesis
Angiogenesis of the developing neoplasia is a key event required for the optimal growth of the tumor and metastasis. MSCs could modulate this phenomenon, as suggested by some reports. By attracting endothelial cells and stimulating their proliferation, MSCs could contribute to metastasis development. This effect is related to expression of proangiogenic factors induced by interaction between carcinoma cells and MSCs. Indeed, when human MSCs are injected i.v. to SCID mice engrafted with melanoma cells, they colonize tumor vessels, which suggests that they could participate to angiogenesis 63. Indirect evidence suggests that MSCs increase tumor growth through an enhancement of angiogenesis, as MSCs expressing truncated soluble vascular endothelial growth factor decoy receptor (tsFlk-1) had no effect on tumor growth, whereas unmanipulated MSCs increased both tumor growth and angiogenesis 55. The direct injection of hSDSCs in brain tumors has also been shown to reduce angiogenesis 53.
MSCs and malignant transformation
As MSCs have the ability to expand, one might wonder whether these cells may also be the seed for new tumors. A number of approaches have been used to begin to answer this question. In particular, several groups analyzed whether MSCs could undergo spontaneous transformation or have attempted to engineer MSCs to determine whether they could be transformed. On the other hand, tumor MSCs have been isolated, which show differentiation abilities that are often close to those of original MSCs.
Are MSCs able to transform in vitro?
The issue of spontaneous transformation of MSCs is a matter of debate and if exists, seems to be an exceptional event. Rubio et al have shown that human AT-MSCs isolated from adipose tissue undergo spontaneous transformation after long term culture (4–5 months) 64. This transformation takes place through two sequential steps, including the up-regulation of c-myc, the down-regulation of p16; Then, the cells display an increased telomerase activity, a deletion of Ink4a/Arf locus and Rb hyperphosphorylation 65. Moreover, a cell population of human BM-MSCs cultured extensively, with a high telomerase activity, is capable of forming solid tumors in multiple organs in mice 66. However, this issue remains controversial, as other studies did not observe transformation of human BM-MSCs 67. Mouse bone-marrow MSCs are also able to undergo spontaneous transformation, but these cells are grown in the presence of a large number of heamtopoietic cells and require many passages before obtaining an homogeneous population, which could explain why mouse cells undergo a higher rate of transformation compared to human MSCs 68, 69. A previous study also reported that gastric cancer could originate from BM-derived cells, presumably MSCs 43. In addition, it was shown that in vitro transformed MSCs form tumors in vivo 70. The transformation of MSCs is associated with chromosomal abnormalities, increased levels of telomerase activity and c-myc expression. In addition, transformed MSCs display a higher sensitivity to anti-cancer drugs such as etoposide, when compared to non-transformed MSCs 69. In conclusion, it is possible that the way MSCs are expanded and long term culture lead to transformation. The safety of using MSCs in humans remains open. The use of MSCs in patients should definitely require precise and limited procedures of expansion to avoid the risk of injecting transformed cells.
Factors inducing transformation of MSCs in vitro
The immortalization and/or transformation of MSCs can also be triggered by the introduction of oncogenes. Indeed, human MSCs can be immortalized using HPV16 E6/E7 genes without neoplastic transformation, although the conditions of culture used by the authors could explain this result 71. These cell lines retain the ability to differentiate into osteoblasts, chondroblasts, adipocytes and neurons 71. On the other hand, the transduction of MSCs with telomerase reverse transcriptase is sufficient to induce their transformation, leading to a population of cells with loss of contact inhibition, anchorage independence, and tumor formation in vivo 72, 73. Human MSCs do not express telomerase reverse transcriptase (hTERT). hTERT expression is indeed repressed in MSCs through hypoacetylation of hTERT promoter 74. Furthermore, human MSCs immortalized with hTERT and Bmi1 (a repressor of p16INK4A) and then transformed with H-RAS display anchorage-independent growth and increased invasion ability 75. They retain adipogenic and chondrogenic differentiation ability, but not the osteogenic potential.
MSCs are targets of oncogenic processes
In patients, other oncogenes have also the potential to transform MSCs. This is the case for EWS-FLI1, which is involved in the etiology of Ewing tumors (ET). ET, a bone tumor observed in adolescents and young adults harbors characteristic translocations which fuse a portion of the EWS gene with encoding DNA binding domain of one of five ETS family genes 76. The resulting EWS-FLI1 chimeric protein is thought to induce transformation. The introduction of EWS-FLI1 into MSCs is sufficient to transform them, and the resulting cells display the hallmarks of Ewing tumors 77. Conversely, Tirode et al. have shown that Ewing cells, inhibited for EWS-FLI1 by a specific shRNA, display a MSCs phenotype with the ability to differentiate into adipogenic and osteogenic lineage, suggesting that Ewing tumors could originate from MSCs progenitors 78.
