Abstract
A novel gene, blaKHM-1, encoding a metallo-β-lactamase, KHM-1, was cloned from a clinical isolate of Citrobacter freundii resistant to most β-lactam antibiotics. Escherichia coli expressing blaKHM-1 was resistant to all broad-spectrum β-lactams except for monobactams and showed reduced susceptibility to carbapenems. Recombinant KHM-1 exhibited EDTA-inhibitable hydrolytic activity against most β-lactams, with an overall preference for cephalosporins.
Acquired metallo-β-lactamases (MBLs) produced by gram-negative bacteria, including Pseudomonas aeruginosa, Acinetobacter spp., and several enterobacteria, confer resistance to all β-lactams except the monobactams (2). Acquired MBLs are categorized on the basis of amino acid sequences into various types (2, 23). The IMP- and VIM-type enzymes are the most common and are found worldwide (2, 8, 23). Recently, four additional types, SPM, GIM, SIM, and AIM, have been found in Brazil (21), Germany (3), Korea (11), and Australia (24), respectively. We report here on the detection of a novel acquired MBL in a clinical isolate of Citrobacter freundii identified in Japan.
C. freundii strain KHM243 was isolated in 1997 from a patient with catheter-associated urinary tract infection at Kyorin University Hospital (Tokyo, Japan). Escherichia coli K-12 strain W1895 was used as the recipient in conjugation experiments. E. coli JM109 (Takara Bio, Shiga, Japan) was used as the host for recombinant plasmids. Plasmid pHSG396 (Takara Bio) was used for the cloning of blaKHM-1 fragments.
Susceptibility to β-lactams was determined by the microdilution method (4). The production of MBL was detected by a double-disk synergy test with disks containing sodium mercaptoacetic acid (MBL production test; Eiken Chemical Co. Ltd., Tokyo, Japan), as described by Arakawa et al. (1).
The transfer of resistance by conjugation was analyzed as described previously (7). E. coli transconjugants were selected on Penassay broth agar (antibiotic medium no. 3; Becton Dickinson, Franklin Lakes, NJ) containing rifampin (200 μg/ml; Wako Pure Chemical Industries, Ltd., Osaka, Japan) and moxalactam (16 μg/ml; Shionogi & Co., Ltd., Osaka, Japan). Plasmid DNA was extracted by an alkaline lysis procedure (9). Plasmid R100 (94.5 kb) (13) from E. coli CSH2, plasmid R478 (275 kb) (6) from E. coli J53, and three cryptic plasmids (200, 60, and 2.4 kb) from Salmonella enterica serovar Enteritidis L119 (15) were used as molecular size markers.
PCR analysis specific for class 1 integrons was performed as described previously (12). DNA sequences flanking blaKHM-1 were determined by inverse PCR (16). Briefly, plasmid DNA extracted from E. coli transconjugant W1895(pCF243) was digested with EcoRV or XspI (Takara Bio). Self-ligated digests were used as the template for an inverse PCR. The upstream and downstream flanking regions of blaKHM-1 were amplified by inverse PCR with two sets of primers: primers 5′-CGATATAACAAGAGCTATTTTCAT-3′ and 5′-GGTATGCGCTGACGATTC-3′ for the upstream region and primers 5′-GGTGTACAGATAAACGCCG-3′ and 5′-TTTATTTGGTGGCTGTTTTGTC-3′ for the downstream region.
The KHM-1 MBL from E. coli JM109(pKHM-1) was purified with HiTrap Q HP and Superdex 200 columns (GE Healthcare Bio-Sciences KK, Tokyo, Japan), as described by Franceschini et al. (5). During the purification procedure, the presence of β-lactamase activity was monitored with 100 μM nitrocefin (Oxoid Ltd., Basingstoke, United Kingdom). The protein concentration was determined with a bicinchoninic acid protein assay kit (Pierce, Rockford, IL). Kinetic analysis was carried out in 50 mM phosphate buffer (pH 7.0) at 25°C with a UV-visible spectrophotometer (V-530; Jasco, Tokyo, Japan). The Km and kcat values and the kcat/Km ratio were determined by analyzing β-lactam hydrolysis under initial-rate conditions by use of the Lineweaver-Burk plot.
