Abstract
The misuse of antibiotics has led our age to a dangerous edge, as antibiotic-resistant pathogens appear to evolve more quickly than antibiotics are invented. Thus, new agents to treat bacterial infection are badly needed. Cationic host defense peptides are on the first line of a host defense system and are thought to be good candidates for treating bacterial infection. Here, a novel cationic host defense peptide, mucroporin, was cloned and characterized from the venom of Lychas mucronatus. The MIC for Staphylococcus aureus was 25 μg/ml, including antibiotic-resistant pathogens. Based on the molecular template of mucroporin, mucroporin-M1 was designed by amino acid substitution. The MIC for S. aureus was 5 μg/ml, including the antibiotic-resistant pathogens methicillin-resistant S. aureus, methicillin-resistant coagulase-negative Staphylococcus, penicillin-resistant S. aureus, and penicillin-resistant S. epidermidis. Moreover, mucroporin-M1 also inhibited gram-negative bacteria. The modes of action of mucroporin and mucroporin-M1 were both rapid killing by disrupting the cell membrane of bacteria, and the number of surviving bacteria was reduced by about 4 to 5 orders of magnitude immediately after peptide delivery. These results showed that mucroporin could be considered a potential anti-infective drug, especially for treating antibiotic-resistant pathogens.
When a new antibiotic comes into use, resistance will follow. During the development period of antibiotics discovery, when resistance to former antibiotics appeared, drug substitution was used to treat resistant pathogens. However, during the last decade, it seems that the frequency of antibiotic resistance, especially the evolution of multidrug-resistant pathogens, is outpacing the development of new antibiotics (44, 48). Among the existing antibiotic-resistant pathogens, methicillin-resistant Staphylococcus aureus (MRSA) is considered the most lethal one. MRSA was first identified as a hospital-acquired pathogen in the 1960s, which was resistant to the entire class of β-lactam agents (20). Over the past 40 years, MRSA infections have become endemic in hospitals worldwide and have recently appeared in communities (13, 20, 38). It was reported that there were roughly 94,000 invasive MRSA cases in the United States, with roughly 19,000 deaths, in 2005, which was higher than the number of deaths caused by AIDS in the same year (29). Facing the so-called “superbug” (29), we have only got one remaining antibiotic weapon: vancomycin. Unfortunately, the overuse of vancomycin in treating MRSA infection and the existence of vancomycin-resistant enterococcus has led to the emergence of vancomycin-intermediate S. aureus (2, 4, 36). Therefore, new kinds of antimicrobial drugs are needed (26).
Cationic host defense peptides are produced by many organisms as part of their host defense system (23, 25, 34). These peptides are potent antimicrobial agents against gram-positive and gram-negative bacteria, fungi, parasites, and some viruses (9, 21), and a few of them have been reported to inhibit MRSA growth (42, 43). Studies indicated that the targets of cationic host defense peptides varied from the outer membrane to the signaling pathway (8, 10, 27), which may be the reason why resistance to cationic host defense peptides is more difficult to attain than resistance to the conventional antibiotics. The broad-spectrum activity, low potential to induce resistance, and the huge family of over 1,300 peptides (19) make cationic host defense peptides an attractive family of compounds that have the potential to be developed as therapeutic agents (7, 30).
Cationic host defense peptides are usually very short, ranging from 10 to 50 amino acid residues with a net positive charge of 2 to 9 (27, 37, 49, 51). Despite their common physiological effects, cationic host defense peptides vary in both sequence and secondary structure (6, 22, 27, 49). Structurally, natural cationic host defense peptides can be classified as: (i) amphipathic α-helix, (ii) β-sheet structures stabilized with two or three disulfide bonds, (iii) extended structures, and (iv) loop structures with one disulfide bond (40). Besides the main antibacterial, antifungal, antiviral, and antitumor functions, there is increasing evidence supporting the idea that cationic peptides have diverse functions in modulating immune responses, especially infection and inflammation (10, 24, 50). Several peptides are in clinical trial periods (1), and cationic host defense peptides present the best alternative to conventional antibiotics.
Cationic host defense peptides have also been found in scorpion hemolymph (14, 18) and venom, including hadrurin (46), scorpine (15), opistoporins, parabutoporin (39), ISCTs (17), pandinins (16), and BmKn2 and BmKb1 (53). The functions of these scorpion-derived peptides vary from cytotoxic (17) to inhibiting bacteria (15, 46) to inhibiting fungus (18, 39).
In our previous study, we characterized two cationic host defense peptides, BmKn2 and BmKb1, derived from the venom of Buthus martensii Karsch (53). Here, we describe a novel cationic defense peptide mucroporin, which is the first cationic host defense peptide characterized from the scorpion Lychas mucronatus. We found that mucroporin can effectively inhibit standard bacteria, especially gram-positive bacteria. The optimized design of mucroporin-M1 by amino acid substitution resulted in the inhibition of both gram-positive and gram-negative bacteria at low concentrations. We chose S. aureus as a model bacteria strain to further explore the mechanism of mucroporin and mucroporin-M1's bioactivity. Some evidence showed that the cell membrane of S. aureus was broken immediately after the treatment of mucroporin or mucroporin-M1. The assay revealed that the inhibitory effect of mucroporin and mucroporin-M1 was exerted by the action mode of rapid killing. Moreover, the in vitro treatment of clinically isolated pathogens showed that mucroporin-M1 is highly capable of inhibiting antibiotic-resistant pathogens, including MRSA, methicillin-resistant coagulase-negative Staphylococcus (MRCNS), etc. Mucroporin and its analogue present potential anti-infective drugs or lead compounds, especially for treating antibiotic-resistant pathogens.
