Abstract
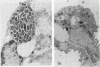
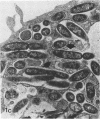
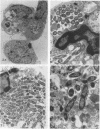
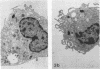
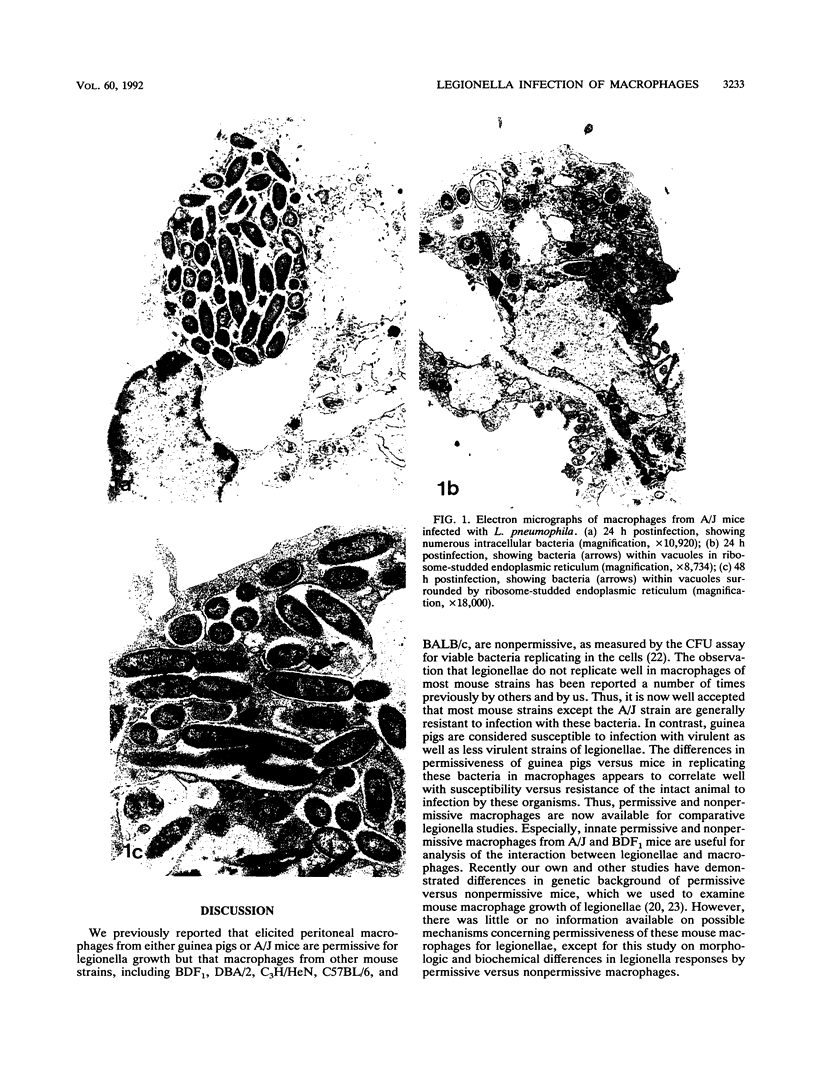
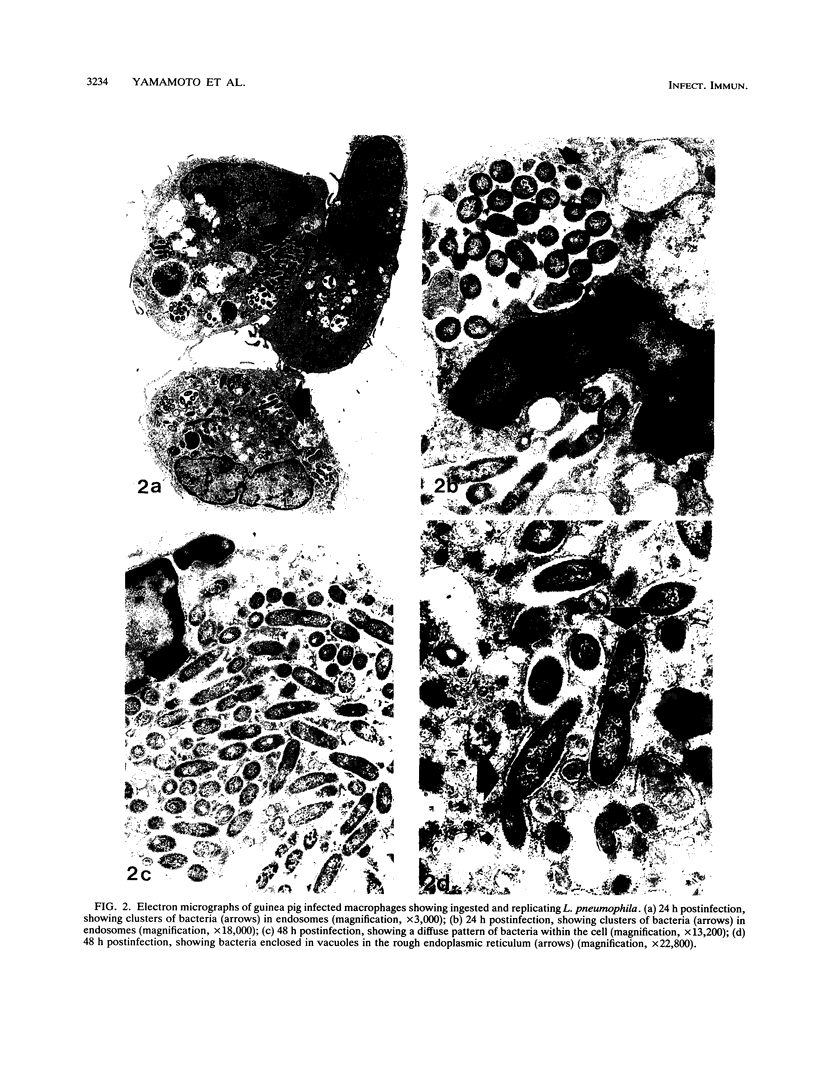
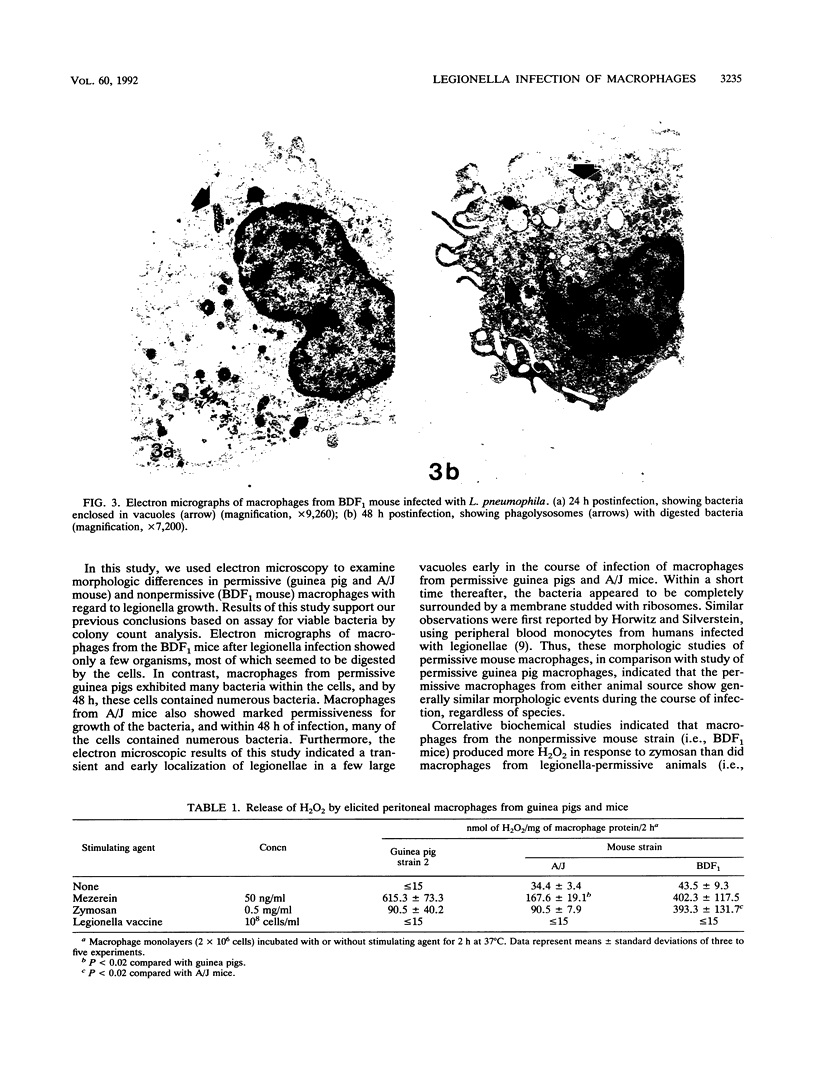
Legionella pneumophila infection of macrophages from permissive guinea pigs and from A/J mice compared with infection of cells from nonpermissive BDF1 mice was studied by electron microscopy. The cells from the BDF1 mice were nonpermissive for legionella growth in vitro and showed few if any bacteria in phagosomes by electron microscopic examination. Similar electron micrographic examination of macrophages from A/J mice permissive for legionella growth showed numerous intact intracellular bacteria within 24 to 48 h of culture and the transition of intracellular bacteria from localization in a few large vacuoles early in the course of infection to later localization in areas surrounded and studded by ribosomes. These electron microscopic observations were similar to those seen in the case of guinea pig macrophages infected with legionellae. Biochemical studies of macrophages from permissive versus nonpermissive animals showed little or no differences in respiratory burst and lysosomal enzyme activity for macrophages from all animals tested. However, when zymosan was used as a stimulant, macrophages from the nonpermissive mouse strain produced a larger amount of H2O2 and O2- than did cells from permissive guinea pigs or A/J mice. However, legionella vaccine itself induced no detectable or very little H2O2 and O2- in macrophages tested from any source. These results suggest that permissiveness of A/J mouse macrophages to legionella growth may involve mechanisms similar to those occurring in guinea pig macrophages in terms of morphologic and possibly even biochemical events. The relatively higher production of reactive oxygens by BDF1 mouse macrophages in response to zymosan correlated with nonpermissiveness for legionella growth, although further analysis is necessary to link these observations.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (first of two parts). N Engl J Med. 1978 Mar 23;298(12):659–668. doi: 10.1056/NEJM197803232981205. [DOI] [PubMed] [Google Scholar]
