Abstract
DDB1 was isolated as a UV-damaged DNA-binding protein, but recent studies established that it plays a role as a component of cullin 4A ubiquitin ligases. Cullin-RING complexes are the largest known ubiquitin ligase family, with hundreds of substrate-specific adaptor subunits and which are defined by characteristic motifs. A common motif for DDB1/cullin 4 ubiquitin ligases, a WDXR motif, was recently reported. Here, we show that Schizosaccharomyces pombe Ddb1 associates with several WD40 repeat proteins that share a novel protein motif designated the DDB-box, a motif essential for interaction with Ddb1 and independent of WD40 repeats, unlike the WDXR motif. We also show that ddb1+ and the putative CSA homolog ckn1+ are involved in transcription-coupled nucleotide excision repair and that the DDB-box is essential for the ckn1+ function in vivo. These data indicate that the DDB-box is another common motif which defines adaptor proteins for DDB1/cullin 4 ubiquitin ligases.
Nucleotide excision repair (NER), one of the most versatile and well-conserved DNA repair mechanisms, removes a broad spectrum of bulky DNA lesions, including cyclobutane pyrimidine dimers (CPDs) and 6-4 photoproducts induced by UV light (UV). Defective or deficient NER underlies hereditary diseases, like xeroderma pigmentosum (XP), Cockayne syndrome (CS), and trichothiodystrophy. XP is an autosomal recessive disorder characterized by severe photosensitivity, abnormal pigmentation, and a high incidence of skin cancers. Mutations in NER genes have been identified in seven (A to G) of the eight complementation groups. CS is also an autosomal recessive disease that is characterized by growth retardation, skeletal and retinal abnormalities, progressive neural retardation, and severe photosensitivity; two genetic complementation groups have been recognized (CS-A and CS-B) (10, 12, 36).
DDB1 was originally isolated as a large subunit of the UV-damaged DNA-binding protein (UV-DDB), which is a heterodimeric DDB1/DDB2 complex associated with the XP-E complementation group. However, recent studies have established a role for DDB1 as a component of cullin 4 ubiquitin ligases (E3s). Among cullin-RING E3s, Skp1-cullin 1-F-box proteins (SCFs) are the best characterized prototypes. In the SCF complex, cullin 1 provides a rigid platform for complex assembly, Roc1 directly interacts with E2s, and Skp1 bridges the cullin 1 and F-box proteins. F-box proteins are the most prominent component of this complex, and they are defined by the presence of a common Skp1-binding motif, the F-box. Most F-box proteins have a single protein-protein-binding domain, such as a WD40 repeat or a leucine-rich repeat, which interacts with substrate proteins that are to be ubiquitinated. Since F-box proteins define the substrate specificity of each SCF, they are also referred to as substrate-specific adaptor proteins. Currently, it is estimated that there are about one hundred SCFs in humans and nine in the fission yeast Schizosaccharomyces pombe (9, 32).
Substrate-specific adaptor proteins in other cullin-RING E3s also share common motifs analogous to the F-box, such as the SOCS-box in cullin 2 and cullin 5 E3s, and the BTB domains in cullin 3 E3s. Concerning a common motif for substrate-specific adaptors for DDB1/cullin 4A E3s, a subdomain of WD40 repeats is reported to comprise a DDB1-binding WDXR motif (1, 16, 17, 21).
DDB1 also interacts with viral proteins. One is the simian virus 5 (SV5) V protein, and V protein together with DDB1 and cullin 4A comprises an E3 (39). Another is the hepatitis B virus X protein (HBx), a key viral regulatory protein (7). Unlike the SV5 V protein, HBx has not been reported to participate in an E3 with DDB1.
