Abstract
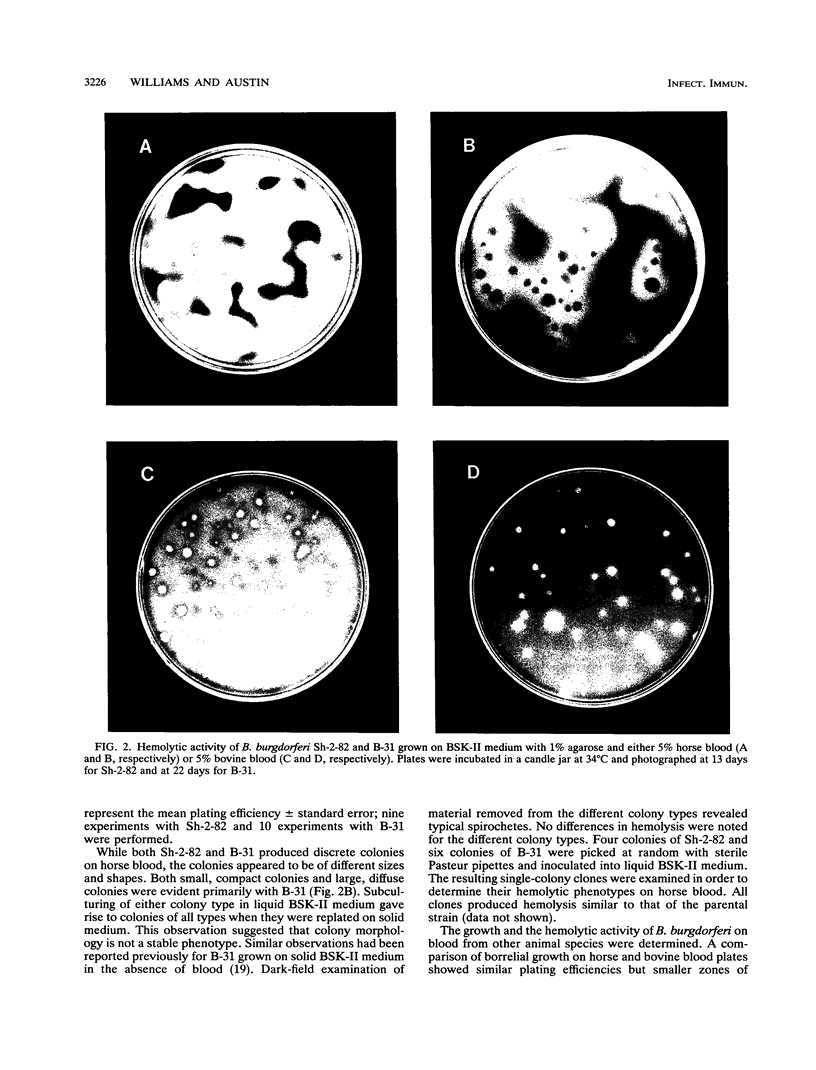
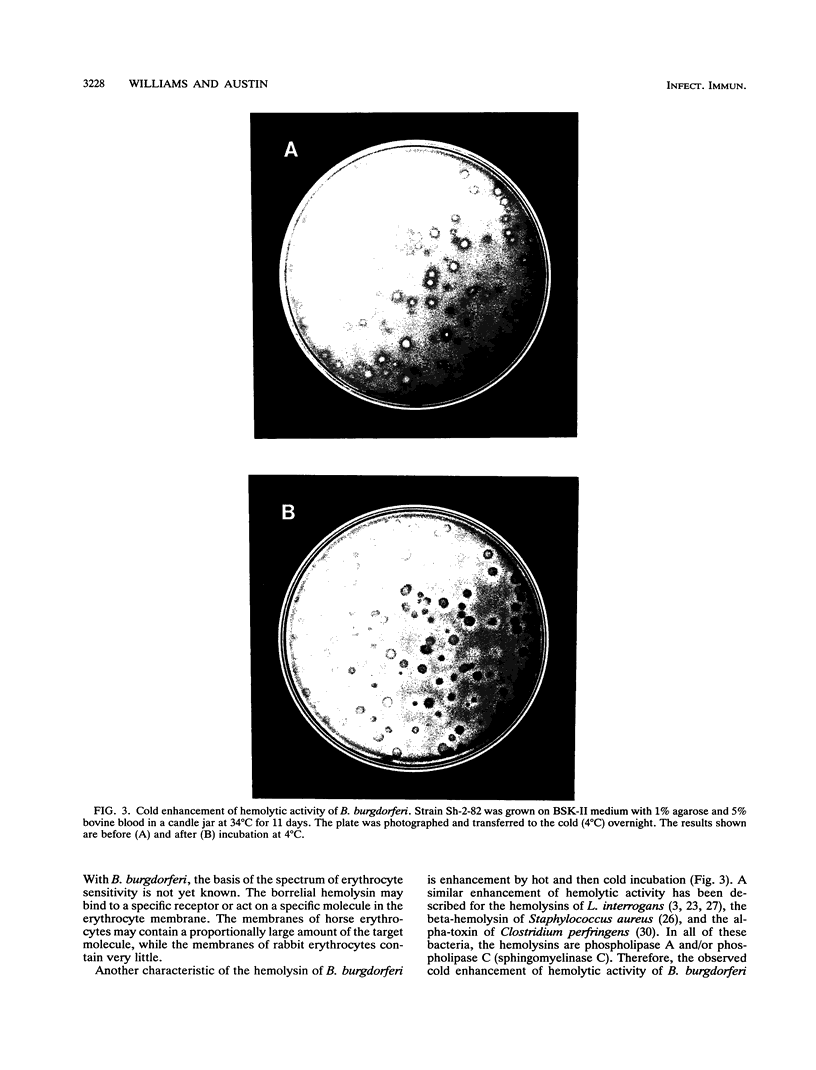
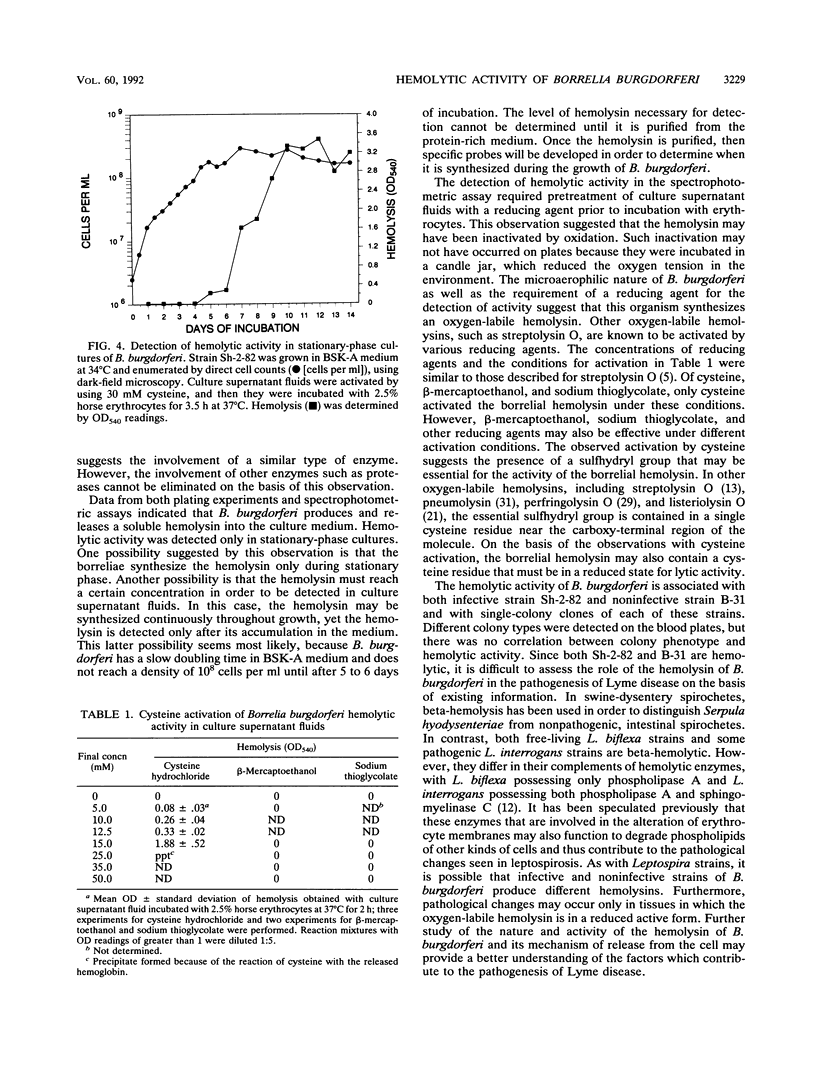
Zones of beta-hemolysis occurred around colonies of Borrelia burgdorferi grown on Barbour-Stoenner-Kelly medium containing agarose and horse blood. Blood plates were inoculated with either the infective strain Sh-2-82 or noninfective strain B-31 in an overlay and incubated in a candle jar. Both strains of B. burgdorferi displayed beta-hemolysis after 1 to 2 weeks of incubation. The hemolytic activity diffused out from the borrelial colonies, eventually resulting in lysis of the entire blood plate. Hemolysis was most pronounced with horse blood and was less intense with bovine, sheep, and rabbit blood. Hemolysis was enhanced by hot-cold incubation, which is typical of phospholipase-like activities in other bacteria. Further characterization of the borrelial hemolysin by using a spectrophotometric assay revealed its presence in the supernatant fluids of stationary-phase cultures. Detection of the borrelial hemolytic activity was dependent on activation of the hemolysin by the reducing agent cysteine. This study provides the first evidence of hemolytic activity associated with B. burgdorferi.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALEXANDER A. D., SMITH O. H., HIATT C. W., GLEISER C. A. Presence of hemolysin in cultures of pathogenic leptospires. Proc Soc Exp Biol Med. 1956 Feb;91(2):205–211. doi: 10.3181/00379727-91-22214. [DOI] [PubMed] [Google Scholar]
- BAUER D. C., EAMES L. N., SLEIGHT S. D., FERGUSON L. C. The significance of leptospiral hemolysin in the pathogenesis of Leptospira pomona infections. J Infect Dis. 1961 Mar-Apr;108:229–236. doi: 10.1093/infdis/108.2.229. [DOI] [PubMed] [Google Scholar]
- Barbour A. G. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984 Jul-Aug;57(4):521–525. [PMC free article] [PubMed] [Google Scholar]
- Bernheimer A. W., Avigad L. S. Streptolysin o: activation by thiols. Infect Immun. 1970 May;1(5):509–510. doi: 10.1128/iai.1.5.509-510.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernheimer A. W., Bey R. F. Copurification of Leptospira interrogans serovar pomona hemolysin and sphingomyelinase C. Infect Immun. 1986 Oct;54(1):262–264. doi: 10.1128/iai.54.1.262-264.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernheimer A. W. Staphylococcal alpha toxin. Ann N Y Acad Sci. 1965 Jul 23;128(1):112–123. doi: 10.1111/j.1749-6632.1965.tb11633.x. [DOI] [PubMed] [Google Scholar]
