Abstract
Protection against cholera has been correlated with the level of serum vibriocidal antibodies. The specificity of these vibriocidal antibodies was mostly to the lipopolysaccharide (LPS). We synthesized conjugates of detoxified LPS with cholera toxin (CT) and other proteins in order to elicit serum LPS antibodies with vibriocidal activity. Treatment with hydrazine (deacylated LPS) reduced the endotoxic properties of the LPS to clinically acceptable levels and resulted in a molecule larger and more antigenic than the saccharide produced by acid hydrolysis. More immunogenic conjugates resulted from multipoint compared with single-point attachment of the deacylated LPS to the protein. The conjugates containing CT had low levels of pyrogen and no toxic activity upon Chinese hamster ovary cells and elicited booster responses of vibriocidal and CT antibodies when injected subcutaneously as saline solutions into mice; the vibriocidal titers were similar to those elicited by comparable doses of cellular vaccines. We suggest how serum vibriocidal antibodies might prevent cholera.
Full text
PDF


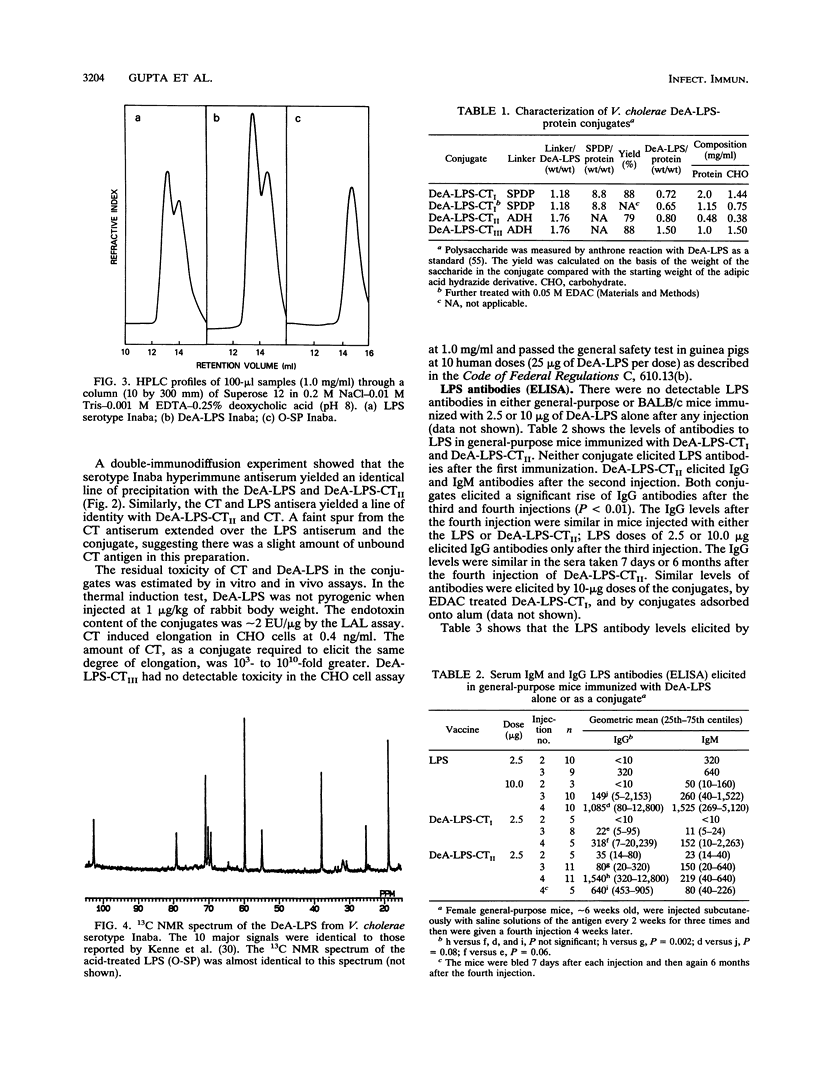
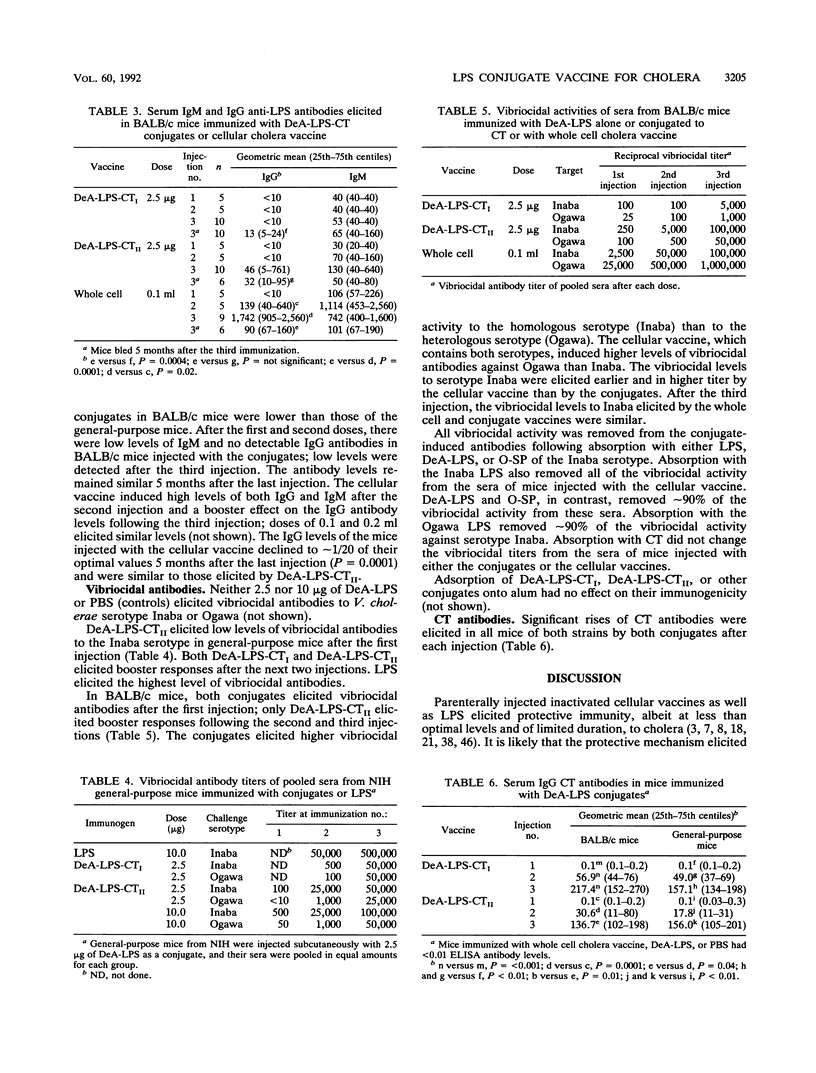



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed A., Bhattacharjee A. K., Mosley W. H. Characteristics of the serum vibriocidal and agglutinating antibodies in cholera cases and in normal residents of the endemic and non-endemic cholera areas. J Immunol. 1970 Aug;105(2):431–441. [PubMed] [Google Scholar]
- Anderson P. W., Pichichero M. E., Insel R. A., Betts R., Eby R., Smith D. H. Vaccines consisting of periodate-cleaved oligosaccharides from the capsule of Haemophilus influenzae type b coupled to a protein carrier: structural and temporal requirements for priming in the human infant. J Immunol. 1986 Aug 15;137(4):1181–1186. [PubMed] [Google Scholar]
- Black R. E., Levine M. M., Clements M. L., Young C. R., Svennerholm A. M., Holmgren J. Protective efficacy in humans of killed whole-vibrio oral cholera vaccine with and without the B subunit of cholera toxin. Infect Immun. 1987 May;55(5):1116–1120. doi: 10.1128/iai.55.5.1116-1120.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brade H. Occurrence of 2-keto-deoxyoctonic acid 5-phosphate in lipopolysaccharides of Vibrio cholerae Ogawa and Inaba. J Bacteriol. 1985 Feb;161(2):795–798. doi: 10.1128/jb.161.2.795-798.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N., Mar V. L., Platler B. W., Schlotterbeck J. D., McGinley M. D., Stoney K. S., Rohde M. F., Kaslow H. R. Site-specific mutagenesis of the catalytic subunit of cholera toxin: substituting lysine for arginine 7 causes loss of activity. Infect Immun. 1991 Nov;59(11):4266–4270. doi: 10.1128/iai.59.11.4266-4270.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash R. A., Music S. I., Libonati J. P., Craig J. P., Pierce N. F., Hornick R. B. Response of man to infection with Vibrio cholerae. II. Protection from illness afforded by previous disease and vaccine. J Infect Dis. 1974 Oct;130(4):325–333. doi: 10.1093/infdis/130.4.325. [DOI] [PubMed] [Google Scholar]