Antibiotic susceptibility testing showed that C. freundii KHM243 was resistant to most β-lactams and showed reduced susceptibility to carbapenems (Table 1). However, KHM243 was susceptible to monobactams (carumonam and aztreonam). The isolate was positive by the MBL production test (data not shown).
TABLE 1.
MICs of β-lactams for C. freundii KHM243, E. coli W1895(pCF243) transconjugant, E. coli JM109(pKHM-1) expressing the KHM-1 MBL, and E. coli host strains
| Antibiotic(s)a | MIC (μg/ml)
|
||||
|---|---|---|---|---|---|
| C. freundii KHM243 | E. coli W1895 (pCF243)b | E. coli W1895 | E. coli JM109 (pKHM-1)c | E. coli JM109 | |
| Ampicillin | 256 | 64 | 8 | 16 | 1 |
| Ampicillin-sulbactam | 64 | 64 | 1 | 16 | 0.5 |
| Ticarcillin | >512 | >512 | 2 | 512 | 2 |
| Ticarcillin-clavulanic acid | 512 | >512 | 4 | 512 | 2 |
| Piperacillin | 4 | 16 | 2 | 4 | 0.25 |
| Cephaloridine | 512 | 128 | 2 | 64 | 1 |
| Cefuroxime | >512 | >512 | 2 | >512 | 8 |
| Ceftazidime | >512 | >512 | 0.125 | >512 | 0.063 |
| Cefotaxime | 64 | >512 | 0.008 | 128 | 0.004 |
| Cefepime | 32 | >512 | 0.002 | 64 | 0.004 |
| Cefozopran | 16 | 256 | 0.016 | 64 | 0.008 |
| Imipenem | 2 | 4 | 0.063 | 0.5 | 0.063 |
| Meropenem | 4 | 4 | 0.004 | 4 | 0.004 |
| Aztreonam | 0.25 | 0.063 | 0.031 | 0.063 | 0.031 |
| Carumonam | 0.25 | 0.125 | 0.031 | 0.063 | 0.031 |
| Cefoxitin | 512 | >512 | 8 | >512 | 8 |
| Cefmetazole | 512 | 512 | 0.5 | >512 | 0.25 |
| Cefotetan | 128 | 512 | 0.125 | >512 | 0.031 |
| Cefbuperazone | 128 | 256 | 0.063 | 512 | 0.031 |
| Cefminox | 512 | >512 | 0.125 | 512 | 0.25 |
| Moxalactam | 256 | >512 | 0.063 | >512 | 0.031 |
| Flomoxef | 64 | 256 | 0.031 | 128 | 0.031 |
The ratio of the ampicillin to sulbactam was 2:1. The ratio of ticarcillin to clavulanic acid was 15:1.
Natural plasmid carrying the bla KHM-1 gene.
Recombinant plasmid constructed by insertion of DNA fragment containing the blaKHM-1 gene into the cloning vector pHSG396.
C. freundii KHM243 has two plasmids, one of approximately 70 kb and one of approximately 200 kb. A conjugation experiment was done with KHM243 and E. coli W1895. W1895 transconjugants that were resistant to β-lactams and that contained a 200-kb plasmid, designated pCF243, were obtained. The transconjugant exhibited a profile of susceptibility to β-lactams similar to that of KHM243, although the MICs for some cephalosporins, including cefotaxime, cefepime, and cefozopran, were significantly higher in the transconjugant than in KHM243 (Table 1).
EcoRI-digested fragments of pCF243 were subcloned into pHSG396 and were transformed into E. coli JM109 cells, and transformants were selected on agar medium containing moxalactam (1 μg/ml). Strain JM109 carrying the plasmid that conferred resistance to moxalactam, named pKHM-1, exhibited a profile of susceptibility to β-lactams similar to the susceptibility profiles of KHM243 and the E. coli W1895 transconjugant carrying pCF243 (Table 1). However, the MICs of some antibiotics, including cefotaxime and cefepime, were lower for the transformant carrying pKHM-1 than the transconjugant. This might be explained by insufficient expression of the gene due to insertion of the DNA fragment with a small 5′-flanking region.