Are MSCs found in tumors identical to bone-marrow MSCs?
Several teams have compared the properties of non-pathological MSCs with those of tumor MSCs. MSCs from MM patients exhibited a normal phenotype, adipogenic and osteogenic differentiation capacity, but a reduced efficiency to inhibit T-cell proliferation 79. The comparison of bone marrow MSCs between healthy patients and MM patients showed a limited number of modifications, as only 145 genes, that are mainly involved in the tumor microenvironment (such as IL-1β, IL-6, SDF-1/CXCL12) were differentially expressed 79, 80. Giant cell tumors of bone (GCT) cells can also differentiate into osteoblasts as well as chondroblasts and adipocytes, suggesting that GCT stromal cells could originate from MSCs 81. In summary, MSCs have the ability to transform spontaneously or to be transformed with natural oncogenes. This could represent a major limitation of their therapeutic use. Moreover, the comparison of MSCs found in healthy patients or patients harboring a cancer seem to have different transcriptomes. It will be essential to determine, whether MSCs found in tumors originate from the site of the tumor itself or, whether they come from the bone marrow.
Cross-talk between MSCs and bone cells
It appears very likely that MSCs could also affect metastasis development, in particular bone metastasis, due to their presence in great amounts in this location. Whether MSCs favors metastasis development or, on the contrary, could counteract osteolysis, remains controversial. Based on several studies, it seems that cancer cells divert MSCs from their original functions to force them to participate in osteolysis. Sohara et al. showed that neuroblastoma cells could induce osteolytic lesions, but that tumor cells could not directly activate osteoclasts 82. This activation occurs through MSCs, but MSCs alone do not have bone-resorbing properties. In vitro, the activation of osteoclasts does not require a direct contact with tumor cells or MSCs, nor the direct interaction of tumor cells with MSCs. The coculture of tumor cells with MSCs increases dramatically the secretion of IL-6 by MSCs, which is an essential mediator of osteoclast activation. Cancer cells also secrete factors such as Dkk1, which in turn prevents MSCs to differentiate into osteoblasts 56. On the other hand, it seems possible to use MSCs as cellular therapy to regenerate the bone. Indeed, MSC inoculation is associated with enhanced bone mineral density and the differentiation of MSCs in osteoblasts, even though these MSCs have mixed effects as tumor growth decreased in some animals but not in others 83.
Innovative cancer therapy through engineering of MSCs
Multipotent stromal cells (MSCs) possess numerous properties that might make them an attractive choice as a cell-mediated gene therapy in human malignancies 46, 50. MSCs have been shown to express transgenes efficiently and for an extended period without any defect in their stem cell properties 84. If their ability to home to tumor sites is further demonstrated, MSCs might represent an attractive tool to deliver anti-cancer drugs to the carcinoma. In order to achieve this, MSCs have been engineered in a number of different ways, either to deliver cytotoxic drugs, to stimulate the immune response or to block angiogenesis.
The first engineered MSCs were modified to express IFN-β and were able to inhibit the growth of melanoma cells in vitro and in vivo 49, 50. Bone marrow MSCs engineered to express IFN-γ are also able to inhibit leukemia cell proliferation in vitro and to trigger their apoptosis 85. Furthermore, MSCs infected with an adenovirus or retrovirus encoding IL-12 and injected i.p. to mice one week before s.c. injection of melanoma cells, hepatoma cells or lung cancer cells are potent inhibitors of tumor growth 86, 87. The observed anti-tumoral effect of MSCs-IL-12 was primarily mediated by activation of NK cells and CD8+ T cells in the inhibition of metastasis formation and primary tumor growth.
In a recent report, adenovirally engineered primary mouse MSCs used to express the immunostimulatory chemokine CX3CL1 were i.v. injected into syngeneic immunocompetent recipient bearing lung metastases of C26 colon carcinoma or B16 melanoma cells. These engineered MSCs were able to target tumoral but not normal tissue, inducing both innate and adaptive anticancer immunity response and thus prolonging the animals’ survival 88. Mouse MSCs adenovirally-transduced to express human NK4, an antagonist of hepatocyte growth factor, exert also potent antitumorigenic effects by inhibiting tumor-associated angiogenesis and lymphoangiogenesis (two processes that are normally mediated by HGF-c-met signaling pathway) and by inducing tumor cell apoptosis 88.
