Abstract
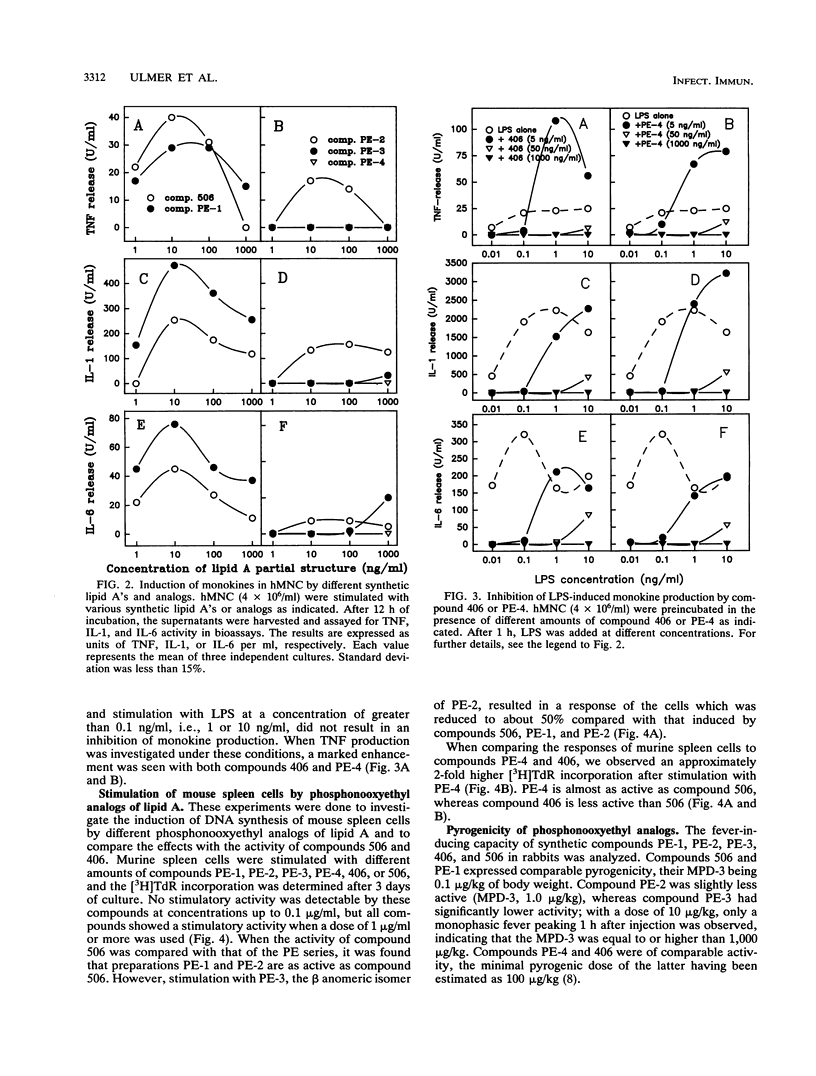
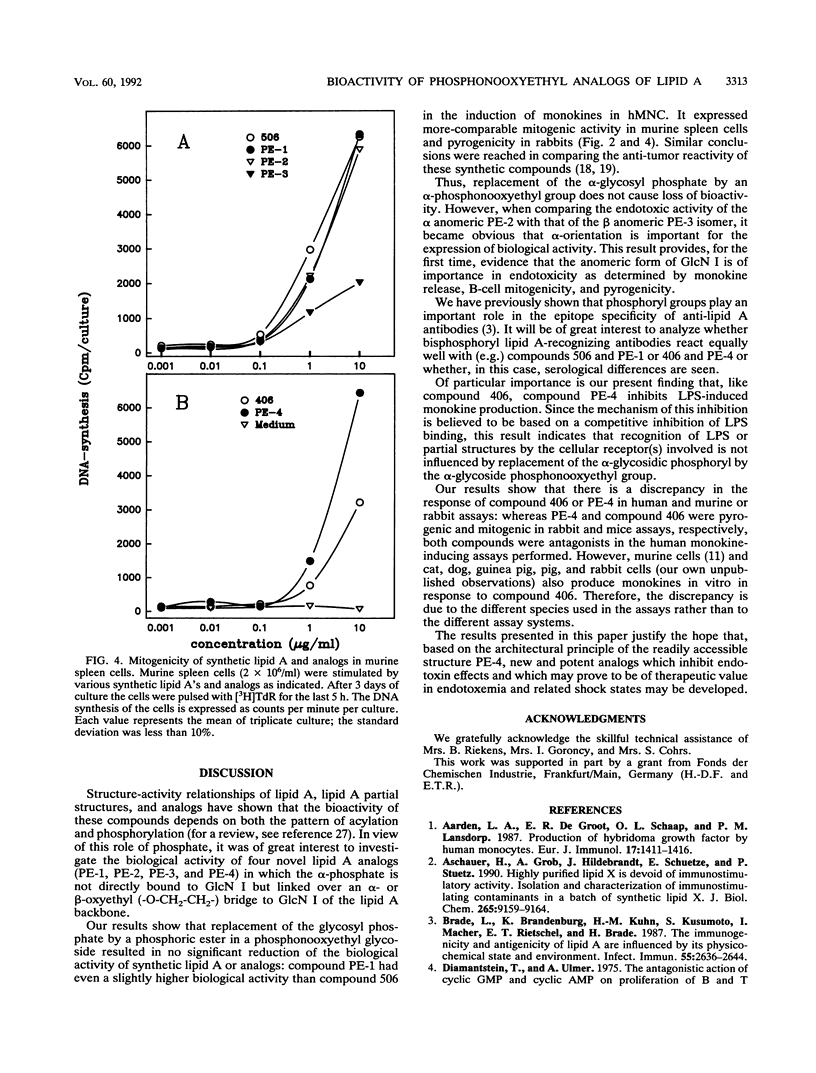
We investigated the biological activity of four new synthetic analogs of lipid A, termed PE-1, PE-2, PE-3, and PE-4. All compounds contain an alpha-oxyethyl-linked (-O-CH2-CH2-) phosphoryl group in position 1 of the reducing glucosaminyl residue (GlcN I) of lipid A. PE-1 is a hexaacylated analog of Escherichia coli lipid A (compound 506). PE-2 differs from PE-1 in carrying two myristic acid residues at GlcN I. PE-3 has the same acylation pattern as PE-2, but GlcN I is present in the beta anomeric form. Finally, PE-4 represents an analog of tetraacyl precursor Ia (compound 406). Structure-activity relationships of these compounds were determined by measuring their capacity to induce tumor necrosis factor alpha, interleukin 1, and interleukin 6 release by human mononuclear cells and to cause mitogenicity of murine spleen cells. The results show that replacement of the glycosidic phosphoryl residue by a phosphonooxyethyl group had no substantial effect on the biological activity of compounds. However, the anomeric configuration of GlcN I was found to be of great biological relevance, as, in general, the alpha anomer (PE-2) expressed high activity, and the beta anomer (PE-3) expressed low mediator-inducing and mitogenic activity. The absence of the 3-hydroxyl groups within the acyl residues at GlcN I in PE-2 was found to only slightly affect the induction of monokines in human mononuclear cells compared with that of PE-1 or lipid A (506). These stable 1-phosphonooxyethyl analogs of lipid A may be candidates in the development of immunomodulators for the treatment of systemic endotoxicosis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aarden L. A., De Groot E. R., Schaap O. L., Lansdorp P. M. Production of hybridoma growth factor by human monocytes. Eur J Immunol. 1987 Oct;17(10):1411–1416. doi: 10.1002/eji.1830171004. [DOI] [PubMed] [Google Scholar]
- Aschauer H., Grob A., Hildebrandt J., Schuetze E., Stuetz P. Highly purified lipid X is devoid of immunostimulatory activity. Isolation and characterization of immunostimulating contaminants in a batch of synthetic lipid X. J Biol Chem. 1990 Jun 5;265(16):9159–9164. [PubMed] [Google Scholar]