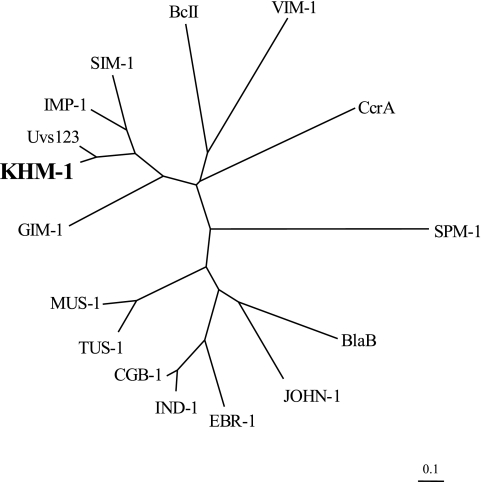
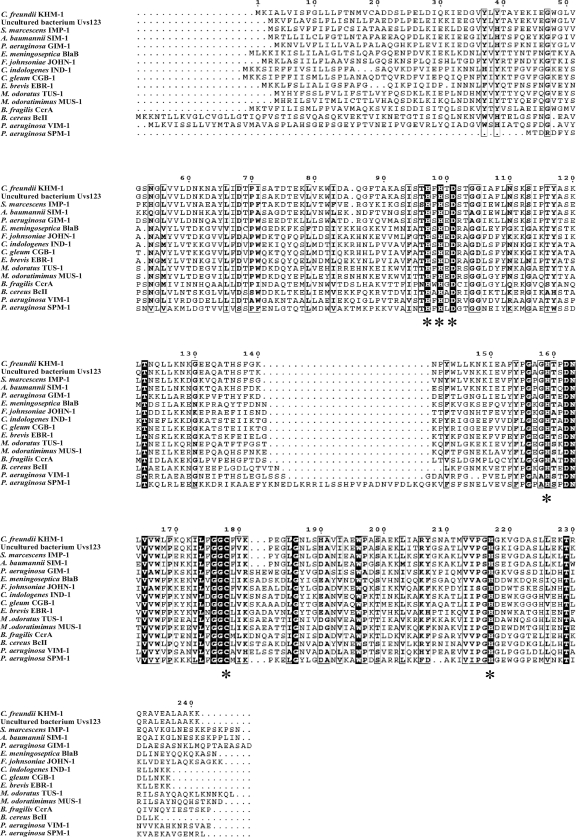
pKHM-1 contained an 837-bp insert with a complete open reading frame (ORF) (data not shown). The 726-bp ORF encoded a putative protein of 241 amino acids. The protein was similar to MBLs, such as Uvs123 from an uncultured bacterium (82% identity) (22), IMP-1 (59% identity) (17), and SIM-1 (59% identity) (11) (Fig. 1). The protein was somewhat less similar to VIM-1 (38% identity) (10), GIM-1 (50% identity) (3), and SPM-1 (46% identity) (21) (Fig. 1). We named the ORF encoding the protein blaKHM-1 and designated the protein KHM-1 (Kyorin Health Science MBL 1). blaKHM-1 was different from the Citrobacter freundii genome in its GC contents (GC contents, 50.27% and 44.63%, respectively) and codon usage (data not shown). KHM-1 contained amino acid motifs conserved in MBL enzymes, including a zinc-binding motif (HXHXD, residues 97 to 101) and three other residues involved in zinc binding (residues 159, 178, and 217) (Fig. 2) (18, 23).
FIG. 1.