A number of laboratories have used conditional replicative adenovirus (CRAds) - loaded MSCs to inhibit tumor growth. Stoff-Khalili et al. showed that intravenous injection of MSCs infected with CRAds (expressing E1A under the control of CXCR4 promoter) strongly decreased the development of pulmonary metastases of breast cancer cells. On the other hand, MSCs alone had no effect on metastasis development 89. In a model of ovarian cancer, intra-peritoneally established xenografts were subsequently injected with MSCs 48. MSCs were infected with an adenovirus (Ad5/3), which has a chimeric fiber where the knob of the Ad5 fiber is replaced by that of the Ad3. MSCs-Ad5/3 exert an oncolytic effect on ovarian cells in vitro and in vivo.
Interestingly, MSCs have been shown to be more resistant to irradiation compared to tumor cells 90. They possess a better antioxidant reactive oxygen species-scavenging capacity and active double-strand break repair, which could facilitate their radioresistance. This could be of great interest for novel anti-cancer therapies. To use such properties, Kucerova et al took advantage of human adipose tissue-derived MSCs (AT-MSCs), which were engineered to express cytosine deaminase (CD-AT-MSCs) 47. Interestingly, AT-MSCs and to a lesser degree, CD-AT-MSCs were less sensitive to 5-FU than cancer cells. In addition, CD-AT-MSCs inhibit the growth of colon carcinoma cells in vitro and in vivo in the presence of 5-FC, whereas AT-MSCs had no effect on cancer cells in the absence and the presence of 5-FC.
III. Conclusion
In summary, the evaluation of the potential use of MSCs in cell-based anti-cancer therapies is just starting. These cells have shown some promise as several studies have reported that a portion (which remains to be defined) of MSCs is able to migrate to the tumor site. However, this homing of MSCs is not selective and it will be important to evaluate possible side effects in organs which are not affected by the disease. In addition, it remains unclear, whether endogenous MSCs found in the tumor site come from the bone marrow or originate from the local mesenchyme. Depending on the types of MSCs or tumor cells, the beneficial anti-tumoral effect of MSCs is highly variable. To elucidate this issue, the precise action of MSCs will need to be studied. The safety of using MSCs could be also questioned as MSCs can also undergo transformation. However, encouraging data come from studies using engineered MSCs, which have proven their efficiency as cell carriers for the in vivo delivery of various clinically relevant anticancer factors. Overall, MSCs represent great hope for cancer therapies, but a thorough evaluation of their potential risk will be pre-required step.
Acknowledgments
We thank Jean-Louis Pasquier for Art work.
Footnotes
Gwendal Lazennec: Manuscript writing, final approval of the manuscript
Christian Jorgensen: Manuscript writing
References
- 1.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 2.Pittenger M, Vanguri P, Simonetti D, et al. Adult mesenchymal stem cells: potential for muscle and tendon regeneration and use in gene therapy. J Musculoskelet Neuronal Interact. 2002;2:309–320. [PubMed] [Google Scholar]
- 3.Makino S, Fukuda K, Miyoshi S, et al. Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest. 1999;103:697–705. doi: 10.1172/JCI5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Phinney DG, Isakova I. Plasticity and therapeutic potential of mesenchymal stem cells in the nervous system. Curr Pharm Des. 2005;11:1255–1265. doi: 10.2174/1381612053507495. [DOI] [PubMed] [Google Scholar]
- 5.Tropel P, Platet N, Platel JC, et al. Functional neuronal differentiation of bone marrow-derived mesenchymal stem cells. Stem Cells. 2006;24:2868–2876. doi: 10.1634/stemcells.2005-0636. [DOI] [PubMed] [Google Scholar]