MATERIALS AND METHODS
cDNA library construction.
The L. mucronatus scorpions were collected in Hainan province, China. Their glands were collected 2 days after electrical extraction of their venom. Total RNA was prepared from the glands by using TRIzol reagent (Invitrogen). Poly(A)-mRNA was purified by a poly(A) tract mRNA isolation system (Promega). A cDNA library was constructed according to the Superscript plasmid system cDNA library construction kit (Gibco-BRL). cDNAs were cloned into pSPORT1 plasmids and transformed into Escherichia coli DH5α cells. Randomly chosen cDNA clones were sequenced to obtain a reliable representation of the toxin content in the venom gland.
Screening of the cDNA library with PCR strategy.
A specific primer was designed and synthesized to screen mucroporin gene (homologue of BmKb1/BmKb2) from the venomous gland cDNA library of L. mucronatus by PCR method as described previously (52). The specific forward and reverse primers were 5′-TCGACCCACGCGTCCG-3′ and 5′-GCGTTTCCTTCGGCC-3′, respectively, corresponding to the digestion sites of the vector and the conserved processing region of the propeptide.
cDNA sequencing and computer analysis.
The plasmids characterized as positive clones were determined by using an ABI Prism 377XL DNA sequencer with a universal T7 promoter primer. Sequence analysis was performed by using BLASTX, DNAMAN, and GENRUNR. All homologue sequences of mucroporin were retrieved from GenBank (http://www.ncbi.nlm.nih.gov/) by the BlastP method. Multiple sequence alignments of mucroporin proteins were carried out by using CLUSTAL X version 1.83 (http://www2.ebi.ac.uk/clustalw/).
Chemical synthesis.
The peptide was synthesized by GL Biochem. (Shanghai), Ltd., China, with a purity of >95%.
Bacterial strains.
E. coli (AB94012), Pseudomonas aeruginosa (AB93066), Bacillus thuringiensis (AB92037), S. aureus (AB94004), Bacillus subtilis (AB91021), and Micrococcus luteus (AB93113) were purchased from the China Center of Type Culture Collection.
Antibiotic-resistant strains were obtained from the 302nd Military Hospital of Beijing, China, including penicillin-resistant S. aureus (PRSA) P1383; penicillin-resistant S. epidermidis (PRSE) P1389; MRSA P1381, P1386, and P1374; and MRCNS P1369. MRCNS 1538 was obtained from the Hubei Maternal and Child Health Hospital, Hubei, China.
Antibiotic-sensitive clinical isolates were also obtained from the 302nd Military Hospital of Beijing, China, including penicillin-sensitive S. aureus (PSSA) P969 and penicillin-sensitive S. epidermidis (PSSE) P1111 and P1368.
MIC determination.
Overnight-cultured bacteria were diluted with Luria-Bertani (LB) medium to about 104 to 106 CFU/ml. This bacterial suspension and serial diluted peptides were added to 96-well plates at ratio of 4:1 in a final volume of 100 μl. The microplates were incubated at 37°C with continuous shaking. After 12 to 16 h, the optical density at 630 nm (OD630) was measured with a microplate reader.
Overnight-cultured clinically isolated strains were diluted with LB medium to 0.5 McFarland. This bacterial suspension and serial diluted peptides were added to 96-well plates at a ratio of 4:1 in a final volume of 100 μl. The microplates were incubated at 37°C with continuous shaking for 12 to 16 h. The OD630 was measured with a microplate reader.
Each concentration reading was repeated three times. The MIC was determined at the concentration at which there was no optical density. All of the above experiments were repeated at least twice.
Bactericidal assay.
Overnight-cultured S. aureus was transferred to LB medium and cultured to exponential phase (OD600 of ∼0.6). A 300-μl portion of peptide solution was added to a 1,200-μl bacterial suspension, and the mixture was incubated at 37°C with continuous shaking. At each time point, 200 μl of treated bacterial suspension was transferred to a sterilized 1.5-ml tube. After centrifugation at 1,000 × g for 5 min, the supernatant was removed, and the pellet was resuspended with 200 μl of LB medium. This bacterial suspension was placed on agar plates and incubated at 37°C until the viable colonies could be counted.
Scanning electron microscopy.
Overnight-cultured S. aureus was transferred to LB medium and cultured to the exponential phase. A portion (300 μl) of the peptide solution was added to a 1,200-μl bacterial suspension, and the mixture was incubated at 37°C with continuous shaking. At 2 min after incubation, the bacterial suspension was centrifuged at 1,000 × g for 5 min, and the pellet was washed with 0.1 M phosphate-buffered saline (PBS) several times and then fixed overnight with 2.5% glutaraldehyde in 0.1 M PBS at 4°C. After fixation, the bacteria were washed with PBS for a minimum of 15 min and then dehydrated by using a series of graded ethyl alcohols (50% for 15 min, 60% for 15 min, 70% for 15 min, 80% for 15 min, 90 for 15 min, and 2 changes of 100% for 10 min each). After this, the samples were mounted on aluminum stubs with adhesive tabs and sputter coated for 3 min by using a polaron. The samples were then ready to view on the Hitachi X650 scanning electron microscope.