Dendrogram showing the similarity of KHM-1 to other MBLs. KHM-1 and MBLs from a variety of organisms were tested. The dendrogram was created with the ClustalW program. Branch lengths correspond to the number of amino acid exchanges of the following MBL proteins (GenBank accession numbers, source organism) of BcII (P04190, from Bacillus cereus), BlaB (CAA65601, from Elizabethkingia meningoseptica), CcrA (P25910, from Bacteroides fragilis), CGB-1 (AAL55263, from Chryseobacterium gleum), EBR-1 (AAN32638, from Empedobacter brevis), GIM-1 (CAF05908, from Pseudomonas aeruginosa), IMP-1 (AAB30289, from Serratia marcescens), IND-1 (AAD20273, from Chryseobacterium indologenes), JOHN-1 (AAK38324, from Flavobacterium johnsoniae), MUS-1 (AAN63647, from Myroides odoratimimus), SIM-1 (AAX76774, from Acinetobacter baumannii), SPM-1 (CAD37801, from P. aeruginosa), TUS-1 (AAN63648, from Myroides odoratus), Uvs123 (AAP70377, from uncultured bacterium), and VIM-1 (CAB46686, from P. aeruginosa).
FIG. 2.
Multiple-sequence alignments of the amino acid sequence of KHM-1 from Citrobacter freundii isolate KHM243 with those of other MBLs. The sequence sources are the same as those indicated in the legend to Fig. 1. Sequence comparison was performed by aligning the protein amino acid sequences by use of the ClustalW program (http://clustalw.ddbj.nig.ac.jp/top-e.html). The residues known to be involved in metal binding are indicated by asterisks. Identical residues are shaded.
The DNA sequences flanking blaKHM-1 were determined from 774 bp upstream to 806 bp downstream of it. No sequence homologies for site-specific cointegration events, ORFs, or transmissible elements was detected within the 774-bp upstream of blaKHM-1. A 360-bp ORF encoding a putative protein of 119 amino acids with 77% identity to hypothetical protein VP1798 of Vibrio parahaemolyticus (14) was located in the fragment 21 to 380 bp downstream of blaKHM-1. Strain KHM243 carried a class 1 integron with an array of two gene cassettes, which carried the aadA2 (20) and aac(6′)-Iae (19) aminoglycoside resistance determinants; however, blaKHM-1 was not detected in this integron.
Analysis of the purified KHM-1 protein by sodium dodecyl sulfate-polyacrylamide gel electrophoresis showed a single 25-kDa band. The activity of KHM-1 against various β-lactams was analyzed with the purified protein. It showed hydrolytic activity against all β-lactams tested except aztreonam (Table 2). Enzymatic activity against aztreonam was undetectable under the experimental conditions adopted. This activity was inhibited by EDTA but was recovered by addition of Zn2+ (data not shown). The kinetic parameters, including Km, kcat, and the kcat/Km ratio, were determined for several different β-lactams (Table 2). Relatively higher values of the kcat/Km ratio (>107 M−1·s−1), as a result of low values of Km and high values of kcat, were observed with the cephalosporins tested (cephaloridine, cefoxitin, cefotaxime, ceftazidime, and moxalactam); and lower values of the kcat/Km ratio (<105 M−1·s−1) were observed with penicillin G, ampicillin, meropenem, and imipenem.
TABLE 2.
Kinetic parameters of β-lactamase KHM-1 with various substrates
| Substrate | Km (μM)a | kcat (s−1)a | kcat/Km (M−1·s−1) |
|---|---|---|---|
| Penicillin G | 1,340 ± 56 | 23 ± 0.9 | 1.7 × 104 |
| Ampicillin | 978 ± 111 | 19 ± 2 | 1.9 × 104 |
| Cephaloridine | 4.4 ± 0.95 | 686 ± 12 | 1.6 × 108 |
| Cefoxitin | 81 ± 4 | 1,178 ± 164 | 1.4 × 107 |
| Cefotaxime | 13 ± 1.5 | 2,181 ± 208 | 1.7 × 108 |
| Ceftazidime | 8 ± 0.4 | 118 ± 3 | 1.5 × 107 |
| Moxalactam | 71 ± 8 | 2,794 ± 260 | 3.9 × 107 |
| Aztreonam | —b | — | — |
| Meropenem | 12 ± 3 | 0.4 ± 0.015 | 3.3 × 104 |
| Imipenem | 268 ± 53 | 15 ± 3 | 5.6 × 104 |
The Km and kcat values represent the means of three independent experiments ± standard deviations.