- 6.Bhatia R, Hare JM. Mesenchymal stem cells: future source for reparative medicine. Congest Heart Fail. 2005;11:87–91. doi: 10.1111/j.1527-5299.2005.03618.x. quiz 92–83. [DOI] [PubMed] [Google Scholar]
- 7.Takashima Y, Era T, Nakao K, et al. Neuroepithelial cells supply an initial transient wave of MSC differentiation. Cell. 2007;129:1377–1388. doi: 10.1016/j.cell.2007.04.028. [DOI] [PubMed] [Google Scholar]
- 8.Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000;109:235–242. doi: 10.1046/j.1365-2141.2000.01986.x. [DOI] [PubMed] [Google Scholar]
- 9.De Ugarte DA, Morizono K, Elbarbary A, et al. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs. 2003;174:101–109. doi: 10.1159/000071150. [DOI] [PubMed] [Google Scholar]
- 10.Gronthos S, Franklin DM, Leddy HA, et al. Surface protein characterization of human adipose tissue-derived stromal cells. J Cell Physiol. 2001;189:54–63. doi: 10.1002/jcp.1138. [DOI] [PubMed] [Google Scholar]
- 11.Zvaifler NJ, Marinova-Mutafchieva L, Adams G, et al. Mesenchymal precursor cells in the blood of normal individuals. Arthritis Res. 2000;2:477–488. doi: 10.1186/ar130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campagnoli C, Roberts IA, Kumar S, et al. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood. 2001;98:2396–2402. doi: 10.1182/blood.v98.8.2396. [DOI] [PubMed] [Google Scholar]
- 13.Tsai MS, Lee JL, Chang YJ, et al. Isolation of human multipotent mesenchymal stem cells from second-trimester amniotic fluid using a novel two-stage culture protocol. Hum Reprod. 2004;19:1450–1456. doi: 10.1093/humrep/deh279. [DOI] [PubMed] [Google Scholar]
- 14.Igura K, Zhang X, Takahashi K, et al. Isolation and characterization of mesenchymal progenitor cells from chorionic villi of human placenta. Cytotherapy. 2004;6:543–553. doi: 10.1080/14653240410005366-1. [DOI] [PubMed] [Google Scholar]
- 15.in ‘t Anker PS, Noort WA, Kruisselbrink AB, et al. Nonexpanded primary lung and bone marrow-derived mesenchymal cells promote the engraftment of umbilical cord blood-derived CD34(+) cells in NOD/SCID mice. Exp Hematol. 2003;31:881–889. doi: 10.1016/s0301-472x(03)00202-9. [DOI] [PubMed] [Google Scholar]
- 16.Ringden O, Le Blanc K. Allogeneic hematopoietic stem cell transplantation: state of the art and new perspectives. Apmis. 2005;113:813–830. doi: 10.1111/j.1600-0463.2005.apm_336.x. [DOI] [PubMed] [Google Scholar]
- 17.Majumdar MK, Thiede MA, Haynesworth SE, et al. Human marrow-derived mesenchymal stem cells (MSCs) express hematopoietic cytokines and support long-term hematopoiesis when differentiated toward stromal and osteogenic lineages. J Hematother Stem Cell Res. 2000;9:841–848. doi: 10.1089/152581600750062264. [DOI] [PubMed] [Google Scholar]
- 18.Liu CH, Hwang SM. Cytokine interactions in mesenchymal stem cells from cord blood. Cytokine. 2005;32:270–279. doi: 10.1016/j.cyto.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 19.De Ugarte DA, Alfonso Z, Zuk PA, et al. Differential expression of stem cell mobilization-associated molecules on multi-lineage cells from adipose tissue and bone marrow. Immunol Lett. 2003;89:267–270. doi: 10.1016/s0165-2478(03)00108-1. [DOI] [PubMed] [Google Scholar]
- 20.Conget PA, Minguell JJ. Phenotypical and functional properties of human bone marrow mesenchymal progenitor cells. J Cell Physiol. 1999;181:67–73. doi: 10.1002/(SICI)1097-4652(199910)181:1<67::AID-JCP7>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 21.Bartholomew A, Sturgeon C, Siatskas M, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42–48. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- 22.Djouad F, Plence P, Bony C, et al. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood. 2003;102:3837–3844. doi: 10.1182/blood-2003-04-1193. [DOI] [PubMed] [Google Scholar]
- 23.Le Blanc K, Tammik C, Rosendahl K, et al. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003;31:890–896. doi: 10.1016/s0301-472x(03)00110-3. [DOI] [PubMed] [Google Scholar]
