Abstract
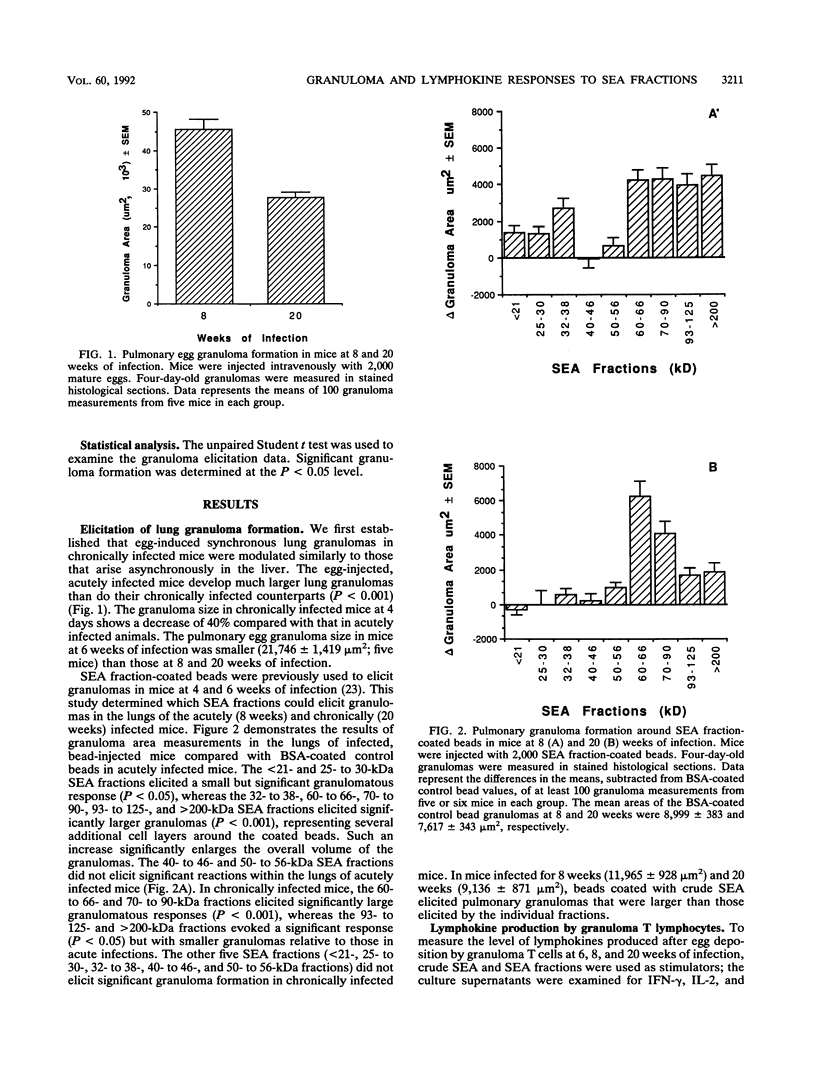
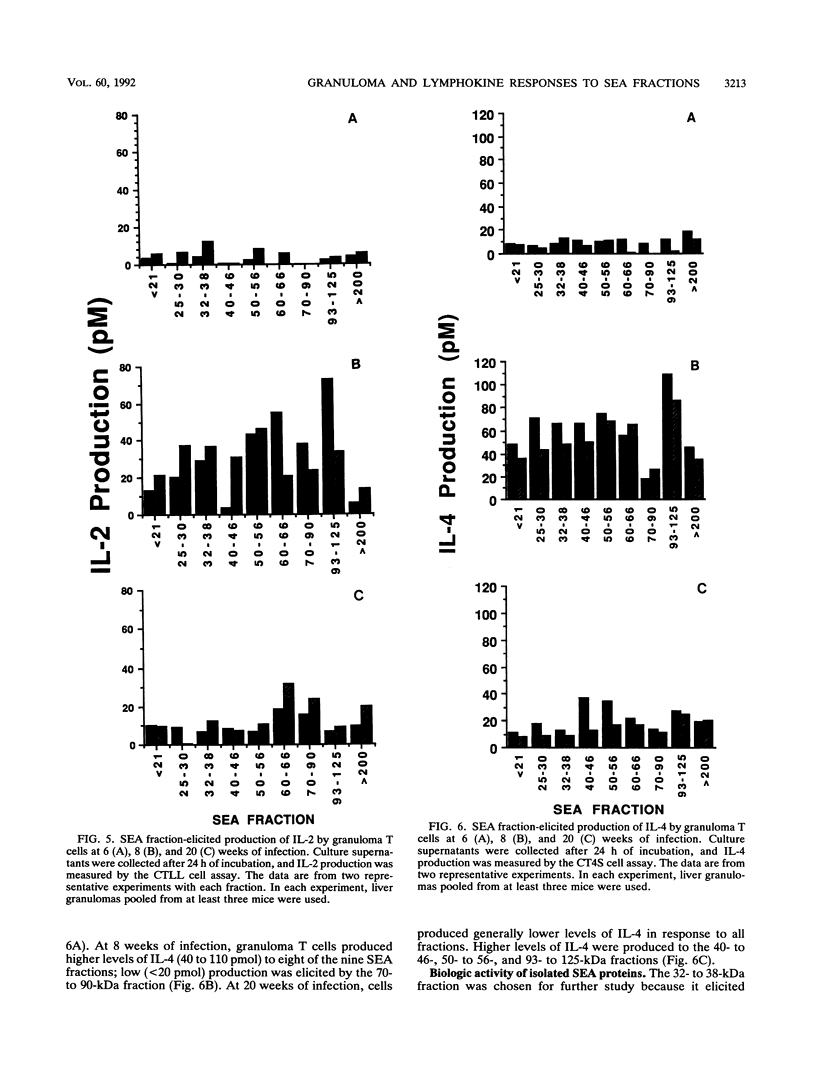
Worm eggs deposited in the livers and intestines of Schistosoma mansoni-infected mice secrete soluble egg antigens (SEA) and induce T cell-mediated circumoval granulomas. In the present study, we fractionated crude SEA by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and tested the fractions for granuloma elicitation and lymphokine production at different stages of the infection. SEA fraction-coupled beads were used to elicit artificial pulmonary granulomas. Acutely infected mice responded with granulomas to seven fractions (less than 21-, 25- to 30-, 32- to 38-, 60- to 66-, 70- to 90-, 93- to 125-, and greater than 200-kDa fractions) of SEA, whereas chronically infected mice responded to four fractions (60- to 66-, 70- to 90-, 93- to 125-, and greater than 200-kDa fractions). In response to both crude and fractionated SEA, granuloma T cells produced high levels of gamma interferon at the preacute (6-week) stage of infection, but production subsequently diminished. Interleukin-2 (IL-2) and IL-4 production peaked at the acute (8-week) stage of infection and concurrently decreased at the chronic (20-week) stage. At the acute stage of the infection, the granulomagenic SEA fractions also elicited IL-2 and IL-4 production; at the chronic stage, IL-2 production and, to a lesser degree, IL-4 production corresponded to SEA fractions that elicited granulomas. Isolated SEA proteins from the 32- to 38-kDa fraction demonstrated differential lymphokine responses: predominant gamma interferon and IL-2 production was elicited by the 32-kDa fraction, whereas the 35- and 38-kDa proteins elicited predominant gamma interferon and IL-4 production. However, all three proteins elicited granuloma formation. The present study reveals changes in granulomatous responses to SEA fractions during the acute and chronic stages of the infection as well as distinct phases of gamma interferon, IL-2, and IL-4 lymphokine production throughout the infection. Based on these results, it is concluded that granuloma formation and IL-2 and IL-4 production are interrelated.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boctor F. N., Nash T. E., Cheever A. W. Isolation of a polysaccharide antigen from Schistosoma mansoni eggs. J Immunol. 1979 Jan;122(1):39–43. [PubMed] [Google Scholar]
- Boros D. L. Immunopathology of Schistosoma mansoni infection. Clin Microbiol Rev. 1989 Jul;2(3):250–269. doi: 10.1128/cmr.2.3.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boros D. L., Pelley R. P., Warren K. S. Spontaneous modulation of granulomatous hypersensitivity in schistosomiasis mansoni. J Immunol. 1975 May;114(5):1437–1441. [PubMed] [Google Scholar]
- Boros D. L., Tomford R., Warren K. S. Induction of granulomatous and elicitation of cutaneous sensitivity by partially purified SEA of Schistosoma mansoni. J Immunol. 1977 Jan;118(1):373–376. [PubMed] [Google Scholar]