NER consists of two subpathways, transcription-coupled repair (TCR), which removes lesions from the transcribed strand of genes actively transcribed by RNA polymerase II, and global genomic repair (GGR), which removes lesions from nontranscribed strands and from transcriptionally silent regions of the genome. The molecular mechanism of TCR has not been fully elucidated, but accumulating observations have inspired several models (36, 37). According to current models, the blockage of transcription elongation by RNA polymerase II at the site of DNA lesions functions as a damage recognition signal and stimulates the excision reaction in a manner dependent on the Cockayne syndrome proteins CSA and CSB. Both CSA and CSB have been implicated in TCR, but their precise roles are still unclear. On the other hand, the largest subunit of RNA polymerase II (Rpb1) is ubiquitinated following UV irradiation in a CSA- and CSB-dependent manner (8), and CSA was recently shown to comprise a ubiquitin ligase complex together with DDB1, cullin 4A, and Roc1 (14). However, other E3s have also been shown to promote ubiquitination of Rpb1 (2, 20, 22), and the relevance of the E3 activity of the CSA complex in TCR is uncertain. CSB homologs in Saccharomyces cerevisiae and S. pombe were previously identified as Rad26 and rhp26+, respectively (40, 42), and a study in S. cerevisiae proved the existence of two alternative forms of TCR, Rad26-dependent TCR and Rad26-independent TCR (25).
S. pombe Ddb1 has been implicated in regulating ribonucleotide reductase activity together with Cdt2, Pcu4, an S. pombe cullin 4 homolog, and the COP9/signalosome, a regulatory complex of cullin-RING E3s. The ddb1+ disruptant has been reported to show defects in mitotic and meiotic cell cycle progression, DNA damage sensitivity, and genome instability because of aberrant regulation of ribonucleotide reductase (5, 6, 18, 28, 45).
Here, we purified the Ddb1 protein complex from S. pombe whole-cell extracts and identified four WD40 repeat proteins in this complex. One is a putative homolog of human CSA, which we named Ckn1. Genetic analysis showed that ddb1+, pcu4+, and ckn1+ function in TCR together with rhp26+. Analysis of another WD40 repeat protein revealed that it contains a sequence that was previously reported for a DDB1-binding region in HBx (3), and this sequence is also conserved among the other WD40 repeat proteins. We show that this common sequence, which we termed the DDB-box for devoted to DDB1 binding, is an important motif for interaction with DDB1, and it is essential for the Ckn1 function in TCR in vivo. Unlike the WDXR motif, the DDB-box is distinct from the WD40 repeats. It is also found in not all DDB1-binding WD40 repeat proteins but a subset of other recently reported DDB1-binding WD40 repeat proteins (1, 16, 17, 21).
MATERIALS AND METHODS
Construction of a Ddb1 overexpression strain.
For purification of the Ddb1 complex, the nmt1-T4 promoter, the three-hemagglutinin-six-histidine (HA3-His6) sequence, a ddb1+ fragment (1 to 357 amino acids), and the ura4+ marker were cloned into pBluescript KS(+) (Stratagene), and the resulting plasmid was linearized and integrated into the ddb1+ locus of a wild-type strain, TP4-1D, to create the ddb1-int strain.
Whole-cell extract preparation.
Whole-cell extracts were prepared from wild-type and ddb1-int strains as described previously (34) with modifications. Cells were cultured until mid-log phase in EMM2 medium (31a) without thiamine, collected by centrifugation, washed with chilled double-distilled water, and resuspended with 1 packed cell volume of buffer A (10 mM HEPES-KOH [pH 7.8], 10 mM KCl, 1.5 mM MgCl2) supplemented with protease inhibitors and a protease inhibitor cocktail (Complete, mini, EDTA-free) (catalog no. 1836170; Roche). The cells were ruptured by two passages through a French press. KCl (2 M) was added to a final concentration of 0.2 M, and proteins were extracted for 30 min at 4°C. The lysates were cleared by centrifugation at 20,500 rpm for 30 min at 2°C (Beckman 45Ti rotor), and the supernatants were centrifuged again at 40,000 rpm for 2 h at 2°C (Beckman 50.2 Ti rotor). The supernatants were dialyzed twice for 1.5 h against buffer D (20 mM HEPES-KOH [pH 7.8], 50 mM KCl, 20% glycerol) supplemented with protease inhibitors. After dialysis, the extracts were centrifuged at 40,000 rpm for 30 min at 2°C (Beckman 70.1 Ti rotor), and the supernatants were stored at −80°C as French press extracts.