- 24.Glennie S, Soeiro I, Dyson PJ, et al. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood. 2005;105:2821–2827. doi: 10.1182/blood-2004-09-3696. [DOI] [PubMed] [Google Scholar]
- 25.Corcione A, Benvenuto F, Ferretti E, et al. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107:367–372. doi: 10.1182/blood-2005-07-2657. [DOI] [PubMed] [Google Scholar]
- 26.Angoulvant D, Clerc A, Benchalal S, et al. Human mesenchymal stem cells suppress induction of cytotoxic response to alloantigens. Biorheology. 2004;41:469–476. [PubMed] [Google Scholar]
- 27.Rasmusson I, Ringden O, Sundberg B, et al. Mesenchymal stem cells inhibit the formation of cytotoxic T lymphocytes, but not activated cytotoxic T lymphocytes or natural killer cells. Transplantation. 2003;76:1208–1213. doi: 10.1097/01.TP.0000082540.43730.80. [DOI] [PubMed] [Google Scholar]
- 28.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 29.Poggi A, Prevosto C, Massaro AM, et al. Interaction between human NK cells and bone marrow stromal cells induces NK cell triggering: role of NKp30 and NKG2D receptors. J Immunol. 2005;175:6352–6360. doi: 10.4049/jimmunol.175.10.6352. [DOI] [PubMed] [Google Scholar]
- 30.Spaggiari GM, Capobianco A, Becchetti S, et al. Mesenchymal stem cell-natural killer cell interactions: evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood. 2006;107:1484–1490. doi: 10.1182/blood-2005-07-2775. [DOI] [PubMed] [Google Scholar]
- 31.Maitra B, Szekely E, Gjini K, et al. Human mesenchymal stem cells support unrelated donor hematopoietic stem cells and suppress T-cell activation. Bone Marrow Transplant. 2004;33:597–604. doi: 10.1038/sj.bmt.1704400. [DOI] [PubMed] [Google Scholar]
- 32.Potian JA, Aviv H, Ponzio NM, et al. Veto-like activity of mesenchymal stem cells: functional discrimination between cellular responses to alloantigens and recall antigens. J Immunol. 2003;171:3426–3434. doi: 10.4049/jimmunol.171.7.3426. [DOI] [PubMed] [Google Scholar]
- 33.Honczarenko M, Le Y, Swierkowski M, et al. Human bone marrow stromal cells express a distinct set of biologically functional chemokine receptors. Stem Cells. 2006;24:1030–1041. doi: 10.1634/stemcells.2005-0319. [DOI] [PubMed] [Google Scholar]
- 34.Kinnaird T, Stabile E, Burnett MS, et al. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res. 2004;94:678–685. doi: 10.1161/01.RES.0000118601.37875.AC. [DOI] [PubMed] [Google Scholar]
- 35.Zacharek A, Chen J, Cui X, et al. Angiopoietin1/Tie2 and VEGF/Flk1 induced by MSC treatment amplifies angiogenesis and vascular stabilization after stroke. J Cereb Blood Flow Metab. 2007;27:1684–1691. doi: 10.1038/sj.jcbfm.9600475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Devine SM, Cobbs C, Jennings M, et al. Mesenchymal stem cells distribute to a wide range of tissues following systemic infusion into nonhuman primates. Blood. 2003;101:2999–3001. doi: 10.1182/blood-2002-06-1830. [DOI] [PubMed] [Google Scholar]
- 37.Gao J, Dennis JE, Muzic RF, et al. The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs. 2001;169:12–20. doi: 10.1159/000047856. [DOI] [PubMed] [Google Scholar]
- 38.Pereira RF, O’Hara MD, Laptev AV, et al. Marrow stromal cells as a source of progenitor cells for nonhematopoietic tissues in transgenic mice with a phenotype of osteogenesis imperfecta. Proc Natl Acad Sci U S A. 1998;95:1142–1147. doi: 10.1073/pnas.95.3.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koc ON, Gerson SL, Cooper BW, et al. Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol. 2000;18:307–316. doi: 10.1200/JCO.2000.18.2.307. [DOI] [PubMed] [Google Scholar]
- 40.Horwitz EM, Gordon PL, Koo WK, et al. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: Implications for cell therapy of bone. Proc Natl Acad Sci U S A. 2002;99:8932–8937. doi: 10.1073/pnas.132252399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Le Blanc K, Rasmusson I, Sundberg B, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 42.Zappia E, Casazza S, Pedemonte E, et al. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005;106:1755–1761. doi: 10.1182/blood-2005-04-1496. [DOI] [PubMed] [Google Scholar]