- Byrd T. F., Horwitz M. A. Interferon gamma-activated human monocytes downregulate transferrin receptors and inhibit the intracellular multiplication of Legionella pneumophila by limiting the availability of iron. J Clin Invest. 1989 May;83(5):1457–1465. doi: 10.1172/JCI114038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman W. H., Kato K., Anstiss C. L., Green S. Human serum beta-glucuronidase; its measurement and some of its properties. Clin Chim Acta. 1967 Mar;15(3):435–447. doi: 10.1016/0009-8981(67)90008-3. [DOI] [PubMed] [Google Scholar]
- Friedman H., Widen R., Klein T., Searls L., Cabrian K. Legionella pneumophila-induced blastogenesis of murine lymphoid cells in vitro. Infect Immun. 1984 Jan;43(1):314–319. doi: 10.1128/iai.43.1.314-319.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A., Silverstein S. C. Legionnaires' disease bacterium (Legionella pneumophila) multiples intracellularly in human monocytes. J Clin Invest. 1980 Sep;66(3):441–450. doi: 10.1172/JCI109874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A. The Legionnaires' disease bacterium (Legionella pneumophila) inhibits phagosome-lysosome fusion in human monocytes. J Exp Med. 1983 Dec 1;158(6):2108–2126. doi: 10.1084/jem.158.6.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs R. F., Locksley R. M., Wilson C. B., Haas J. E., Klebanoff S. J. Interaction of primate alveolar macrophages and Legionella pneumophila. J Clin Invest. 1984 Jun;73(6):1515–1523. doi: 10.1172/JCI111357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein T. W., Yamamoto Y., Brown H. K., Friedman H. Interferon-gamma induced resistance to Legionella pneumophila in susceptible A/J mouse macrophages. J Leukoc Biol. 1991 Jan;49(1):98–103. doi: 10.1002/jlb.49.1.98. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Locksley R. M., Jacobs R. F., Wilson C. B., Weaver W. M., Klebanoff S. J. Susceptibility of Legionella pneumophila to oxygen-dependent microbicidal systems. J Immunol. 1982 Nov;129(5):2192–2197. [PubMed] [Google Scholar]