- Bundoc V. G., Barbour A. G. Clonal polymorphisms of outer membrane protein OspB of Borrelia burgdorferi. Infect Immun. 1989 Sep;57(9):2733–2741. doi: 10.1128/iai.57.9.2733-2741.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chorváth B. Phospholipase A activity in Leptospira biflexa. Biologia (Bratisl) 1970;25(9):587–591. [PubMed] [Google Scholar]
- Honda T., Finkelstein R. A. Purification and characterization of a hemolysin produced by Vibrio cholerae biotype El Tor: another toxic substance produced by cholera vibrios. Infect Immun. 1979 Dec;26(3):1020–1027. doi: 10.1128/iai.26.3.1020-1027.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasărov L. B. Degradiation of the erythrocyte phospholipids and haemolysis of the erythrocytes of different animal species by leptospirae. J Med Microbiol. 1970 Feb;3(1):29–37. doi: 10.1099/00222615-3-1-29. [DOI] [PubMed] [Google Scholar]
- Kehoe M. A., Miller L., Walker J. A., Boulnois G. J. Nucleotide sequence of the streptolysin O (SLO) gene: structural homologies between SLO and other membrane-damaging, thiol-activated toxins. Infect Immun. 1987 Dec;55(12):3228–3232. doi: 10.1128/iai.55.12.3228-3232.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent K. A., Lemcke R. M., Lysons R. J. Production, purification and molecular weight determination of the haemolysin of Treponema hyodysenteriae. J Med Microbiol. 1988 Nov;27(3):215–224. doi: 10.1099/00222615-27-3-215. [DOI] [PubMed] [Google Scholar]
- Kinyon J. M., Harris D. L., Glock R. D. Enteropathogenicity of various isolates of Treponema hyodysenteriae. Infect Immun. 1977 Feb;15(2):638–646. doi: 10.1128/iai.15.2.638-646.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoop F. C. Investigation of a hemolysin produced by enteropathogenic Treponema hyodysenteriae. Infect Immun. 1981 Jan;31(1):193–198. doi: 10.1128/iai.31.1.193-198.