RESULTS
Sequence analysis.
After systemic screening of 500 clones from the venomous cDNA library of L. mucronatus, we obtained eight positive clones. Sequencing of all of these positive clones revealed a novel precursor, termed mucroporin, with high similarities to BmKb1 and BmKb2 and a number of antimicrobial peptides from other scorpion species (Fig. 1). The mucroporin precursor consists of three parts: a 5′ untranslated region (5′UTR), an open reading frame (ORF), and a 3′UTR. The 5′ and 3′UTRs of mucroporin are 10 and 120 bp in length, respectively. At the 3′UTR end of the cDNA, a single AATAAA polyadenylation signal is found 12 bp upstream of the poly(A) tail. An ORF of 222 bp encodes a precursor of 74 amino acid residues (Fig. 2).
FIG. 1.
cDNA and protein sequences of mucroporin (accession no. EU669864). The deduced amino acid residues are shown below the corresponding nucleotide sequences. The signal peptide is in italics, and the propeptide is underlined. The potential polyadenylation signal AATAAA is double underlined.
FIG. 2.
Protein sequence alignments of mucroporin with AP4_TITCO, AP5_TITCO, AP6_TITCO, caerin-1, caerin-2, BmKb1, and BmKb2. The percentage of sequence similarity relative to mucroporin is indicated to the right of each sequence. The signal peptide is in italics. The propeptide is underlined.
Mucroporin is composed of a putative 22 residues, followed by a presumed 17-residue mature peptide, and an uncommon acidic propeptide at the C terminus. The 35-residue propeptide starts with a conserved posttranslational processing signal, Gly-Arg-Arg, at positions 40 to 42. Conventionally, removal of the propeptide with such processing signal would result in a mature peptide with C-terminal amidation as described previously (17).
Mucroporin-M1 (LFRLIKSLIKRLVSAFK) was designed based on the protein sequence of mucroporin for the purpose of enhancing the net positive charge of the hydrophilic side. Mucroporin and mucroporin-M1 was synthesized by GL Biochem, Ltd., Shanghai, China, with reliable quality. The molecule weights measured with mass spectrum (1,731.22 and 2,031.57, respectively) matched with the calculated molecule weights of the amidated mucroporin and mucroporin-M1 (1,731.13 and 2,031.58, respectively) very well.
MIC.
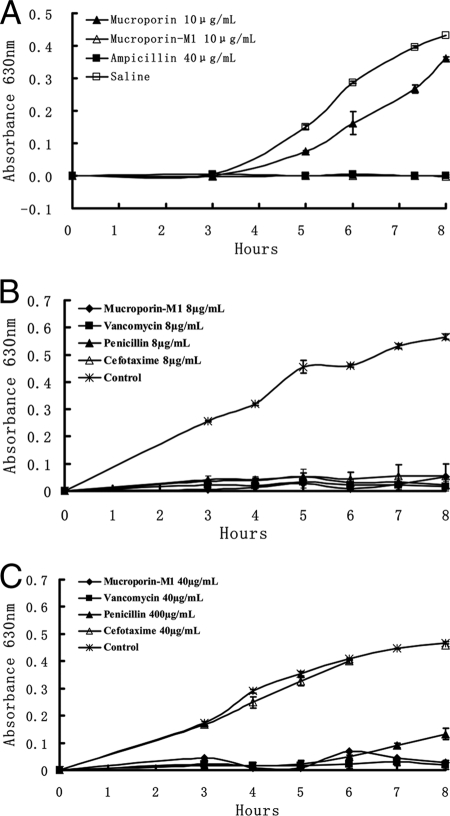
The effect of mucroporin and mucroporin-M1 on bacteria was studied by the microdilution method. As shown in Table 1, it can be seen that mucroporin was more effective on gram-positive bacteria than on gram-negative bacteria. In all, the MICs of mucroporin were 25 μg/ml for S. aureus AB94004, 25 μg/ml for B. thuringiensis AB92037, and 50 μg/ml for B. subtilis AB91021. The MICs for mucroporin-M1 were 5 μg/ml for S. aureus AB94004, 25 μg/ml for B. thuringiensis AB92037, and 25 μg/ml for B. subtilis AB91021. In addition, E. coli AB94012 and P. aeruginosa AB93066 were both insensitive to 100 μg of mucroporin/ml, while the MICs of mucroporin-M1 were 12.5 μg/ml for E. coli AB94012 and 100 μg/ml for P. aeruginosa AB93066. As shown in Fig. 3A, we investigated the growth of S. aureus AB94004 8 h after peptide treatment. It was found that S. aureus AB94004 treated with mucroporin reproduced faster than S. aureus AB94004 treated with mucroporin-M1 at the same concentration. These results indicated that the modification of the mucroporin sequence not only enhanced its in vitro activity but also expanded its antibacterial spectrum.
TABLE 1.
Antibacterial effects of mucroporin and mucroporin-M1
| Strain | MIC (μg/ml)
|
||
|---|---|---|---|
| Mucroporin | Mucroporin-M1 | Penicillin | |
| Escherichia coli AB94012 | >100 | 12.5 | |
| Pseudomonas aeruginosa AB93066 | >100 | 100 | |
| Bacillus thuringiensis AB92037 | 25 | 25 | 12.5 |
| Bacillus subtilis AB91021 | 50 | 25 | 25 |
| Staphylococcus aureus AB94004 | 25 | 5 | 2 |
| 1538a | 25 | 5 | 25 |
Strain 1538 was MRCNS, a clinical isolate obtained from the Hubei Maternal and Child Health Hospital.