Affinity purification of HA3-His6-Ddb1 and associated proteins.
All experiments were done with a standard buffer (20 mM HEPES-KOH [pH 7.8], 100 mM KCl, 0.1% NP-40, 10% glycerol) supplemented with protease inhibitors. The standard buffer, imidazole (final concentration of 2.5 mM), and Ni-nitrilotriacetic acid agarose (catalog no. 30210; Qiagen) were added to French press extracts prepared from wild-type or ddb1-int strains corresponding to approximately 50 g cell weight, and the mixtures were incubated for 2 h at 4°C. The agarose resins were packed into columns (Bio-Rad) and washed with 20 column volumes of standard buffer containing 20 mM imidazole, and bound proteins were eluted with standard buffer containing 250 mM imidazole. Anti-HA affinity matrix (catalog no. 1815016; Roche) was added to eluted fractions, and the mixtures were incubated for 2 h at 4°C. After the mixtures were washed extensively, bound proteins were eluted from the matrix by boiling in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer. Partially purified fractions were separated on a 4 to 20% gradient gel and stained with Coomassie brilliant blue. The protein bands visualized were excised and digested with trypsin. The resulting digestion products were analyzed by nanocapillary liquid chromatography coupled to tandem mass spectrometry (nanoLC-MS-MS) using Agilent series 1100 LC/MSD trap SL. Proteins were identified by correlation of MS-MS spectra with entries in Swiss-Prot and NCBI nr using the MASCOT program.
Protein purification.
Myc-Ddb1 and HA-FLAG-tagged Wdr21 (HF-Wdr21) were expressed in S. pombe from pREP41/42N vectors, and French press extracts were prepared as described above. French press extracts were supplemented with the standard buffer described above containing 300 mM KCl and passed through HiTrap DEAE fast flow (catalog no. 17-5154-01; GE Healthcare Bio-Sciences Corp.). Flowthrough fractions were diluted to a KCl concentration of 100 mM and loaded onto M2 agarose (catalog no. A2220; Sigma), and bound proteins were eluted by incubation with FLAG epitope peptides. Elution fractions were applied to a Mini S column (catalog no. 17-0687-01; GE Healthcare Bio-Sciences Corp.) equilibrated with standard buffer containing 100 mM KCl, and bound proteins were eluted with a linear KCl gradient. Myc-Ddb1 and HF-Wdr21 were recovered in fractions around 300 mM KCl. Myc-Ddb1 and HF-Wdr21 were loaded onto a Superdex 200 column (catalog no. 17-1089-01; GE Healthcare Bio-Sciences Corp.) equilibrated with standard buffer containing 200 mM KCl. The column was calibrated with bovine serum albumin, aldolase, and ferritin for molecular mass estimation.
Myc-Ddb1 and hexahistidine-tagged Ckn1 (His-Ckn1) were expressed in insect cells with the Bac-to-Bac baculovirus expression system (catalog no. 10359-016; Invitrogen) according to the manufacturer's instructions, and cell lysates were prepared as previously described (35). Cell lysates were diluted with buffer (20 mM NaPi [pH 7.2], 300 mM NaCl, 0.1% NP-40, 10% glycerol) supplemented with protease inhibitors and applied to Ni-nitrilotriacetic acid Superflow (catalog no. 30410; Qiagen), and bound proteins were eluted with a linear imidazole gradient. Fractions containing Ddb1/Ckn1 were identified by Western blotting, collected and dialyzed against buffer E (20 mM Tris-HCl [pH 7.8] at 4°C, 0.1% NP-40, 10% glycerol, 0.2 mM EDTA, 0.5 mM dithiothreitol) containing 100 mM NaCl. The fractions were applied to a Mono Q column (catalog no. 17-0671-01; GE Healthcare Bio-Sciences Corp.) equilibrated with buffer E containing 100 mM NaCl. After the column was washed with the same buffer, bound proteins were eluted with a linear NaCl gradient. Myc-Ddb1 and His-Ckn1 from the Mono Q column were then loaded onto a Superdex 200 column equilibrated with buffer E containing 250 mM NaCl.
Gene disruptions, epitope tagging, and other genetic techniques.