- Chu C. Y., Liu B. K., Watson D., Szu S. S., Bryla D., Shiloach J., Schneerson R., Robbins J. B. Preparation, characterization, and immunogenicity of conjugates composed of the O-specific polysaccharide of Shigella dysenteriae type 1 (Shiga's bacillus) bound to tetanus toxoid. Infect Immun. 1991 Dec;59(12):4450–4458. doi: 10.1128/iai.59.12.4450-4458.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens J. D., Sack D. A., Chakraborty J., Rao M. R., Ahmed F., Harris J. R., van Loon F., Khan M. R., Yunis M., Huda S. Field trial of oral cholera vaccines in Bangladesh: evaluation of anti-bacterial and anti-toxic breast-milk immunity in response to ingestion of the vaccines. Vaccine. 1990 Oct;8(5):469–472. doi: 10.1016/0264-410x(90)90248-k. [DOI] [PubMed] [Google Scholar]
- Clemens J. D., Sack D. A., Harris J. R., Van Loon F., Chakraborty J., Ahmed F., Rao M. R., Khan M. R., Yunus M., Huda N. Field trial of oral cholera vaccines in Bangladesh: results from three-year follow-up. Lancet. 1990 Feb 3;335(8684):270–273. doi: 10.1016/0140-6736(90)90080-o. [DOI] [PubMed] [Google Scholar]
- Clemens J. D., van Loon F., Sack D. A., Chakraborty J., Rao M. R., Ahmed F., Harris J. R., Khan M. R., Yunus M., Huda S. Field trial of oral cholera vaccines in Bangladesh: serum vibriocidal and antitoxic antibodies as markers of the risk of cholera. J Infect Dis. 1991 Jun;163(6):1235–1242. doi: 10.1093/infdis/163.6.1235. [DOI] [PubMed] [Google Scholar]
- DeMaria A., Jr, Johns M. A., Berberich H., McCabe W. R. Immunization with rough mutants of Salmonella minnesota: initial studies in human subjects. J Infect Dis. 1988 Aug;158(2):301–311. doi: 10.1093/infdis/158.2.301. [DOI] [PubMed] [Google Scholar]
- Elson C. O., Ealding W. Generalized systemic and mucosal immunity in mice after mucosal stimulation with cholera toxin. J Immunol. 1984 Jun;132(6):2736–2741. [PubMed] [Google Scholar]
- FINKELSTEIN R. A., POWELL C. J., Jr, WOODROW J. C., KREVANS J. R. SEROLOGICAL RESPONSES IN MAN TO A SINGLE SMALL DOSE OF CHOLERA VACCINE WITH SPECIAL REFERENCE TO THE LACK OF INFLUENCE OF ABO BLOOD GROUP ON NATURAL ANTIBODY OR IMMUNOLOGICAL RESPONSIVENESS. Bull Johns Hopkins Hosp. 1965 Mar;116:152–160. [PubMed] [Google Scholar]