FIG. 3.
Time growth curves of S. aureus treated by mucroporin or mucroporin-M1. The growth of S. aureus was measured at 630 nm. (A) Comparison of mucroporin and mucroporin-M1 activities against S. aureus AB94004. (B) Comparison of mucroporin-M1 and antibiotics activities against clinical isolate MRSA P1386. (C) Comparison of mucroporin-M1 and antibiotics activities against clinical isolate PSSA P969.
The antibiotic-resistant pathogens were clinical isolates, all of which were tested with traditional antibiotics to determine the resistance before experiments. As shown in Table 1, mucroporin and mucroporin-M1 can both inhibit MRCNS 1538 growth. Comparing the MICs of penicillin to 1538 and S. aureus AB94004, we found that MRCNS 1538 was resistant to penicillin. However, the MIC of mucroporin for 1538 was fivefold greater than that of mucroporin-M1. This result further demonstrated that the modification of the mucroporin amino acid residues enhanced its in vitro activity.
As shown in Table 2, mucroporin-M1 was as effective against antibiotic-resistant pathogens (MRSA, MRCNS, PRSA, and PRSE) as it was against antibiotic-sensitive pathogens (PSSA and PSSE). The MICs of mucroporin-M1 for penicillin-resistant strains P1383 (PRSA) and P1389 (PRSE) were 10 and 8 μg/ml, respectively, while the MICs of mucroporin-M1 were 40 μg/ml for penicillin-sensitive strain P969 (PSSA) and 20 μg/ml for P1111 and 8 μg/ml for P1368 (PSSE). In addition, the MICs of mucroporin-M1 for methicillin-resistant strains were 20 μg/ml for P1381 (MRSA), 25 μg/ml for P1386 (MRSA), 8 μg/ml for P1374 (MRSA), and 8 μg/ml for P1369 (MRCNS), which was at the same level as for methicillin-sensitive strains. The MICs of penicillin G salt, cefotaxime, and vancomycin against penicillin-sensitive strains, penicillin-resistant strains, and methicillin-resistant strains were all determined to define susceptibility. Furthermore, as shown in Fig. 3B and C, 400 μg of penicillin/ml and 40 μg of cefotaxime/ml cannot effectively inhibit P1386 strain (MRSA) growth during the first 8 h after drug delivery, whereas growth was totally inhibited by a 40-μg/ml dose of vancomycin and mucroporin-M1. Comparatively, an 8-μg/ml dose of each antibiotic and mucroporin-M1 effectively inhibited P969 (PSSA) growth. These results indicated that mucroporin-M1 can effectively inhibit antibiotic-resistant pathogens, including MRSA.
TABLE 2.
MICs of mucroporin-M1 for clinical isolates
| Straina | MIC (μg/ml)
|
|||
|---|---|---|---|---|
| Mucroporin-M1 | Vancomycin | Penicillin | Cefotaxime | |
| Penicillin resistant | ||||
| P1383 (PRSA) | 10 | 8 | 10,000 | 5 |
| P1389 (PRSE) | 8 | 8 | 10,000 | 2 |
| Methicillin resistant | ||||
| P1381 (MRSA) | 20 | 8 | 5,000 | 400 |
| P1386 (MRSA) | 25 | 4 | 10,000 | 100 |
| P1374 (MRSA) | 8 | 8 | 4,000 | 400 |
| P1369 (MRCNS) | 8 | 8 | 20,000 | 400 |
| Penicillin sensitive | ||||
| P969 (PSSA) | 40 | 8 | 4 | 5 |
| P1111 (PSSE) | 20 | 8 | 40 | 5 |
| P1368 (PSSE) | 8 | 8 | 40 | 10 |
All strains were clinical isolates obtained from the 302nd Military Hospital, Beijing, China.
Killing assay.
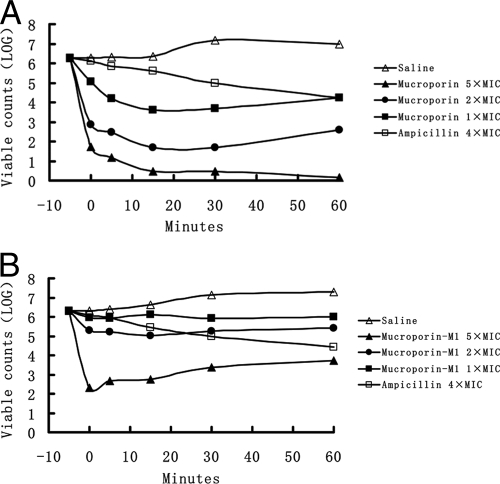
To study the functional mechanisms of mucroporin and mucroporin-M1, we performed a killing assay for mucroporin and mucroporin-M1 on the selected model strain S. aureus. One-, two-, or fivefold the MIC of mucroporin or mucroporin-M1 for S. aureus was directly added into exponential-phase bacteria, and the survival rate was then determined by counting the viable colony on the plates. The results revealed that the viable colony was decreased dramatically in a very short time, and the reaction started as soon as the bacteria mixed with these peptides. The killing curves (Fig. 4) show that the killing rate corresponded with the increasing peptide concentration. In contrast, the fivefold greater MIC of ampicillin sodium (40 μg/ml) did not make a dramatic decrease in survival rate, a finding which was consistent with its molecular mechanism of inhibiting bacterial wall synthesis. This result suggested that mucroporin and mucroporin-M1 exerted killing effects on microorganisms, which was probably the reason it can effectively inhibit the growth of microorganisms.