For disruption of ddb1+, a 2.4-kb ClaI-PvuI fragment from ddb1+ was replaced with the ura4+ marker. For creation of the pcu4+ disruptant, a 1.25-kb BclI-ClaI fragment from pcu4+ was replaced with the ura4+ marker. For disruption of wdr21+ and ckn1+, ura4+ markers were inserted into the PflMI site of wdr21+ and the central PstI site of ckn1+. A 1.4-kb KpnI-BcnI fragment of wdr21+ and a 1.45-kb EcoRI-AccI fragment of ckn1+ were replaced with ura4+ markers to create null alleles of wdr21+ and ckn1+, but these null mutants showed the same phenotypes as observed for the wdr21+ and ckn1+ disruptants (data not shown). Transformations were carried out with TP4-1D/5A diploid cells, and gene disruptions were confirmed by Southern blotting. The disruptants were backcrossed three times against wild-type strains.
For creation of the ddb1-HH strain, the His6-HA3 (HH) sequence, the nmt1+ polyadenylation signal, and the ura4+ marker were inserted into the C terminus of chromosomal ddb1+. For creation of ckn1+ DDB-box mutant strains, a 0.5-kb EcoRV-PstI fragment of ckn1+, which contains the 5′ nontranslated region and N terminus of the coding region, was cloned into pBluescript together with ura4+. Mutations were introduced into the ckn1+ fragment on this plasmid with the QuikChange XL site-directed mutagenesis kit (catalog no. 200516; Invitrogen) to create pBS-ura4+-ckn1+-DDB-box mutant plasmids. These plasmids were linearized and integrated into the ckn1+ locus of strain TP4-1D. The resulting strains were counterselected with 5-fluoroorotic acid to obtain ckn1+-DDB-box mutant strains.
UV survival assay and strand-specific repair assay.
The UV survival assay and a strand-specific repair assay to monitor CPD removal were performed as previously described (13). The UV survival curves were obtained with stationary-phase cells, except where otherwise noted.
Coimmunoprecipitation analysis.
Abbreviations for the affinity tags are as follows: HH, HA3-His6; MH, Myc4-His10; and HF, HA-FLAG. Please see Materials and Methods in the supplemental material for each vector.
S. pombe native extracts for coimmunoprecipitation analysis were prepared by transforming pREP42N-MH-Wdr21, Ckn1, Cdt2, or Iqwd1 into TP4-1D together with the vector pREP41-HH-Ddb1 or pREP41-HH. Cells were cultured until mid-log phase in EMM2 medium without thiamine and harvested by centrifugation. Cells were ruptured by vortexing with glass beads (catalog no. G8772; Sigma) in lysis buffer (20 mM HEPES-KOH [pH 7.8], 150 mM KCl, 0.1% NP-40) supplemented with protease inhibitors. Extracts were cleared by centrifugation, and supernatants were supplemented with a quarter volume of lysis buffer containing 80% glycerol and stored at −80°C as native extracts.
Human Myc-DDB1 and HF-WDR21 were expressed in insect cells with the Bac-to-Bac baculovirus expression system (catalog no. 10359-016; Invitrogen) according to the manufacturer's instructions, and cell lysates were prepared as previously described (35). Coimmunoprecipitations were performed in the standard buffer described above containing 150 or 200 mM KCl.
RESULTS
Purification of the Ddb1 complex.
We first purified the Ddb1 complex from S. pombe whole-cell extracts and identified constituent proteins. For this purpose, we created the ddb1-int strain, in which Ddb1 is overexpressed from its chromosomal locus under the control of the nmt1-T4 promoter. The amount of HH-Ddb1 protein in the ddb1-int strain is about 20-fold higher than that of endogenous Ddb1 (data not shown).