- Boros D. L., Warren K. S. Delayed hypersensitivity-type granuloma formation and dermal reaction induced and elicited by a soluble factor isolated from Schistosoma mansoni eggs. J Exp Med. 1970 Sep 1;132(3):488–507. doi: 10.1084/jem.132.3.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. P., Remold H. G., Warren K. S., David J. R. Partial purification of antigens from eggs of Schistosoma mansoni that elicit delayed hypersensitivity. J Immunol. 1977 Oct;119(4):1275–1278. [PubMed] [Google Scholar]
- Carter C. E., Colley D. G. Partial purification and characterization of Schistosoma mansoni soluble egg antigen with Con A-Sepharose chromatography. J Immunol. 1979 Jun;122(6):2204–2209. [PubMed] [Google Scholar]
- Chensue S. W., Boros D. L. Modulation of granulomatous hypersensitivity. I. Characterization of T lymphocytes involved in the adoptive suppression of granuloma formation in Schistosoma mansoni-infected mice. J Immunol. 1979 Sep;123(3):1409–1414. [PubMed] [Google Scholar]
- Chensue S. W., Kunkel S. L., Higashi G. I., Ward P. A., Boros D. L. Production of superoxide anion, prostaglandins, and hydroxyeicosatetraenoic acids by macrophages from hypersensitivity-type (Schistosoma mansoni egg) and foreign body-type granulomas. Infect Immun. 1983 Dec;42(3):1116–1125. doi: 10.1128/iai.42.3.1116-1125.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chensue S. W., Terebuh P. D., Warmington K. S., Hershey S. D., Evanoff H. L., Kunkel S. L., Higashi G. I. Role of IL-4 and IFN-gamma in Schistosoma mansoni egg-induced hypersensitivity granuloma formation. Orchestration, relative contribution, and relationship to macrophage function. J Immunol. 1992 Feb 1;148(3):900–906. [PubMed] [Google Scholar]
- Colley D. G. Immune responses to a soluble schistosomal egg antigen preparation during chronic primary infection with Schistosoma mansoni. J Immunol. 1975 Jul;115(1):150–156. [PubMed] [Google Scholar]
- Dunne D. W., Lucas S., Bickle Q., Pearson S., Madgwick L., Bain J., Doenhoff M. J. Identification and partial purification of an antigen (omega 1) from Schistosoma mansoni eggs which is putatively hepatotoxic in T-cell deprived mice. Trans R Soc Trop Med Hyg. 1981;75(1):54–71. doi: 10.1016/0035-9203(81)90013-4. [DOI] [PubMed] [Google Scholar]
- Firestein G. S., Roeder W. D., Laxer J. A., Townsend K. S., Weaver C. T., Hom J. T., Linton J., Torbett B. E., Glasebrook A. L. A new murine CD4+ T cell subset with an unrestricted cytokine profile. J Immunol. 1989 Jul 15;143(2):518–525. [PubMed] [Google Scholar]
- Grzych J. M., Pearce E., Cheever A., Caulada Z. A., Caspar P., Heiny S., Lewis F., Sher A. Egg deposition is the major stimulus for the production of Th2 cytokines in murine schistosomiasis mansoni. J Immunol. 1991 Feb 15;146(4):1322–1327. [PubMed] [Google Scholar]