—, no hydrolysis was detected under conditions with a substrate concentration of up to 1 mM and an enzyme concentration of up to 840 nM.
During 1997 and 1998, 104, 13, and 5 clinical isolates of C. freundii, C. koseri, and other Citrobacter spp., respectively, were collected in the hospital and were screened for imipenem resistance. Of these, four isolates of C. freundii showed reduced susceptibilities to imipenem (MICs, >8 μg/ml). However, blaKHM-1 was not detected in any of these isolates except the one from the patient infected with strain KHM243. A laboratory-based survey of other isolates of the family Enterobacteriaceae is in progress to detect blaKHM-1.
Nucleotide sequence accession number.
The nucleotide sequence data for blaKHM-1 and its flanking region from 774 bp upstream to 806 bp downstream reported here have been deposited in the EMBL/GenBank/DDBJ databases under accession number AB443628.
Acknowledgments
This study was supported in part by the Ministry of Health, Labor and Welfare of Japan (grants H18-Shinko-011, H19-Shinko-011, and H20-Shinko-011).
We thank M. Nakano (Jichi Medical School, Tochigi, Japan) for helpful comments on the manuscript.
Footnotes
Published ahead of print on 2 September 2008.
REFERENCES
- 1.Arakawa, Y., N. Shibata, K. Shibayama, H. Kurokawa, T. Yagi, H. Fujiwara, and M. Goto. 2000. Convenient test for screening metallo-β-lactamase-producing gram-negative bacteria by using thiol compounds. J. Clin. Microbiol. 38:40-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bush, K. 2001. New beta-lactamases in gram-negative bacteria: diversity and impact on the selection of antimicrobial therapy. Clin. Infect. Dis. 32:1085-1089. [DOI] [PubMed] [Google Scholar]
- 3.Castanheira, M., M. A. Toleman, R. N. Jones, F. J. Schmidt, and T. R. Walsh. 2004. Molecular characterization of a β-lactamase gene, blaGIM-1, encoding a new subclass of metallo-β-lactamase. Antimicrob. Agents Chemother. 48:4654-4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clinical and Laboratory Standards Institute. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A7. Clinical and Laboratory Standards Institute, Wayne, PA.
- 5.Franceschini, N., B. Caravelli, J. D. Docquier, M. Galleni, J. M. Frère, G. Amicosante, and G. M. Rossolini. 2000. Purification and biochemical characterization of the VIM-1 metallo-β-lactamase. Antimicrob. Agents Chemother. 44:3003-3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gilmour, M. W., N. R. Thomson, M. Sanders, J. Parkhill, and D. E. Taylor. 2004. The complete nucleotide sequence of the resistance plasmid R478: defining the backbone components of incompatibility group H conjugative plasmids through comparative genomics. Plasmid 52:182-202. [DOI] [PubMed] [Google Scholar]
- 7.Inoue, M., J. Itoh, and S. Mitsuhashi. 1983. pMS76, a plasmid capable of amplification by treatment with chloramphenicol. Plasmid 9:86-97. [DOI] [PubMed] [Google Scholar]
- 8.Jacoby, G. A., and L. S. Munoz-Price. 2005. The new β-lactamases. N. Engl. J. Med. 352:380-391. [DOI] [PubMed] [Google Scholar]
- 9.Kado, C. I., and S. T. Liu. 1981. Rapid procedure for detection and isolation of large and small plasmids. J. Bacteriol. 145:1365-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lauretti, L., M. L. Riccio, A. Mazzariol, G. Cornaglia, G. Amicosante, R. Fontana, and G. M. Rossolini. 1999. Cloning and characterization of blaVIM, a new integron-borne metallo-β-lactamase gene from a Pseudomonas aeruginosa clinical isolate. Antimicrob. Agents Chemother. 43:1584-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee, K., J. H. Yum, D. Yong, H. M. Lee, H. D. Kim, J. D. Docquier, G. M. Rossolini, and Y. Chong. 