- Fattom A., Lue C., Szu S. C., Mestecky J., Schiffman G., Bryla D., Vann W. F., Watson D., Kimzey L. M., Robbins J. B. Serum antibody response in adult volunteers elicited by injection of Streptococcus pneumoniae type 12F polysaccharide alone or conjugated to diphtheria toxoid. Infect Immun. 1990 Jul;58(7):2309–2312. doi: 10.1128/iai.58.7.2309-2312.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisatsune K., Haishima Y., Iguchi T., Kondo S. Lipopolysaccharides of non-cholera vibrios possessing common antigen factor to 01 Vibrio cholerae. Adv Exp Med Biol. 1990;256:189–197. doi: 10.1007/978-1-4757-5140-6_16. [DOI] [PubMed] [Google Scholar]
- Holmgren J., Svennerholm A. M. Mechanisms of disease and immunity in cholera: a review. J Infect Dis. 1977 Aug;136 (Suppl):S105–S112. doi: 10.1093/infdis/136.supplement.s105. [DOI] [PubMed] [Google Scholar]
- Jertborn M., Svennerholm A. M., Holmgren J. Saliva, breast milk, and serum antibody responses as indirect measures of intestinal immunity after oral cholera vaccination or natural disease. J Clin Microbiol. 1986 Aug;24(2):203–209. doi: 10.1128/jcm.24.2.203-209.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabir S. Preparation and immunogenicity of a bivalent cell-surface protein-polysaccharide conjugate of Vibrio cholerae. J Med Microbiol. 1987 Feb;23(1):9–18. doi: 10.1099/00222615-23-1-9. [DOI] [PubMed] [Google Scholar]
- Kaur J., Burrows W., Furlong M. A. Immunity to cholera: antibody response in the lower ileum of the rabbit. J Infect Dis. 1971 Oct;124(4):359–366. doi: 10.1093/infdis/124.4.359. [DOI] [PubMed] [Google Scholar]
- Kazemi M., Finkelstein R. A. Study of epitopes of cholera enterotoxin-related enterotoxins by checkerboard immunoblotting. Infect Immun. 1990 Jul;58(7):2352–2360. doi: 10.1128/iai.58.7.2352-2360.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenne L., Lindberg B., Unger P., Gustafsson B., Holme T. Structural studies of the Vibrio cholerae O-antigen. Carbohydr Res. 1982 Mar 1;100:341–349. doi: 10.1016/s0008-6215(00)81047-2. [DOI] [PubMed] [Google Scholar]
- LANDY M., GAINES S., SEAL J. R., WHITSIDE J. E. Antibody responses of man to three types of antityphoid immunizing agents: heat-phenol fluid vaccine, acetone-dehydrated vaccine, and isolated Vi and O antigens. Am J Public Health Nations Health. 1954 Dec;44(12):1572–1579. doi: 10.2105/ajph.44.12.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. M., Black R. E., Clements M. L., Lanata C., Sears S., Honda T., Young C. R., Finkelstein R. A. Evaluation in humans of attenuated Vibrio cholerae El Tor Ogawa strain Texas Star-SR as a live oral vaccine. Infect Immun. 1984 Feb;43(2):515–522. doi: 10.1128/iai.43.2.515-522.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugowski C., Romanowska E. Chemical studies on Shigella sonnei lipid A. Eur J Biochem. 1974 Oct 2;48(2):319–323. doi: 10.1111/j.1432-1033.1974.tb03771.x. [DOI] [PubMed] [Google Scholar]