FIG. 4.
Killing assay. A killing assay was conducted to determine the count of the surviving bacteria without the supernatant. The set point in the picture refers to the initial bacterial count: 0 min was defined as the time of the first sample collection, which was immediately after mixing bacteria and mucroporin or mucroporin-M1, and the other samples were collected at 5, 15, 30, and 60 min. All of the counts represent the average of three dishes. The experiment was repeated and showed the same trend. (A) Mucroporin at different concentrations; (B) mucroporin-M1 at different concentrations.
Scanning electron microscopy.
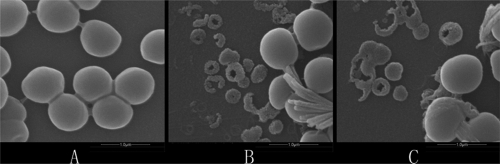
S. aureus cells treated with mucroporin or mucroporin-M1 were observed with a scanning electron microscope to evaluate individual bacterium changes (Fig. 5). As shown in Fig. 5B and C, S. aureus cells were found broken, and the cell contents were assembled as small particles, which was direct evidence supporting the idea that the bacterial membrane was broken after treatment with mucroporin or mucroporin-M1.
FIG. 5.
Scanning electron microscopic images of S. aureus treated with mucroporin or mucroporin-M1. (A) Negative control; (B) 10 min after mucroporin treatment; (C) 10 min after mucroporin-M1 treatment.
The results of the in vitro bactericidal activity and scanning electron microscopy provided solid evidence that the bacterial membrane was broken immediately after mucroporin or mucroporin-M1 treatment and that the inhibitory effect of mucroporin and mucroporin-M1 on bacteria was through the action mode of rapid killing.
DISCUSSION
Toxins are specific poisonous substances developed by venomous animals for predation and defense and are composed of various proteins and/or polypeptides. Recently, the output of data by cDNA library sequencing and proteomics profiling research (28) has revealed large numbers of peptide toxins from animal venoms, including ion channel modulators, bradykinin potentiating peptides, cationic host defense peptides, etc. Some of these have been developed and have shown favorable in vivo activity (33), which suggests that the large number of venom peptides is a rich source of potential therapeutic drugs.
Scorpion toxins have been recognized as potential therapeutic drugs for many years, especially as ion channel modulators. For example, margatoxin, from the scorpion Centruroides margaritatus, was the first peptide to be tested in vivo and is a potent blocker of the voltage-gated potassium channels Kv1.1, Kv1.2, and Kv1.3 (5). Margatoxin depolarizes the T cells of both pigs and humans in vitro and also inhibits the delayed-type hypersensitivity reaction to tuberculin in mini-pigs, as determined by both the size of induration and the extent of T-cell infiltration (31). Another toxin, chlorotoxin, derived from the venom of the scorpion Leiurus quengestriatus, displays an extraordinary feature that specifically targets glioma cells through MMP-2, the primary receptor highly expressed on the glioma cell membrane. The radioactive 131I-labeled chlorotoxin analogue has cytolytic activity and therefore the potential to selectively affect tumors and gliomas of neuroectodermal origin (35, 45). On the basis of these findings, TransMolecular, Inc., is running trials with 131I-TM-601 (131I-chlorotoxin) as an investigational new drug for the treatment of gliomas.
Thus far, several cationic host defense peptides of scorpions have been isolated and characterized (16-18, 39, 46). In our present study, the first cationic host defense peptide from L. mucronatus was characterized. It has been shown that mucroporin exerted an inhibitory effect, especially on gram-positive bacteria strains, including clinically isolated pathogens. However, the activity of mucroporin was not very high; this is why we designed the sequence for mucroporin-M1. The principal design aim was to replace the amino acid residues at the hydrophilic site with positively charged residues. As a result, the antibacterial activity was improved, including the activity against gram-positive bacteria, gram-negative bacteria, and clinically isolated antibiotic-resistant pathogens (MRSA, MRCNS, etc.). This result showed us that mucroporin was an ideal template for anti-infective drug design.
Vancomycin is considered the most effective drug for the treatment of MRSA infection, but vancomycin-resistant S. aureus strains have also been identified (2, 3). Thus, the treatment of MRSA infection will be a difficult problem in the near future. Cationic host defense peptides may offer us a solution. The results showed that the in vitro effect of mucroporin-M1 on MRSA and MRCNS were at the same level as vancomycin. Clinical trials of cationic host defense peptides have been ongoing for many years (11, 12, 47), including a phase IIIa trial utilizing these peptides in topical treatment (32, 40). However, none of them has obtained U.S. Food and Drug Administration approval for their various clinical indications thus far (21, 41). Designed peptides with high effectiveness and low toxicity are our future goal.
In conclusion, we showed here that mucroporin and mucroporin-M1, a native cationic host defense peptide and its analogue, have shown specific effects on inhibiting bacteria. These peptides kill S. aureus very quickly. Mucroporin-M1 can effectively inhibit hospital-acquired MRSA, MRCNS, PRSA, and PRSE. This antibacterial activity suggests that mucroporin may be a good template for anti-infective drug design.