- Hamburger J., Lustigman S., Siongok T. K., Ouma J. H., Mahmoud A. A. Characterization of a purified glycoprotein from Schistosoma mansoni eggs: specificity, stability, and the involvement of carbohydrate and peptide moieties in its serologic activity. J Immunol. 1982 Apr;128(4):1864–1869. [PubMed] [Google Scholar]
- Hang L. M., Boros D. L., Warren K. S. Induction of immunological hyporesponsiveness to granulomatous hypersensitivity in Schistosoma mansoni infection. J Infect Dis. 1974 Nov;130(5):515–522. doi: 10.1093/infdis/130.5.515. [DOI] [PubMed] [Google Scholar]
- Harn D. A., Danko K., Quinn J. J., Stadecker M. J. Schistosoma mansoni: the host immune response to egg antigens. I. Partial characterization of cellular and humoral responses to pI fractions of soluble egg antigens. J Immunol. 1989 Mar 15;142(6):2061–2066. [PubMed] [Google Scholar]
- Harrison D. J., Carter C. E., Colley D. G. Immunoaffinity purification of Schistosoma mansoni soluble egg antigens. J Immunol. 1979 Jun;122(6):2210–2217. [PubMed] [Google Scholar]
- Henderson G. S., Conary J. T., Summar M., McCurley T. L., Colley D. G. In vivo molecular analysis of lymphokines involved in the murine immune response during Schistosoma mansoni infection. I. IL-4 mRNA, not IL-2 mRNA, is abundant in the granulomatous livers, mesenteric lymph nodes, and spleens of infected mice. J Immunol. 1991 Aug 1;147(3):992–997. [PubMed] [Google Scholar]
- Loveless S. E., Wellhausen S. R., Boros D. L., Heppner G. H. Tumoricidal macrophages isolated from liver granulomas of Schistosoma mansoni-infected mice. J Immunol. 1982 Jan;128(1):284–288. [PubMed] [Google Scholar]
- Lukacs N. W., Boros D. L. Identification of larval cross-reactive and egg-specific antigens involved in granuloma formation in murine schistosomiasis mansoni. Infect Immun. 1991 Sep;59(9):3237–3242. doi: 10.1128/iai.59.9.3237-3242.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukacs N. W., Boros D. L. Splenic and granuloma T-lymphocyte responses to fractionated soluble egg antigens of Schistosoma mansoni-infected mice. Infect Immun. 1991 Mar;59(3):941–948. doi: 10.1128/iai.59.3.941-948.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustigman S., Mahmoud A. A., Hamburger J. Glycopeptides in soluble egg antigen of Schistosoma mansoni: isolation, characterization, and elucidation of their immunochemical and immunopathological relation to the major egg glycoprotein (MEG). J Immunol. 1985 Mar;134(3):1961–1967. [PubMed] [Google Scholar]
- Mathew R. C., Boros D. L. Anti-L3T4 antibody treatment suppresses hepatic granuloma formation and abrogates antigen-induced interleukin-2 production in Schistosoma mansoni infection. Infect Immun. 1986 Dec;54(3):820–826. doi: 10.1128/iai.54.3.820-826.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew R. C., Ragheb S., Boros D. L. Recombinant IL-2 therapy reverses diminished granulomatous responsiveness in anti-L3T4-treated, Schistosoma mansoni-infected mice. J Immunol. 1990 Jun 1;144(11):4356–4361. [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Mosmann T. R., Moore K. W. The role of IL-10 in crossregulation of TH1 and TH2 responses. Immunol Today. 1991 Mar;12(3):A49–A53. doi: 10.1016/S0167-5699(05)80015-5. [DOI] [PubMed] [Google Scholar]
