Abstract
Spirochetes are associated with destructive periodontal diseases, and one cultivatable oral species, Treponema denticola, binds to mammalian cells and perturbs metabolism. To evaluate the cytoskeletal responses and attachment functions of human gingival fibroblasts (HGF) exposed to T. denticola, monolayers of HGF were incubated with T. denticola strains ATCC 35405, e, and e' in serum-free medium. HGF retracted pseudopods, rounded up, and ultimately detached from the substratum. Scanning electron microscopy showed spirochetes in close contact with HGF surfaces; occasionally, bacteria were partially submerged between folds in the HGF membrane. Blebbing and numerous microvilli formed on the cell surface as the HGF retracted. By confocal microscopy, spirochetes were detected in contact with the HGF surface but were never found on the ventral surface of fibroblasts between the substratum and cell. Morphological alterations were associated with and preceded by actin assembly, as measured by microscopic fluorimetry: there was a 263% increase in actin fluorescence over controls within 30 min. Detachment of fibroblasts from the substratum was related to incubation time and was dependent on the concentration of T. denticola. Detachment was observed for all strains tested and was also dependent on the viability of T. denticola: UV light, heat, and metronidazole treatment markedly reduced the HGF detachment response. Detachment was also significantly reduced by the protease inhibitor phenylmethylsulfonyl fluoride. HGF viability was not significantly affected by coincubation with spirochetes, as measured by lactate dehydrogenase release. Thus, T. denticola induces rapid cytoskeletal remodelling followed by cell detachment, which might be stimulated by a bacterially associated protease but is not likely directly mediated by proteolytic degradation at the cell-substratum adhesive contact points.
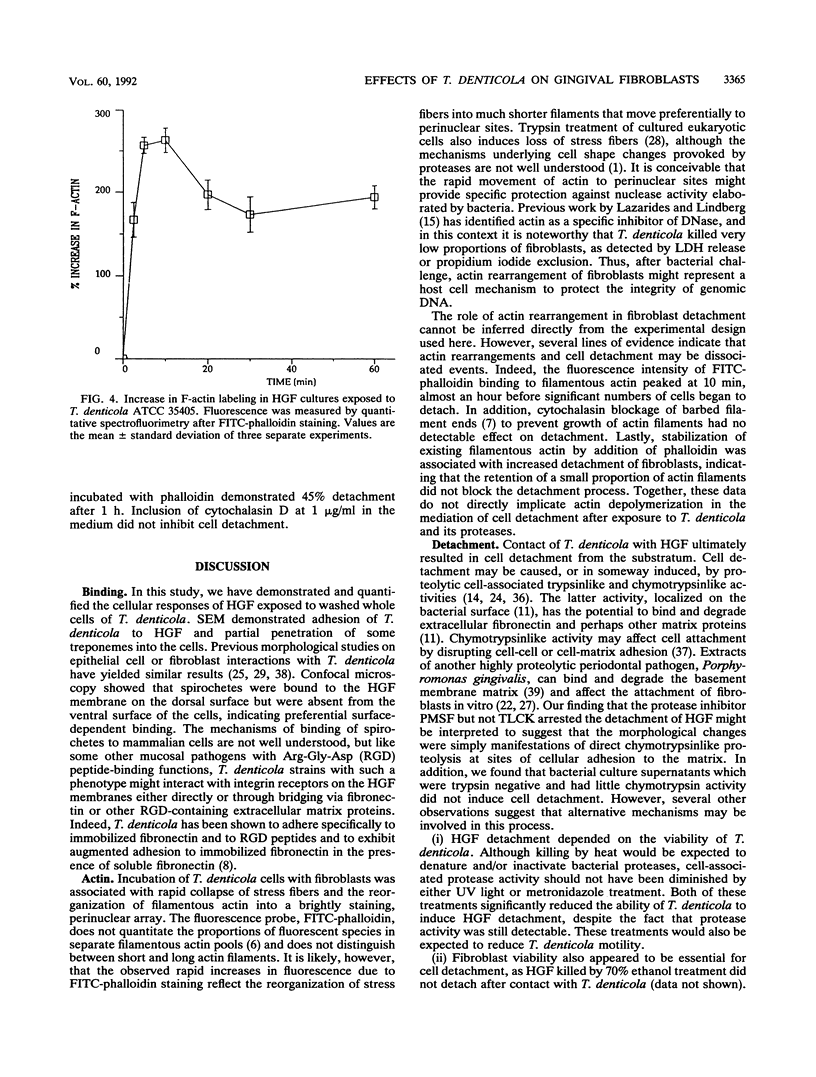
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aggeler J. Cytoskeletal dynamics in rabbit synovial fibroblasts: II. Reformation of stress fibers in cells rounded by treatment with collagenase-inducing agents. Cell Motil Cytoskeleton. 1990;16(2):121–132. doi: 10.1002/cm.970160206. [DOI] [PubMed] [Google Scholar]
- Boehringer H., Berthold P. H., Taichman N. S. Studies on the interaction of human neutrophils with plaque spirochetes. J Periodontal Res. 1986 May;21(3):195–209. doi: 10.1111/j.1600-0765.1986.tb01452.x. [DOI] [PubMed] [Google Scholar]
- Boehringer H., Taichman N. S., Shenker B. J. Suppression of fibroblast proliferation by oral spirochetes. Infect Immun. 1984 Jul;45(1):155–159. doi: 10.1128/iai.45.1.155-159.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunette D. M., Melcher A. H., Moe H. K. Culture and origin of epithelium-like and fibroblast-like cells from porcine periodontal ligament explants and cell suspensions. Arch Oral Biol. 1976;21(7):393–400. doi: 10.1016/0003-9969(76)90001-7. [DOI] [PubMed] [Google Scholar]