Selected bands in the Ddb1 protein complex (Fig. 1A) were analyzed by mass spectrometry, and proteins that significantly matched ddb1-int-specific peptides are listed in Fig. 1B. The presence of Pcu4 and the probable heat shock protein Ssa2, proteins that were previously reported to interact with Ddb1 (29), underscores the integrity of our preparation. Among the other identified proteins, four have WD40 repeats. One is Cdt2, which is important for mitotic and premeiotic DNA replication in S. pombe and which has been implicated in the regulation of ribonucleotide reductase as an adaptor protein for the Ddb1/Pcu4 E3 (28, 44). Another protein, encoded by open reading frame (ORF) SPBC577.09, has a molecular mass of 46 kDa and is a putative S. pombe homolog of human CSA. We named this protein Ckn1 for Cockayne syndrome 1, because an unrelated S. pombe protein was already named Csa1. A third protein, encoded by ORF SPAC12g12.10, has a molecular mass of 48 kDa. We named this protein Wdr21, in reference to a putative sequence homolog in humans, WDR21. Human WDR21 and S. pombe Wdr21 share a unique N-terminal peptide sequence in addition to WD40 repeats (see Fig. S1 in the supplemental material). The last WD40 repeat protein, encoded by ORF SPBC609.03, has a molecular mass of 92 kDa. We named this protein Iqwd1, in reference to the putative human sequence homolog IQWD1, which was previously called NRIP (38). Human IQWD1 interacts with human DDB1 when expressed in insect cells (data not shown). Liu et al. reported an association of Ddb1/Pcu4 with the COP9/signalosome (29), but we did not identify COP9/signalosome subunits in our preparation, except for Csn1 and Csn2, which were detected in minor fractions (data not shown).
FIG. 1.
Identification of four WD40 repeat proteins in the Ddb1 protein complex. (A) The nmt1-T4 promoter and HA3-His6 tag were fused to the N terminus of chromosomal ddb1+ to generate the strain ddb1-int, which mildly overexpresses HA3-His6-Ddb1 (HH-Ddb1), and the Ddb1 complex was purified by virtue of the affinity tags. Purified fractions were separated by SDS-PAGE, and the gels were silver stained. (B) Selected bands were analyzed by nanoLC-MS-MS, and identified proteins are listed. Bands 1 to 8 in panel A are shown in panel B.
Ddb1 forms heterodimeric protein complexes with Ckn1 and Wdr21.
Ddb1 was ectopically coexpressed in S. pombe with the identified WD40 repeat proteins, and interactions between Ddb1 and these proteins were confirmed by immunoprecipitation of whole-cell extracts (Fig. 2A) (see Fig. S2A and B in the supplemental material). On a glycerol gradient, coexpressed Ddb1 and Ckn1 migrated to the same extent as aldolase, suggesting that Ddb1 and Ckn1 comprise a heterodimeric complex (see Fig. S3A in the supplemental material). On the other hand, coexpressed Ddb1 and Wdr21 sedimented much faster than coexpressed Ddb1 and Ckn1. Coexpressed Ddb1 and Wdr21 also migrated as a complex with a higher molecular mass when whole-cell extracts were resolved by gel filtration (data not shown). Because one explanation for this faster migration is that other proteins may associate with the Ddb1/Wdr21 complex, we purified Ddb1 and Wdr21 from S. pombe whole-cell extracts as a protein complex. However, the purified Ddb1/Wdr21 complex showed a molecular mass of 160 kDa, as judged by gel filtration analysis (Fig. 2B). Similarly, the Ddb1/Ckn1 complex, which was expressed in insect cells and purified from lysates, showed a molecular mass of 160 kDa (Fig. 2C), and we conclude that Ddb1 forms heterodimeric complexes with Ckn1 and Wdr21. We considered that it was not essential to express Ddb1 and Ckn1 in S. pombe and purify them from large culture volumes, because the Ddb1/Wdr21 complex that we purified is also an ectopically overexpressed complex. Glycerol gradient fractionation of whole-cell extracts showed that endogenous Ddb1 forms a much broader peak than did overexpressed Ddb1 and Ddb1/Ckn1, suggesting that endogenous Ddb1 is incorporated into heterogeneous complexes (see Fig. S3 in the supplemental material). Endogenous Wdr21 and Ckn1 were undetectable in crude extracts.
FIG. 2.