- Owhashi M., Horii Y., Imai J., Ishii A., Nawa Y. Purification and physicochemical characterization of Schistosoma mansoni egg allergen recognized by mouse sera obtained at an acute stage of infection. Int Arch Allergy Appl Immunol. 1986;81(2):129–135. doi: 10.1159/000234121. [DOI] [PubMed] [Google Scholar]
- Pelley R. P., Pelley R. J., Hamburger J., Peters P. A., Warren K. S. Schistosoma mansoni soluble egg antigens. I. Identification and purification of three major antigens, and the employment of radioimmunoassay for their further characterization. J Immunol. 1976 Nov;117(5 Pt 1):1553–1560. [PubMed] [Google Scholar]
- Phillips S. M., Lammie P. J. Immunopathology of granuloma formation and fibrosis in schistosomiasis. Parasitol Today. 1986 Nov;2(11):296–302. doi: 10.1016/0169-4758(86)90123-7. [DOI] [PubMed] [Google Scholar]
- Ragheb S., Boros D. L. Characterization of granuloma T lymphocyte function from Schistosoma mansoni-infected mice. J Immunol. 1989 May 1;142(9):3239–3246. [PubMed] [Google Scholar]
- Sher A., Fiorentino D., Caspar P., Pearce E., Mosmann T. Production of IL-10 by CD4+ T lymphocytes correlates with down-regulation of Th1 cytokine synthesis in helminth infection. J Immunol. 1991 Oct 15;147(8):2713–2716. [PubMed] [Google Scholar]
- Street N. E., Schumacher J. H., Fong T. A., Bass H., Fiorentino D. F., Leverah J. A., Mosmann T. R. Heterogeneity of mouse helper T cells. Evidence from bulk cultures and limiting dilution cloning for precursors of Th1 and Th2 cells. J Immunol. 1990 Mar 1;144(5):1629–1639. [PubMed] [Google Scholar]
- Weinstock J. V., Boros D. L. Heterogeneity of the granulomatous response in the liver, colon, ileum, and ileal Peyer's patches to schistosome eggs in murine schistosomiasis mansoni. J Immunol. 1981 Nov;127(5):1906–1909. [PubMed] [Google Scholar]
- Weiss J. B., Aronstein W. S., Strand M. Schistosoma mansoni: stimulation of artificial granuloma formation in vivo by carbohydrate determinants. Exp Parasitol. 1987 Oct;64(2):228–236. doi: 10.1016/0014-4894(87)90147-0. [DOI] [PubMed] [Google Scholar]
- Weiss J. B., Strand M. Characterization of developmentally regulated epitopes of Schistosoma mansoni egg glycoprotein antigens. J Immunol. 1985 Aug;135(2):1421–1429. [PubMed] [Google Scholar]
- Wellhausen S. R., Boros D. L. Comparison of Fc, C3 receptors and Ia antigens on the inflammatory macrophage isolated from vigorous or immunomodulated liver granulomas of schistosome-infected mice. J Reticuloendothel Soc. 1981 Sep;30(3):191–203. [PubMed] [Google Scholar]
- Yamashita T., Boros D. L. Changing patterns of lymphocyte proliferation, IL-2 production and utilization, and IL-2 receptor expression in mice infected with Schistosoma mansoni. J Immunol. 1990 Jul 15;145(2):724–731. [PubMed] [Google Scholar]