- Pick E., Keisari Y. Superoxide anion and hydrogen peroxide production by chemically elicited peritoneal macrophages--induction by multiple nonphagocytic stimuli. Cell Immunol. 1981 Apr;59(2):301–318. doi: 10.1016/0008-8749(81)90411-1. [DOI] [PubMed] [Google Scholar]
- Taylor J. L., Sedmak J. J., Jameson P., Lin Y. G., Grossberg S. E. Markedly enhanced production of gamma interferon in murine T lymphocytes treated with lentil lectin and the diterpene ester, mezerein. J Interferon Res. 1984 Summer;4(3):315–327. doi: 10.1089/jir.1984.4.315. [DOI] [PubMed] [Google Scholar]
- Thomas E. L., Lehrer R. I., Rest R. F. Human neutrophil antimicrobial activity. Rev Infect Dis. 1988 Jul-Aug;10 (Suppl 2):S450–S456. doi: 10.1093/cid/10.supplement_2.s450. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y., Klein T. W., Friedman H. Legionella pneumophila growth in macrophages from susceptible mice is genetically controlled. Proc Soc Exp Biol Med. 1991 Apr;196(4):405–409. doi: 10.3181/00379727-196-43207. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y., Klein T. W., Newton C. A., Widen R., Friedman H. Differential growth of Legionella pneumophila in guinea pig versus mouse macrophage cultures. Infect Immun. 1987 Jun;55(6):1369–1374. doi: 10.1128/iai.55.6.1369-1374.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y., Klein T. W., Newton C. A., Widen R., Friedman H. Growth of Legionella pneumophila in thioglycolate-elicited peritoneal macrophages from A/J mice. Infect Immun. 1988 Feb;56(2):370–375. doi: 10.1128/iai.56.2.370-375.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S., Goto Y., Mizuguchi Y., Nomoto K., Skamene E. Genetic control of natural resistance in mouse macrophages regulating intracellular Legionella pneumophila multiplication in vitro. Infect Immun. 1991 Jan;59(1):428–432. doi: 10.1128/iai.59.1.428-432.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S., Mizuguchi Y., Nikaido Y., Mitsuyama M., Nomoto K. Fate of Legionella pneumophila Philadelphia-1 strain in resident, elicited, activated, and immune peritoneal macrophages of guinea pigs. Infect Immun. 1987 Oct;55(10):2477–2482. doi: 10.1128/iai.55.10.2477-2482.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]