- Cassimeris L., McNeill H., Zigmond S. H. Chemoattractant-stimulated polymorphonuclear leukocytes contain two populations of actin filaments that differ in their spatial distributions and relative stabilities. J Cell Biol. 1990 Apr;110(4):1067–1075. doi: 10.1083/jcb.110.4.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A. Effects of cytochalasin and phalloidin on actin. J Cell Biol. 1987 Oct;105(4):1473–1478. doi: 10.1083/jcb.105.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson J. R., Ellen R. P. Tip-oriented adherence of Treponema denticola to fibronectin. Infect Immun. 1990 Dec;58(12):3924–3928. doi: 10.1128/iai.58.12.3924-3928.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B. B. Cell adhesion and invasion mechanisms in microbial pathogenesis. Curr Opin Cell Biol. 1990 Oct;2(5):815–820. doi: 10.1016/0955-0674(90)90078-s. [DOI] [PubMed] [Google Scholar]
- Finlay B. B., Ruschkowski S., Dedhar S. Cytoskeletal rearrangements accompanying salmonella entry into epithelial cells. J Cell Sci. 1991 Jun;99(Pt 2):283–296. doi: 10.1242/jcs.99.2.283. [DOI] [PubMed] [Google Scholar]
- Grenier D., Uitto V. J., McBride B. C. Cellular location of a Treponema denticola chymotrypsinlike protease and importance of the protease in migration through the basement membrane. Infect Immun. 1990 Feb;58(2):347–351. doi: 10.1128/iai.58.2.347-351.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isberg R. R. Discrimination between intracellular uptake and surface adhesion of bacterial pathogens. Science. 1991 May 17;252(5008):934–938. doi: 10.1126/science.1674624. [DOI] [PubMed] [Google Scholar]
- Knowles G. C., McKeown M., Sodek J., McCulloch C. A. Mechanism of collagen phagocytosis by human gingival fibroblasts: importance of collagen structure in cell recognition and internalization. J Cell Sci. 1991 Apr;98(Pt 4):551–558. doi: 10.1242/jcs.98.4.551. [DOI] [PubMed] [Google Scholar]
- Laughon B. E., Syed S. A., Loesche W. J. API ZYM system for identification of Bacteroides spp., Capnocytophaga spp., and spirochetes of oral origin. J Clin Microbiol. 1982 Jan;15(1):97–102. doi: 10.1128/jcm.15.1.97-102.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarides E., Lindberg U. Actin is the naturally occurring inhibitor of deoxyribonuclease I. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4742–4746. doi: 10.1073/pnas.71.12.4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Listgarten M. A., Helldén L. Relative distribution of bacteria at clinically healthy and periodontally diseased sites in humans. J Clin Periodontol. 1978 May;5(2):115–132. doi: 10.1111/j.1600-051x.1978.tb01913.x. [DOI] [PubMed] [Google Scholar]
- Loesche W. J., Syed S. A., Morrison E. C., Kerry G. A., Higgins T., Stoll J. Metronidazole in periodontitis. I. Clinical and bacteriological results after 15 to 30 weeks. J Periodontol. 1984 Jun;55(6):325–335. doi: 10.1902/jop.1984.55.6.325. [DOI] [PubMed] [Google Scholar]
- Loesche W. J. The role of spirochetes in periodontal disease. Adv Dent Res. 1988 Nov;2(2):275–283. doi: 10.1177/08959374880020021201. [DOI] [PubMed] [Google Scholar]
- Mousquès T., Listgarten M. A., Phillips R. W. Effect of scaling and root planing on the composition of the human subgingival microbial flora. J Periodontal Res. 1980 Mar;15(2):144–151. doi: 10.1111/j.1600-0765.1980.tb00268.x. [DOI] [PubMed] [Google Scholar]