- Brade L., Brandenburg K., Kuhn H. M., Kusumoto S., Macher I., Rietschel E. T., Brade H. The immunogenicity and antigenicity of lipid A are influenced by its physicochemical state and environment. Infect Immun. 1987 Nov;55(11):2636–2644. doi: 10.1128/iai.55.11.2636-2644.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feist W., Ulmer A. J., Musehold J., Brade H., Kusumoto S., Flad H. D. Induction of tumor necrosis factor-alpha release by lipopolysaccharide and defined lipopolysaccharide partial structures. Immunobiology. 1989 Oct;179(4-5):293–307. doi: 10.1016/S0171-2985(89)80036-1. [DOI] [PubMed] [Google Scholar]
- Feist W., Ulmer A. J., Wang M. H., Musehold J., Schlüter C., Gerdes J., Herzbeck H., Brade H., Kusumoto S., Diamantstein T. Modulation of lipopolysaccharide-induced production of tumor necrosis factor, interleukin 1, and interleukin 6 by synthetic precursor Ia of lipid A. FEMS Microbiol Immunol. 1992 Jan;4(2):73–89. doi: 10.1111/j.1574-6968.1992.tb04973.x. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. Preparation and properties of a standardized lipopolysaccharide from salmonella abortus equi (Novo-Pyrexal). Zentralbl Bakteriol Orig A. 1979 Apr;243(2-3):226–244. [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Golenbock D. T., Hampton R. Y., Qureshi N., Takayama K., Raetz C. R. Lipid A-like molecules that antagonize the effects of endotoxins on human monocytes. J Biol Chem. 1991 Oct 15;266(29):19490–19498. [PubMed] [Google Scholar]
- Golenbock D. T., Leggett J. E., Rasmussen P., Craig W. A., Raetz C. R., Proctor R. A. Lipid X protects mice against fatal Escherichia coli infection. Infect Immun. 1988 Apr;56(4):779–784. doi: 10.1128/iai.56.4.779-784.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golenbock D. T., Will J. A., Raetz C. R., Proctor R. A. Lipid X ameliorates pulmonary hypertension and protects sheep from death due to endotoxin. Infect Immun. 1987 Oct;55(10):2471–2476. doi: 10.1128/iai.55.10.2471-2476.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton R. Y., Golenbock D. T., Penman M., Krieger M., Raetz C. R. Recognition and plasma clearance of endotoxin by scavenger receptors. Nature. 1991 Jul 25;352(6333):342–344. doi: 10.1038/352342a0. [DOI] [PubMed] [Google Scholar]