- Manning P. A., Heuzenroeder M. W., Yeadon J., Leavesley D. I., Reeves P. R., Rowley D. Molecular cloning and expression in Escherichia coli K-12 of the O antigens of the Inaba and Ogawa serotypes of the Vibrio cholerae O1 lipopolysaccharides and their potential for vaccine development. Infect Immun. 1986 Aug;53(2):272–277. doi: 10.1128/iai.53.2.272-277.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGroarty E. J., Rivera M. Growth-dependent alterations in production of serotype-specific and common antigen lipopolysaccharides in Pseudomonas aeruginosa PAO1. Infect Immun. 1990 Apr;58(4):1030–1037. doi: 10.1128/iai.58.4.1030-1037.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neoh S. H., Rowley D. Protection of infant mice against cholera by antibodies to three antigens of Vibrio cholerae. J Infect Dis. 1972 Jul;126(1):41–47. doi: 10.1093/infdis/126.1.41. [DOI] [PubMed] [Google Scholar]
- Neoh S. H., Rowley D. The antigens of Vibrio cholerae involved in the vibriocidal action of antibody and complement. J Infect Dis. 1970 May;121(5):505–513. doi: 10.1093/infdis/121.5.505. [DOI] [PubMed] [Google Scholar]
- Parsot C., Taxman E., Mekalanos J. J. ToxR regulates the production of lipoproteins and the expression of serum resistance in Vibrio cholerae. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1641–1645. doi: 10.1073/pnas.88.5.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson J. W. Synergistic protection against experimental cholera by immunization with cholera toxoid and vaccine. Infect Immun. 1979 Nov;26(2):528–533. doi: 10.1128/iai.26.2.528-533.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce N. F., Cray W. C., Jr, Kaper J. B., Mekalanos J. J. Determinants of immunogenicity and mechanisms of protection by virulent and mutant Vibrio cholerae O1 in rabbits. Infect Immun. 1988 Jan;56(1):142–148. doi: 10.1128/iai.56.1.142-148.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raziuddin S. Toxic and immunological properties of the lipopolysaccharides (O-antigens) from Vibrio el-tor. Immunochemistry. 1978 Sep;15(9):611–614. doi: 10.1016/0161-5890(78)90032-9. [DOI] [PubMed] [Google Scholar]
- Redmond J. W. The structure of the O-antigenic side chain of the lipopolysaccharide of Vibrio cholerae 569B (Inaba). Biochim Biophys Acta. 1979 May 1;584(2):346–352. doi: 10.1016/0304-4165(79)90280-0. [DOI] [PubMed] [Google Scholar]
- Robbins J. B., Schneerson R. Polysaccharide-protein conjugates: a new generation of vaccines. J Infect Dis. 1990 May;161(5):821–832. doi: 10.1093/infdis/161.5.821. [DOI] [PubMed] [Google Scholar]
- Sack D. A., Clemens J. D., Huda S., Harris J. R., Khan M. R., Chakraborty J., Yunus M., Gomes J., Siddique O., Ahmed F. Antibody responses after immunization with killed oral cholera vaccines during the 1985 vaccine field trial in Bangladesh. J Infect Dis. 1991 Aug;164(2):407–411. doi: 10.1093/infdis/164.2.407. [DOI] [PubMed] [Google Scholar]