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kothary M. H., Kreger A. S. Purification and characterization of an extracellular cytolysin produced by Vibrio damsela. Infect Immun. 1985 Jul;49(1):25–31. doi: 10.1128/iai.49.1.25-31.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtti T. J., Munderloh U. G., Johnson R. C., Ahlstrand G. G. Colony formation and morphology in Borrelia burgdorferi. J Clin Microbiol. 1987 Nov;25(11):2054–2058. doi: 10.1128/jcm.25.11.2054-2058.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemcke R. M., Burrows M. R. Studies on a haemolysin produced by Treponema hyodysenteriae. J Med Microbiol. 1982 May;15(2):205–214. doi: 10.1099/00222615-15-2-205. [DOI] [PubMed] [Google Scholar]
- Mengaud J., Vicente M. F., Chenevert J., Pereira J. M., Geoffroy C., Gicquel-Sanzey B., Baquero F., Perez-Diaz J. C., Cossart P. Expression in Escherichia coli and sequence analysis of the listeriolysin O determinant of Listeria monocytogenes. Infect Immun. 1988 Apr;56(4):766–772. doi: 10.1128/iai.56.4.766-772.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard B., Massicotte L., Saheb S. A. Effet du ribonucléate de sodium sur la croissance et l'activité hémolytique de Treponema hyodysenteriae. Experientia. 1979 Apr 15;35(4):484–486. doi: 10.1007/BF01922721. [DOI] [PubMed] [Google Scholar]
- RUSSELL C. M. A hemolysin associated with leptospirae. J Immunol. 1956 Dec;77(6):405–409. [PubMed] [Google Scholar]
- Schwan T. G., Burgdorfer W., Garon C. F. Changes in infectivity and plasmid profile of the Lyme disease spirochete, Borrelia burgdorferi, as a result of in vitro cultivation. Infect Immun. 1988 Aug;56(8):1831–1836. doi: 10.1128/iai.56.8.1831-1836.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segers R. P., van der Drift A., de Nijs A., Corcione P., van der Zeijst B. A., Gaastra W. Molecular analysis of a sphingomyelinase C gene from Leptospira interrogans serovar hardjo. Infect Immun. 1990 Jul;58(7):2177–2185. doi: 10.1128/iai.58.7.2177-2185.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamm L. V., Charon N. W. Plate assay for detection of Leptospira interrogans serovar pomona hemolysin. J Clin Microbiol. 1979 Oct;10(4):590–592. doi: 10.1128/jcm.10.4.590-592.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton T. B., Jensen N. S., Casey T. A., Tordoff L. A., Dewhirst F. E., Paster B. J. Reclassification of Treponema hyodysenteriae and Treponema innocens in a new genus, Serpula gen. nov., as Serpula hyodysenteriae comb. nov. and Serpula innocens comb. nov. Int J Syst Bacteriol. 1991 Jan;41(1):50–58. doi: 10.1099/00207713-41-1-50. [DOI] [PubMed] [Google Scholar]