- Kovach N. L., Yee E., Munford R. S., Raetz C. R., Harlan J. M. Lipid IVA inhibits synthesis and release of tumor necrosis factor induced by lipopolysaccharide in human whole blood ex vivo. J Exp Med. 1990 Jul 1;172(1):77–84. doi: 10.1084/jem.172.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusama T., Soga T., Ono Y., Kumazawa E., Shioya E., Osada Y., Kusumoto S., Shiba T. Synthesis and biological activities of analogs of a lipid A biosynthetic precursor: 1-O-phosphonooxyethyl-4'-O-phosphono-disaccharides with (R)-3-hydroxytetradecanoyl or tetradecanoyl groups at positions 2, 3, 2' and 3'. Chem Pharm Bull (Tokyo) 1991 Aug;39(8):1994–1999. doi: 10.1248/cpb.39.1994. [DOI] [PubMed] [Google Scholar]
- Kusama T., Soga T., Shioya E., Nakayama K., Nakajima H., Osada Y., Ono Y., Kusumoto S., Shiba T. Synthesis and antitumor activity of lipid A analogs having a phosphonooxyethyl group with alpha- or beta-configuration at position 1. Chem Pharm Bull (Tokyo) 1990 Dec;38(12):3366–3372. doi: 10.1248/cpb.38.3366. [DOI] [PubMed] [Google Scholar]
- Loppnow H., Brade H., Dürrbaum I., Dinarello C. A., Kusumoto S., Rietschel E. T., Flad H. D. IL-1 induction-capacity of defined lipopolysaccharide partial structures. J Immunol. 1989 May 1;142(9):3229–3238. [PubMed] [Google Scholar]
- Loppnow H., Brade L., Brade H., Rietschel E. T., Kusumoto S., Shiba T., Flad H. D. Induction of human interleukin 1 by bacterial and synthetic lipid A. Eur J Immunol. 1986 Oct;16(10):1263–1267. doi: 10.1002/eji.1830161013. [DOI] [PubMed] [Google Scholar]
- Loppnow H., Flad H. D., Dürrbaum I., Musehold J., Fetting R., Ulmer A. J., Herzbeck H., Brandt E. Detection of interleukin 1 with human dermal fibroblasts. Immunobiology. 1989 Jun;179(2-3):283–291. doi: 10.1016/S0171-2985(89)80023-3. [DOI] [PubMed] [Google Scholar]
- Loppnow H., Libby P., Freudenberg M., Krauss J. H., Weckesser J., Mayer H. Cytokine induction by lipopolysaccharide (LPS) corresponds to lethal toxicity and is inhibited by nontoxic Rhodobacter capsulatus LPS. Infect Immun. 1990 Nov;58(11):3743–3750. doi: 10.1128/iai.58.11.3743-3750.