Acknowledgments
We thank Xu Xiuling and Robert Thompson from Burnham Institute for Medical Research for language correction of this article. We thank Panyong Mao (302nd Military Hospital of Beijing, China) and Weipeng Wang (Hubei Maternal and Child Health Hospital, Hubei, China) for providing clinical pathogens.
This study was supported by grants from the National Natural Sciences Foundation of China to W. Li and Z. Cao (30530140 and 30570045), the Basic Project of Ministry of Science and Technology of China to W. Li (2007FY210800), and the Youth Chenguang Project of Science and Technology of Wuhan City to Z. Cao (20065004116-06).
Footnotes
Published ahead of print on 8 September 2008.
REFERENCES
- 1.Andres, E., and J. L. Dimarcq. 2004. Cationic antimicrobial peptides: update of clinical development. J. Intern. Med. 255:519-520. [DOI] [PubMed] [Google Scholar]
- 2.Appelbaum, P. C. 2006. The emergence of vancomycin-intermediate and vancomycin-resistant Staphylococcus aureus. Clin. Microbiol. Infect. 12(Suppl. 1):16-23. [DOI] [PubMed] [Google Scholar]
- 3.Appelbaum, P. C. 2006. MRSA-the tip of the iceberg. Clin. Microbiol. Infect. 12(Suppl. 2):3-10. [DOI] [PubMed] [Google Scholar]
- 4.Avery, R., M. Kalaycio, B. Pohlman, R. Sobecks, E. Kuczkowski, S. Andresen, S. Mossad, J. Shamp, J. Curtis, J. Kosar, K. Sands, M. Serafin, and B. Bolwell. 2005. Early vancomycin-resistant enterococcus (VRE) bacteremia after allogeneic bone marrow transplantation is associated with a rapidly deteriorating clinical course. Bone Marrow Transplant. 35:497-499. [DOI] [PubMed] [Google Scholar]
- 5.Bianchi, L., B. Wible, A. Arcangeli, M. Taglialatela, F. Morra, P. Castaldo, O. Crociani, B. Rosati, L. Faravelli, M. Olivotto, and E. Wanke. 1998. Herg encodes a K+ current highly conserved in tumors of different histogenesis: a selective advantage for cancer cells? Cancer Res. 58:815-822. [PubMed] [Google Scholar]
- 6.Boman, H. G. 1995. Peptide antibiotics and their role in innate immunity. Annu. Rev. Immunol. 13:61-92. [DOI] [PubMed] [Google Scholar]
- 7.Bradshaw, J. 2003. Cationic antimicrobial peptides: issues for potential clinical use. Bio Drugs 17:233-240. [DOI] [PubMed] [Google Scholar]
- 8.Brogden, K. A. 2005. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 3:238-250. [DOI] [PubMed] [Google Scholar]
- 9.Brogden, K. A., M. Ackermann, P. B. McCray, Jr., and B. F. Tack. 2003. Antimicrobial peptides in animals and their role in host defenses. Int. J. Antimicrob. Agents 22:465-478. [DOI] [PubMed] [Google Scholar]
- 10.Brown, K. L., and R. E. Hancock. 2006. Cationic host defense (antimicrobial) peptides. Curr. Opin. Immunol. 18:24-30. [DOI] [PubMed] [Google Scholar]
- 11.Chalekson, C. P., M. W. Neumeister, and J. Jaynes. 2002. Improvement in burn wound infection and survival with antimicrobial peptide D2A21 (Demegel). Plast. Reconstr. Surg. 109:1338-1343. [DOI] [PubMed] [Google Scholar]
- 12.Chalekson, C. P., M. W. Neumeister, and J. Jaynes. 2003. Treatment of infected wounds with the antimicrobial peptide D2A21. J. Trauma 54:770-774. [DOI] [PubMed] [Google Scholar]
- 13.Chambers, H. F. 2005. Community-associated MRSA-resistance and virulence converge. N. Engl. J. Med. 352:1485-1487. [DOI] [PubMed] [Google Scholar]
- 14.Cociancich, S., M. Goyffon, F. Bontems, P. Bulet, F. Bouet, A. Menez, and J. Hoffmann. 1993. Purification and characterization of a scorpion defensin, a 4-kDa antibacterial peptide presenting structural similarities with insect defensins and scorpion toxins. Biochem. Biophys. Res. Commun. 194:17-22. [DOI] [PubMed] [Google Scholar]
- 15.Conde, R., F. Z. Zamudio, M. H. Rodriguez, and L. D. Possani. 2000. Scorpine, an anti-malaria and antibacterial agent purified from scorpion venom. FEBS Lett. 471:165-168. [DOI] [PubMed] [Google Scholar]
- 16.Corzo, G., P. Escoubas, E. Villegas, K. J. Barnham, W. He, R. S. Norton, and T. Nakajima. 2001. Characterization of unique amphipathic antimicrobial peptides from venom of the scorpion Pandinus imperator. Biochem. J. 359:35-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dai, L., A. Yasuda, H. Naoki, G. Corzo, M. Andriantsiferana, and T. Nakajima. 2001. IsCT, a novel cytotoxic linear peptide from scorpion Opisthacanthus madagascariensis. Biochem. Biophys. Res. Commun. 286:820-825. [DOI] [PubMed] [Google Scholar]