- Nitzan D., Sperry J. F., Wilkins T. D. Fibrinolytic activity of oral anaerobic bacteria. Arch Oral Biol. 1978;23(6):465–470. doi: 10.1016/0003-9969(78)90078-x. [DOI] [PubMed] [Google Scholar]
- Ohta K., Makinen K. K., Loesche W. J. Purification and characterization of an enzyme produced by Treponema denticola capable of hydrolyzing synthetic trypsin substrates. Infect Immun. 1986 Jul;53(1):213–220. doi: 10.1128/iai.53.1.213-220.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen I. Attachment of Treponema denticola to cultured human epithelial cells. Scand J Dent Res. 1984 Feb;92(1):55–63. doi: 10.1111/j.1600-0722.1984.tb00860.x. [DOI] [PubMed] [Google Scholar]
- Pender N., McCulloch C. A. Quantitation of actin polymerization in two human fibroblast sub-types responding to mechanical stretching. J Cell Sci. 1991 Sep;100(Pt 1):187–193. doi: 10.1242/jcs.100.1.187. [DOI] [PubMed] [Google Scholar]
- Phillips J. R., Nadim H. S., Layman D. L. Alterations in cell morphology and cytoskeletal proteins in gingival fibroblasts exposed to a Bacteroides gingivalis extract. J Periodontal Res. 1990 Nov;25(6):339–346. doi: 10.1111/j.1600-0765.1990.tb00925.x. [DOI] [PubMed] [Google Scholar]
- Reijntjens F. M., Mikx F. H., Wolters-Lutgerhorst J. M., Maltha J. C. Adherence of oral treponemes and their effect on morphological damage and detachment of epithelial cells in vitro. Infect Immun. 1986 Feb;51(2):642–647. doi: 10.1128/iai.51.2.642-647.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenker B. J., Listgarten M. A., Taichman N. S. Suppression of human lymphocyte responses by oral spirochetes: a monocyte-dependent phenomenon. J Immunol. 1984 Apr;132(4):2039–2045. [PubMed] [Google Scholar]
- Socransky S. S. Microbiology of periodontal disease -- present status and future considerations. J Periodontol. 1977 Sep;48(9):497–504. doi: 10.1902/jop.1977.48.9.497. [DOI] [PubMed] [Google Scholar]
- Taichman N. S., Klass J. E., Shenker B. J., Macarak E. J., Boehringer H., Tsai C. C. Suspected periodontopathic organisms alter in vitro proliferation of endothelial cells. J Periodontal Res. 1984 Nov;19(6):583–586. doi: 10.1111/j.1600-0765.1984.tb01319.x. [DOI] [PubMed] [Google Scholar]
- Uitto V. J., Grenier D., Chan E. C., McBride B. C. Isolation of a chymotrypsinlike enzyme from Treponema denticola. Infect Immun. 1988 Oct;56(10):2717–2722. doi: 10.1128/iai.56.10.2717-2722.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A., Holt S. C. Interaction of Treponema denticola TD-4, GM-1, and MS25 with human gingival fibroblasts. Infect Immun. 1990 Jun;58(6):1720–1729. doi: 10.1128/iai.58.6.1720-1729.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler J. R., Matarese V., Hoover C. I., Kramer R. H., Murray P. A. An in vitro model to study bacterial invasion of periodontal tissues. J Periodontol. 1988 Jan;59(1):40–45. doi: 10.1902/jop.1988.59.1.40. [DOI] [PubMed] [Google Scholar]
- Wymann M. P., Kernen P., Bengtsson T., Andersson T., Baggiolini M., Deranleau D. A. Corresponding oscillations in neutrophil shape and filamentous actin content. J Biol Chem. 1990 Jan 15;265(2):619–622. [PubMed] [Google Scholar]