- 43.Houghton J, Stoicov C, Nomura S, et al. Gastric cancer originating from bone marrow-derived cells. Science. 2004;306:1568–1571. doi: 10.1126/science.1099513. [DOI] [PubMed] [Google Scholar]
- 44.Nakamura K, Ito Y, Kawano Y, et al. Antitumor effect of genetically engineered mesenchymal stem cells in a rat glioma model. Gene Ther. 2004;11:1155–1164. doi: 10.1038/sj.gt.3302276. [DOI] [PubMed] [Google Scholar]
- 45.Nakamizo A, Marini F, Amano T, et al. Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 2005;65:3307–3318. doi: 10.1158/0008-5472.CAN-04-1874. [DOI] [PubMed] [Google Scholar]
- 46.Khakoo AY, Pati S, Anderson SA, et al. Human mesenchymal stem cells exert potent antitumorigenic effects in a model of Kaposi’s sarcoma. J Exp Med. 2006;203:1235–1247. doi: 10.1084/jem.20051921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kucerova L, Altanerova V, Matuskova M, et al. Adipose Tissue-Derived Human Mesenchymal Stem Cells Mediated Prodrug Cancer Gene Therapy. Cancer Res. 2007;67:6304–6313. doi: 10.1158/0008-5472.CAN-06-4024. [DOI] [PubMed] [Google Scholar]
- 48.Komarova S, Kawakami Y, Stoff-Khalili MA, et al. Mesenchymal progenitor cells as cellular vehicles for delivery of oncolytic adenoviruses. Mol Cancer Ther. 2006;5:755–766. doi: 10.1158/1535-7163.MCT-05-0334. [DOI] [PubMed] [Google Scholar]
- 49.Studeny M, Marini FC, Champlin RE, et al. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Cancer Res. 2002;62:3603–3608. [PubMed] [Google Scholar]
- 50.Studeny M, Marini FC, Dembinski JL, et al. Mesenchymal stem cells: potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. J Natl Cancer Inst. 2004;96:1593–1603. doi: 10.1093/jnci/djh299. [DOI] [PubMed] [Google Scholar]
- 51.Maestroni GJ, Hertens E, Galli P. Factor(s) from nonmacrophage bone marrow stromal cells inhibit Lewis lung carcinoma and B16 melanoma growth in mice. Cell Mol Life Sci. 1999;55:663–667. doi: 10.1007/s000180050322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ohlsson LB, Varas L, Kjellman C, et al. Mesenchymal progenitor cell-mediated inhibition of tumor growth in vivo and in vitro in gelatin matrix. Exp Mol Pathol. 2003;75:248–255. doi: 10.1016/j.yexmp.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 53.Pisati F, Belicchi M, Acerbi F, et al. Effect of human skin-derived stem cells on vessel architecture, tumor growth, and tumor invasion in brain tumor animal models. Cancer Res. 2007;67:3054–3063. doi: 10.1158/0008-5472.CAN-06-1384. [DOI] [PubMed] [Google Scholar]
- 54.Zhu W, Xu W, Jiang R, et al. Mesenchymal stem cells derived from bone marrow favor tumor cell growth in vivo. Exp Mol Pathol. 2006;80:267–274. doi: 10.1016/j.yexmp.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 55.Kyriakou CA, Yong KL, Benjamin R, et al. Human mesenchymal stem cells (hMSCs) expressing truncated soluble vascular endothelial growth factor receptor (tsFlk-1) following lentiviral-mediated gene transfer inhibit growth of Burkitt’s lymphoma in a murine model. J Gene Med. 2006;8:253–264. doi: 10.1002/jgm.840. [DOI] [PubMed] [Google Scholar]
- 56.Gunn WG, Conley A, Deininger L, et al. A crosstalk between myeloma cells and marrow stromal cells stimulates production of DKK1 and interleukin-6: a potential role in the development of lytic bone disease and tumor progression in multiple myeloma. Stem Cells. 2006;24:986–991. doi: 10.1634/stemcells.2005-0220. [DOI] [PubMed] [Google Scholar]
- 57.Djouad F, Bony C, Apparailly F, et al. Earlier onset of syngeneic tumors in the presence of mesenchymal stem cells. Transplantation. 2006;82:1060–1066. doi: 10.1097/01.tp.0000236098.13804.0b. [DOI] [PubMed] [Google Scholar]