- 18.Ehret-Sabatier, L., D. Loew, M. Goyffon, P. Fehlbaum, J. A. Hoffmann, A. van Dorsselaer, and P. Bulet. 1996. Characterization of novel cysteine-rich antimicrobial peptides from scorpion blood. J. Biol. Chem. 271:29537-29544. [DOI] [PubMed] [Google Scholar]
- 19.Fjell, C. D., R. E. Hancock, and A. Cherkasov. 2007. AMPer: a database and an automated discovery tool for antimicrobial peptides. Bioinformatics 23:1148-1155. [DOI] [PubMed] [Google Scholar]
- 20.Fridkin, S. K., J. C. Hageman, M. Morrison, L. T. Sanza, K. Como-Sabetti, J. A. Jernigan, K. Harriman, L. H. Harrison, R. Lynfield, and M. M. Farley. 2005. Methicillin-resistant Staphylococcus aureus disease in three communities. N. Engl. J. Med. 352:1436-1444. [DOI] [PubMed] [Google Scholar]
- 21.Gordon, Y. J., E. G. Romanowski, and A. M. McDermott. 2005. A review of antimicrobial peptides and their therapeutic potential as anti-infective drugs. Curr. Eye Res. 30:505-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hancock, R. E. 1997. Peptide antibiotics. Lancet 349:418-422. [DOI] [PubMed] [Google Scholar]
- 23.Hancock, R. E., K. L. Brown, and N. Mookherjee. 2006. Host defence peptides from invertebrates: emerging antimicrobial strategies. Immunobiology 211:315-322. [DOI] [PubMed] [Google Scholar]
- 24.Hancock, R. E., and G. Diamond. 2000. The role of cationic antimicrobial peptides in innate host defences. Trends Microbiol. 8:402-410. [DOI] [PubMed] [Google Scholar]
- 25.Hancock, R. E., and R. Lehrer. 1998. Cationic peptides: a new source of antibiotics. Trends Biotechnol. 16:82-88. [DOI] [PubMed] [Google Scholar]
- 26.Jain, R., and L. H. Danziger. 2004. Multidrug-resistant Acinetobacter infections: an emerging challenge to clinicians. Ann. Pharmacother. 38:1449-1459. [DOI] [PubMed] [Google Scholar]
- 27.Jenssen, H., P. Hamill, and R. E. Hancock. 2006. Peptide antimicrobial agents. Clin. Microbiol. Rev. 19:491-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kennedy, A. D., M. Otto, K. R. Braughton, A. R. Whitney, L. Chen, B. Mathema, J. R. Mediavilla, K. A. Byrne, L. D. Parkins, F. C. Tenover, B. N. Kreiswirth, J. M. Musser, and F. R. DeLeo. 2008. Epidemic community-associated methicillin-resistant Staphylococcus aureus: recent clonal expansion and diversification. Proc. Natl. Acad. Sci. USA 105:1327-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klevens, R. M., M. A. Morrison, J. Nadle, S. Petit, K. Gershman, S. Ray, L. H. Harrison, R. Lynfield, G. Dumyati, J. M. Townes, A. S. Craig, E. R. Zell, G. E. Fosheim, L. K. McDougal, R. B. Carey, and S. K. Fridkin. 2007. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 298:1763-1771. [DOI] [PubMed] [Google Scholar]
- 30.Koczulla, A. R., and R. Bals. 2003. Antimicrobial peptides: current status and therapeutic potential. Drugs 63:389-406. [DOI] [PubMed] [Google Scholar]
- 31.Koo, G. C., J. T. Blake, A. Talento, M. Nguyen, S. Lin, A. Sirotina, K. Shah, K. Mulvany, D. Hora, Jr., P. Cunningham, D. L. Wunderler, O. B. McManus, R. Slaughter, R. Bugianesi, J. Felix, M. Garcia, J. Williamson, G. Kaczorowski, N. H. Sigal, M. S. Springer, and W. Feeney. 1997. Blockade of the voltage-gated potassium channel Kv1.3 inhibits immune responses in vivo. J. Immunol. 158:5120-5128. [PubMed] [Google Scholar]
- 32.Lamb, H. M., and L. R. Wiseman. 1998. Pexiganan acetate. Drugs 56:1047-1054. [DOI] [PubMed] [Google Scholar]
- 33.Lewis, R. J., and M. L. Garcia. 2003. Therapeutic potential of venom peptides. Nat. Rev. Drug Discov. 2:790-802. [DOI] [PubMed] [Google Scholar]
- 34.Li, J., X. Xu, C. Xu, W. Zhou, K. Zhang, H. Yu, Y. Zhang, Y. Zheng, H. H. Rees, R. Lai, D. Yang, and J. Wu. 2007. Anti-infection peptidomics of amphibian skin. Mol. Cell Proteomics 6:882-894. [DOI] [PubMed] [Google Scholar]
- 35.Lyons, S. A., J. O'Neal, and H. Sontheimer. 2002. Chlorotoxin, a scorpion-derived peptide, specifically binds to gliomas and tumors of neuroectodermal origin. Glia 39:162-173. [DOI] [PubMed] [Google Scholar]
- 36.Marothi, Y. A., H. Agnihotri, and D. Dubey. 2005. Enterococcal resistance-an overview. Indian J. Med. Microbiol. 23:214-219. [PubMed] [Google Scholar]