2005. Novel acquired metallo-β-lactamase gene, blaSIM-1, in a class 1 integron from Acinetobacter baumannii clinical isolates from Korea. Antimicrob. Agents Chemother. 49:4485-4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levesque, C., L. Piche, C. Larose, and P. H. Roy. 1995. PCR mapping of integrons reveals several novel combinations of resistance genes. Antimicrob. Agents Chemother. 39:185-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liebert, C. A., R. M. Hall, and A. O. Summers. 1999. Transposon Tn21, flagship of the floating genome. Microbiol. Mol. Biol. Rev. 63:507-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Makino, K., K. Oshima, K. Kurokawa, K. Yokoyama, T. Uda, K. Tagomori, Y. Iijima, M. Najima, M. Nakano, A. Yamashita, Y. Kubota, S. Kimura, T. Yasunaga, T. Honda, H. Shinagawa, M. Hattori, and T. Iida. 2003. Genome sequence of Vibrio parahaemolyticus: a pathogenic mechanism distinct from that of V. cholera. Lancet 361:743-749. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura, M., S. Sato, T. Ohya, A. Suzuki, and S. Ikeda. 1985. Possible relationship of a 36-megadalton Salmonella Enteritidis plasmid to virulence in mice. Infect. Immun. 47:831-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ochman, H., A. S. Gerber, and D. L. Hartl. 1988. Genetic applications of an inverse polymerase chain reaction. Genetics 120:621-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osano, E., Y. Arakawa, R. Wacharotayankun, M. Ohta, T. Horii, H. Ito, F. Yoshimura, and N. Kato. 1994. Molecular characterization of an enterobacterial metallo beta-lactamase found in a clinical isolate of Serratia marcescens that shows imipenem resistance. Antimicrob. Agents Chemother. 38:71-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rasumussen, B. A., and K. Bush. 1997. Carbapenem-hydrolyzing β-lactamases. Antimicrob. Agents Chemother. 41:223-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sekiguchi, J., T. Asagi, T. Miyoshi-Akiyama, T. Fujino, I. Kobayashi, K. Morita, Y. Kikuchi, T. Kuratsuji, and T. Kirikae. 2005. Multidrug-resistant Pseudomonas aeruginosa strain that caused an outbreak in a neurosurgery ward and its aac(6′)-Iae gene cassette encoding a novel aminoglycoside acetyltransferase. Antimicrob. Agents Chemother. 49:3734-3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taito, R. C., H. Rempel, R. L. Rodriguez, and C. I. Kado. 1985. The aminoglycoside-resistance operon of the plasmid pSa: nucleotide sequence of the streptomycin-spectinomycin resistance gene. Gene 36:97-104. [DOI] [PubMed] [Google Scholar]
- 21.Toleman, M. A., A. M. Simm, T. A. Murphy, A. C. Gales, D. J. Biedenbach, R. N. Jones, and T. R. Walsh. 2002. Molecular characterization of SPM-1, a novel metallo-β-lactamase isolated in Latin America: report from the SENTRY antimicrobial programme. J. Antimicrob. Chemother. 50:673-679. [DOI] [PubMed] [Google Scholar]
- 22.Voget, S., C. Leggewie, A. Uesbeck, C. Raasch, K.-E. Jaeger, and W. R. Streit. 2003. Prospecting for novel biocatalysts in a soil metagenome. Appl. Environ. Microbiol. 69:6235-6242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walsh, T. R., M. A. Toleman, L. Poirel, and P. Nordmann. 2005. Metallo-β-lactamases: the quiet before the storm? Clin. Microbiol. Rev. 18:306-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Young, D., J. M. Bell, B. Ritchie, R. Pratt, M. A. Toleman, and T. R. Walsh. 2007. A novel sub-group methalo-β-lactamase (MBL), AIM-1 emerges in Pseudomonas aeruginosa (PSA) from Australia, abstr. C1-593, p. 75. Abstr. 47th Intersci. Cof. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.