- 58.Fierro FA, Sierralta WD, Epunan MJ, et al. Marrow-derived mesenchymal stem cells: role in epithelial tumor cell determination. Clin Exp Metastasis. 2004;21:313–319. doi: 10.1023/b:clin.0000046130.79363.33. [DOI] [PubMed] [Google Scholar]
- 59.Sasser AK, Sullivan NJ, Studebaker AW, et al. Interleukin-6 is a potent growth factor for ER-{alpha}-positive human breast cancer. Faseb J. 2007 doi: 10.1096/fj.07-8832com. [DOI] [PubMed] [Google Scholar]
- 60.Karnoub AE, Dash AB, Vo AP, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 61.Ramasamy R, Lam EW, Soeiro I, et al. Mesenchymal stem cells inhibit proliferation and apoptosis of tumor cells: impact on in vivo tumor growth. Leukemia. 2007;21:304–310. doi: 10.1038/sj.leu.2404489. [DOI] [PubMed] [Google Scholar]
- 62.Dwyer RM, Potter-Beirne SM, Harrington KA, et al. Monocyte chemotactic protein-1 secreted by primary breast tumors stimulates migration of mesenchymal stem cells. Clin Cancer Res. 2007;13:5020–5027. doi: 10.1158/1078-0432.CCR-07-0731. [DOI] [PubMed] [Google Scholar]
- 63.Sun B, Zhang S, Ni C, et al. Correlation between melanoma angiogenesis and the mesenchymal stem cells and endothelial progenitor cells derived from bone marrow. Stem Cells Dev. 2005;14:292–298. doi: 10.1089/scd.2005.14.292. [DOI] [PubMed] [Google Scholar]
- 64.Rubio D, Garcia-Castro J, Martin MC, et al. Spontaneous human adult stem cell transformation. Cancer Res. 2005;65:3035–3039. doi: 10.1158/0008-5472.CAN-04-4194. [DOI] [PubMed] [Google Scholar]
- 65.Rubio D, Garcia S, Paz MF, et al. Molecular characterization of spontaneous mesenchymal stem cell transformation. PLoS ONE. 2008;3:e1398. doi: 10.1371/journal.pone.0001398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang Y, Huso DL, Harrington J, et al. Outgrowth of a transformed cell population derived from normal human BM mesenchymal stem cell culture. Cytotherapy. 2005;7:509–519. doi: 10.1080/14653240500363216. [DOI] [PubMed] [Google Scholar]
- 67.Bernardo ME, Zaffaroni N, Novara F, et al. Human Bone Marrow Derived Mesenchymal Stem Cells Do Not Undergo Transformation after Long-term In vitro Culture and Do Not Exhibit Telomere Maintenance Mechanisms. Cancer Res. 2007;67:9142–9149. doi: 10.1158/0008-5472.CAN-06-4690. [DOI] [PubMed] [Google Scholar]
- 68.Sudres M, Norol F, Trenado A, et al. Bone marrow mesenchymal stem cells suppress lymphocyte proliferation in vitro but fail to prevent graft-versus-host disease in mice. J Immunol. 2006;176:7761–7767. doi: 10.4049/jimmunol.176.12.7761. [DOI] [PubMed] [Google Scholar]
- 69.Miura M, Miura Y, Padilla-Nash HM, et al. Accumulated chromosomal instability in murine bone marrow mesenchymal stem cells leads to malignant transformation. Stem Cells. 2006;24:1095–1103. doi: 10.1634/stemcells.2005-0403. [DOI] [PubMed] [Google Scholar]
- 70.Li H, Fan X, Kovi RC, et al. Spontaneous expression of embryonic factors and p53 point mutations in aged mesenchymal stem cells: a model of age-related tumorigenesis in mice. Cancer Res. 2007;67:10889–10898. doi: 10.1158/0008-5472.CAN-07-2665. [DOI] [PubMed] [Google Scholar]
- 71.Hung SC, Yang DM, Chang CF, et al. Immortalization without neoplastic transformation of human mesenchymal stem cells by transduction with HPV16 E6/E7 genes. Int J Cancer. 2004;110:313–319. doi: 10.1002/ijc.20126. [DOI] [PubMed] [Google Scholar]
- 72.Serakinci N, Guldberg P, Burns JS, et al. Adult human mesenchymal stem cell as a target for neoplastic transformation. Oncogene. 2004;23:5095–5098. doi: 10.1038/sj.onc.1207651. [DOI] [PubMed] [Google Scholar]
- 73.Burns JS, Abdallah BM, Guldberg P, et al. Tumorigenic heterogeneity in cancer stem cells evolved from long-term cultures of telomerase-immortalized human mesenchymal stem cells. Cancer Res. 2005;65:3126–3135. doi: 10.1158/0008-5472.CAN-04-2218. [DOI] [PubMed] [Google Scholar]
- 74.Serakinci N, Hoare SF, Kassem M, et al. Telomerase promoter reprogramming and interaction with general transcription factors in the human mesenchymal stem cell. Regen Med. 2006;1:125–131. doi: 10.2217/17460751.1.1.125. [DOI] [PubMed] [Google Scholar]