- Sommer A., Mosley W. H. Ineffectiveness of cholera vaccination as an epidemic control measure. Lancet. 1973 Jun 2;1(7814):1232–1235. doi: 10.1016/s0140-6736(73)90540-0. [DOI] [PubMed] [Google Scholar]
- Svennerholm A. M., Holmgren J. Synergistic protective effect in rabbits of immunization with Vibrio cholerae lipopolysaccharide and toxin/toxoid. Infect Immun. 1976 Mar;13(3):735–740. doi: 10.1128/iai.13.3.735-740.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szu S. C., Li X. R., Schneerson R., Vickers J. H., Bryla D., Robbins J. B. Comparative immunogenicities of Vi polysaccharide-protein conjugates composed of cholera toxin or its B subunit as a carrier bound to high- or lower-molecular-weight Vi. Infect Immun. 1989 Dec;57(12):3823–3827. doi: 10.1128/iai.57.12.3823-3827.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szu S. C., Schneerson R., Robbins J. B. Rabbit antibodies to the cell wall polysaccharide of Streptococcus pneumoniae fail to protect mice from lethal challenge with encapsulated pneumococci. Infect Immun. 1986 Nov;54(2):448–455. doi: 10.1128/iai.54.2.448-455.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TREVELYAN W. E., HARRISON J. S. Studies on yeast metabolism. I. Fractionation and microdetermination of cell carbohydrates. Biochem J. 1952 Jan;50(3):298–303. doi: 10.1042/bj0500298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M. The analysis of lipopolysaccharide (endotoxin) in meningococcal polysaccharide vaccines by silver staining following SDS-polyacrylamide gel electrophoresis. J Biol Stand. 1986 Jan;14(1):25–33. doi: 10.1016/s0092-1157(86)80006-3. [DOI] [PubMed] [Google Scholar]
- Waldman R. H., Bencic Z., Sinha R., Deb B. C., Sakazaki R., Tamura K., Mukerjee S., Ganguly R. Cholera immunology. II. Serum and intestinal secretion antibody response after naturally occurring cholera. J Infect Dis. 1972 Oct;126(4):401–407. doi: 10.1093/infdis/126.4.401. [DOI] [PubMed] [Google Scholar]
- Wernet P., Breu H., Knop J., Rowley D. Antibacterial action of specific IgA and transport of IgM, IgA, and IgG from serum into the small intestine. J Infect Dis. 1971 Aug;124(2):223–226. doi: 10.1093/infdis/124.2.223. [DOI] [PubMed] [Google Scholar]
- Winner L., 3rd, Mack J., Weltzin R., Mekalanos J. J., Kraehenbuhl J. P., Neutra M. R. New model for analysis of mucosal immunity: intestinal secretion of specific monoclonal immunoglobulin A from hybridoma tumors protects against Vibrio cholerae infection. Infect Immun. 1991 Mar;59(3):977–982. doi: 10.1128/iai.59.3.977-982.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]