- Tweten R. K. Nucleotide sequence of the gene for perfringolysin O (theta-toxin) from Clostridium perfringens: significant homology with the genes for streptolysin O and pneumolysin. Infect Immun. 1988 Dec;56(12):3235–3240. doi: 10.1128/iai.56.12.3235-3240.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Heyningen W. E. The biochemistry of the gas gangrene toxins: Partial purification of the toxins of Cl. welchii, type A. Separation of alpha and theta toxins. Biochem J. 1941 Nov;35(10-11):1257–1269. doi: 10.1042/bj0351257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. A., Allen R. L., Falmagne P., Johnson M. K., Boulnois G. J. Molecular cloning, characterization, and complete nucleotide sequence of the gene for pneumolysin, the sulfhydryl-activated toxin of Streptococcus pneumoniae. Infect Immun. 1987 May;55(5):1184–1189. doi: 10.1128/iai.55.5.1184-1189.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka H., Shimatani S., Tanaka M., Katsu T., Ono B., Shinoda S. Susceptibility of erythrocytes from several animal species to Vibrio vulnificus hemolysin. FEMS Microbiol Lett. 1989 Oct 15;52(3):251–255. doi: 10.1016/0378-1097(89)90206-1. [DOI] [PubMed] [Google Scholar]
- Yanagihara Y., Kojima T., Mifuchi I. Hemolytic activity of Leptospira interrogans serovar canicola cultured in protein-free medium. Microbiol Immunol. 1982;26(7):547–556. [PubMed] [Google Scholar]
- Zen-Yoji H., Hitokoto H., Morozumi S., Le Clair R. A. Purification and characterization o;f a hemolysin produced by Vibrio parahaemolyticus. J Infect Dis. 1971 Jun;123(6):665–667. doi: 10.1093/infdis/123.6.665. [DOI] [PubMed] [Google Scholar]
- del Real G., Segers R. P., van der Zeijst B. A., Gaastra W. Cloning of a hemolysin gene from Leptospira interrogans serovar hardjo. Infect Immun. 1989 Aug;57(8):2588–2590. doi: 10.1128/iai.57.8.2588-2590.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]