- 75.Shima Y, Okamoto T, Aoyama T, et al. In vitro transformation of mesenchymal stem cells by oncogenic H-rasVal12. Biochem Biophys Res Commun. 2007;353:60–66. doi: 10.1016/j.bbrc.2006.11.137. [DOI] [PubMed] [Google Scholar]
- 76.Arvand A, Denny CT. Biology of EWS/ETS fusions in Ewing’s family tumors. Oncogene. 2001;20:5747–5754. doi: 10.1038/sj.onc.1204598. [DOI] [PubMed] [Google Scholar]
- 77.Riggi N, Cironi L, Provero P, et al. Development of Ewing’s sarcoma from primary bone marrow-derived mesenchymal progenitor cells. Cancer Res. 2005;65:11459–11468. doi: 10.1158/0008-5472.CAN-05-1696. [DOI] [PubMed] [Google Scholar]
- 78.Tirode F, Laud-Duval K, Prieur A, et al. Mesenchymal stem cell features of Ewing tumors. Cancer Cell. 2007;11:421–429. doi: 10.1016/j.ccr.2007.02.027. [DOI] [PubMed] [Google Scholar]
- 79.Arnulf B, Lecourt S, Soulier J, et al. Phenotypic and functional characterization of bone marrow mesenchymal stem cells derived from patients with multiple myeloma. Leukemia. 2007;21:158–163. doi: 10.1038/sj.leu.2404466. [DOI] [PubMed] [Google Scholar]
- 80.Corre J, Mahtouk K, Attal M, et al. Bone marrow mesenchymal stem cells are abnormal in multiple myeloma. Leukemia. 2007;21:1079–1088. doi: 10.1038/sj.leu.2404621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wulling M, Delling G, Kaiser E. The origin of the neoplastic stromal cell in giant cell tumor of bone. Hum Pathol. 2003;34:983–993. doi: 10.1053/s0046-8177(03)00413-1. [DOI] [PubMed] [Google Scholar]
- 82.Sohara Y, Shimada H, Minkin C, et al. Bone marrow mesenchymal stem cells provide an alternate pathway of osteoclast activation and bone destruction by cancer cells. Cancer Res. 2005;65:1129–1135. doi: 10.1158/0008-5472.CAN-04-2853. [DOI] [PubMed] [Google Scholar]
- 83.Yaccoby S, Wezeman MJ, Zangari M, et al. Inhibitory effects of osteoblasts and increased bone formation on myeloma in novel culture systems and a myelomatous mouse model. Haematologica. 2006;91:192–199. [PMC free article] [PubMed] [Google Scholar]
- 84.Caplan AI, Bruder SP. Mesenchymal stem cells: building blocks for molecular medicine in the 21st century. Trends Mol Med. 2001;7:259–264. doi: 10.1016/s1471-4914(01)02016-0. [DOI] [PubMed] [Google Scholar]
- 85.Li X, Lu Y, Huang W, et al. In vitro effect of adenovirus-mediated human Gamma Interferon gene transfer into human mesenchymal stem cells for chronic myelogenous leukemia. Hematol Oncol. 2006;24:151–158. doi: 10.1002/hon.779. [DOI] [PubMed] [Google Scholar]
- 86.Chen XC, Wang R, Zhao X, et al. Prophylaxis against carcinogenesis in three kinds of unestablished tumor models via IL12-gene-engineered MSCs. Carcinogenesis. 2006;27:2434–2441. doi: 10.1093/carcin/bgl069. [DOI] [PubMed] [Google Scholar]
- 87.Elzaouk L, Moelling K, Pavlovic J. Anti-tumor activity of mesenchymal stem cells producing IL-12 in a mouse melanoma model. Exp Dermatol. 2006;15:865–874. doi: 10.1111/j.1600-0625.2006.00479.x. [DOI] [PubMed] [Google Scholar]
- 88.Kanehira M, Xin H, Hoshino K, et al. Targeted delivery of NK4 to multiple lung tumors by bone marrow-derived mesenchymal stem cells. Cancer Gene Ther. 2007 doi: 10.1038/sj.cgt.7701079. [DOI] [PubMed] [Google Scholar]
- 89.Stoff-Khalili MA, Rivera AA, Mathis JM, et al. Mesenchymal stem cells as a vehicle for targeted delivery of CRAds to lung metastases of breast carcinoma. Breast Cancer Res Treat. 2007 doi: 10.1007/s10549-006-9449-8. [DOI] [PubMed] [Google Scholar]
- 90.Chen MF, Lin CT, Chen WC, et al. The sensitivity of human mesenchymal stem cells to ionizing radiation. Int J Radiat Oncol Biol Phys. 2006;66:244–253. doi: 10.1016/j.ijrobp.2006.03.062. [DOI] [PubMed] [Google Scholar]
- 91.Kang Y, Siegel PM, Shu W, et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3:537–549. doi: 10.1016/s1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]