- 37.Martin, E., T. Ganz, and R. I. Lehrer. 1995. Defensins and other endogenous peptide antibiotics of vertebrates. J. Leukoc. Biol. 58:128-136. [DOI] [PubMed] [Google Scholar]
- 38.Miller, L. G., F. Perdreau-Remington, G. Rieg, S. Mehdi, J. Perlroth, A. S. Bayer, A. W. Tang, T. O. Phung, and B. Spellberg. 2005. Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. N. Engl. J. Med. 352:1445-1453. [DOI] [PubMed] [Google Scholar]
- 39.Moerman, L., S. Bosteels, W. Noppe, J. Willems, E. Clynen, L. Schoofs, K. Thevissen, J. Tytgat, J. Van Eldere, J. Van Der Walt, and F. Verdonck. 2002. Antibacterial and antifungal properties of alpha-helical, cationic peptides in the venom of scorpions from southern Africa. Eur. J. Biochem. 269:4799-4810. [DOI] [PubMed] [Google Scholar]
- 40.Mookherjee, N., and R. E. Hancock. 2007. Cationic host defense peptides: innate immune regulatory peptides as a novel approach for treating infections. Cell Mol. Life Sci. 64:922-933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moore, A. 2003. The big and small of drug discovery. Biotech versus pharma: advantages and drawbacks in drug development. EMBO Rep. 4:114-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mygind, P. H., R. L. Fischer, K. M. Schnorr, M. T. Hansen, C. P. Sonksen, S. Ludvigsen, D. Raventos, S. Buskov, B. Christensen, L. De Maria, O. Taboureau, D. Yaver, S. G. Elvig-Jorgensen, M. V. Sorensen, B. E. Christensen, S. Kjaerulff, N. Frimodt-Moller, R. I. Lehrer, M. Zasloff, and H. H. Kristensen. 2005. Plectasin is a peptide antibiotic with therapeutic potential from a saprophytic fungus. Nature 437:975-980. [DOI] [PubMed] [Google Scholar]
- 43.Qiu, X. Q., H. Wang, X. F. Lu, J. Zhang, S. F. Li, G. Cheng, L. Wan, L. Yang, J. Y. Zuo, Y. Q. Zhou, H. Y. Wang, X. Cheng, S. H. Zhang, Z. R. Ou, Z. C. Zhong, J. Q. Cheng, Y. P. Li, and G. Y. Wu. 2003. An engineered multidomain bactericidal peptide as a model for targeted antibiotics against specific bacteria. Nat. Biotechnol. 21:1480-1485. [DOI] [PubMed] [Google Scholar]
- 44.Shea, K. M. 2003. Antibiotic resistance: what is the impact of agricultural uses of antibiotics on children's health? Pediatrics 112:253-258. [PubMed] [Google Scholar]
- 45.Soroceanu, L., Y. Gillespie, M. B. Khazaeli, and H. Sontheimer. 1998. Use of chlorotoxin for targeting of primary brain tumors. Cancer Res. 58:4871-4879. [PubMed] [Google Scholar]
- 46.Torres-Larios, A., G. B. Gurrola, F. Z. Zamudio, and L. D. Possani. 2000. Hadrurin, a new antimicrobial peptide from the venom of the scorpion Hadrurus aztecus. Eur. J. Biochem. 267:5023-5031. [DOI] [PubMed] [Google Scholar]
- 47.Trotti, A., A. Garden, P. Warde, P. Symonds, C. Langer, R. Redman, T. F. Pajak, T. R. Fleming, M. Henke, J. Bourhis, D. I. Rosenthal, E. Junor, A. Cmelak, F. Sheehan, J. Pulliam, P. Devitt-Risse, H. Fuchs, M. Chambers, B. O'Sullivan, and K. K. Ang. 2004. A multinational, randomized phase III trial of iseganan HCl oral solution for reducing the severity of oral mucositis in patients receiving radiotherapy for head-and-neck malignancy. Int. J. Radiat Oncol. Biol. Phys 58:674-681. [DOI] [PubMed] [Google Scholar]
- 48.Verhoef, J. 2003. Antibiotic resistance: the pandemic. Adv. Exp. Med. Biol. 531:301-313. [DOI] [PubMed] [Google Scholar]
- 49.Wang, Z., and G. Wang. 2004. APD: the antimicrobial peptide database. Nucleic Acids Res. 32:D590-D592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang, D., A. Biragyn, L. W. Kwak, and J. J. Oppenheim. 2002. Mammalian defensins in immunity: more than just microbicidal. Trends Immunol. 23:291-296. [DOI] [PubMed] [Google Scholar]
- 51.Zasloff, M. 2002. Antimicrobial peptides of multicellular organisms. Nature 415:389-395. [DOI] [PubMed] [Google Scholar]
- 52.Zeng, X. C., and S. X. Wang. Evidence that BmTXK beta-BmKCT cDNA from Chinese scorpion Buthus martensii Karsch is an artifact generated in the reverse transcription process. FEBS Lett. 520:183-185. [DOI] [PubMed]
- 53.Zeng, X. C., S. X. Wang, Y. Zhu, S. Y. Zhu, and W. X. Li. 2004. Identification and functional characterization of novel scorpion venom peptides with no disulfide bridge from Buthus martensii Karsch. Peptides 25:143-150. [DOI] [PubMed] [Google Scholar]