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohlman T. H., Munford R. S., Harlan J. M. Deacylated lipopolysaccharide inhibits neutrophil adherence to endothelium induced by lipopolysaccharide in vitro. J Exp Med. 1987 May 1;165(5):1393–1402. doi: 10.1084/jem.165.5.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi N., Takayama K., Kurtz R. Diphosphoryl lipid A obtained from the nontoxic lipopolysaccharide of Rhodopseudomonas sphaeroides is an endotoxin antagonist in mice. Infect Immun. 1991 Jan;59(1):441–444. doi: 10.1128/iai.59.1.441-444.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietschel E. T., Galanos C. Lipid A antiserum-mediated protection against lipopolysaccharide- and lipid A-induced fever and skin necrosis. Infect Immun. 1977 Jan;15(1):34–49. doi: 10.1128/iai.15.1.34-49.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama K., Qureshi N., Beutler B., Kirkland T. N. Diphosphoryl lipid A from Rhodopseudomonas sphaeroides ATCC 17023 blocks induction of cachectin in macrophages by lipopolysaccharide. Infect Immun. 1989 Apr;57(4):1336–1338. doi: 10.1128/iai.57.4.1336-1338.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmer A. J., Scholz W., Ernst M., Brandt E., Flad H. D. Isolation and subfractionation of human peripheral blood mononuclear cells (PBMC) by density gradient centrifugation on Percoll. Immunobiology. 1984 May;166(3):238–250. doi: 10.1016/S0171-2985(84)80042-X. [DOI] [PubMed] [Google Scholar]
- Wang M. H., Flad H. D., Feist W., Brade H., Kusumoto S., Rietschel E. T., Ulmer A. J. Inhibition of endotoxin-induced interleukin-6 production by synthetic lipid A partial structures in human peripheral blood mononuclear cells. Infect Immun. 1991 Dec;59(12):4655–4664. doi: 10.1128/iai.59.12.4655-4664.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M. H., Flad H. D., Feist W., Musehold J., Kusumoto S., Brade H., Gerdes J., Rietschel H. T., Ulmer A. J. Inhibition of endotoxin or lipid A-induced tumor necrosis factor production by synthetic lipid A partial structures in human peripheral blood mononuclear cells. Lymphokine Cytokine Res. 1992 Feb;11(1):23–31. [PubMed] [Google Scholar]
- Wright S. D. Multiple receptors for endotoxin. Curr Opin Immunol. 1991 Feb;3(1):83–90. doi: 10.1016/0952-7915(91)90082-c. [DOI] [PubMed] [Google Scholar]