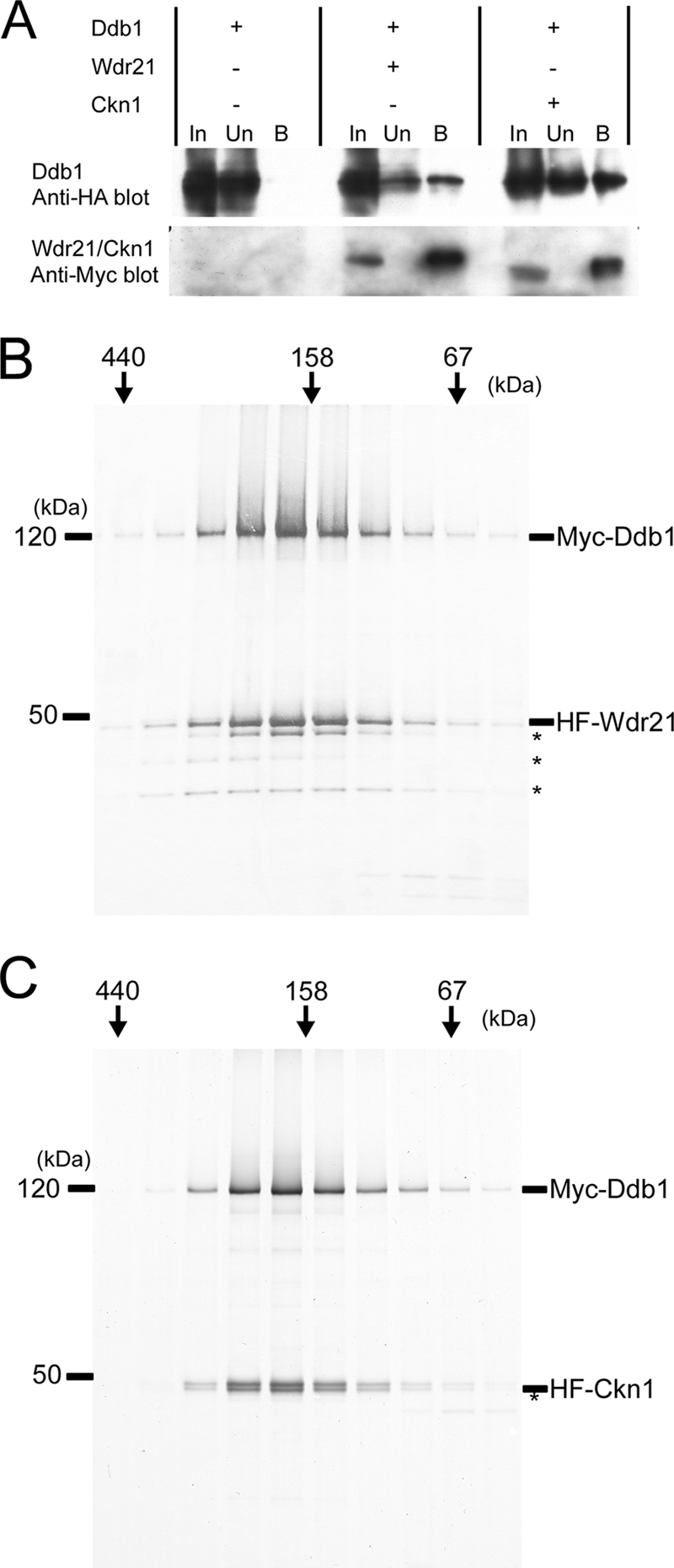
Ddb1/Wdr21 and Ddb1/Ckn1 form heterodimeric complexes. (A) Ddb1 and Wdr21 or Ckn1 were coexpressed in S. pombe from pREP41/42N vectors. MH-Wdr21 or MH-Ckn1 was immunoprecipitated from French press extracts with an anti-Myc antibody, and coimmunoprecipitation of HH-Ddb1 was confirmed by an anti-HA antibody. Twenty percent input is shown. In, input; Un, unbound; B, bound. (B) Ddb1 and Wdr21 were coexpressed in S. pombe and purified from a whole-cell extract as described in Materials and Methods. Fractions resolved by gel filtration were subjected to SDS-PAGE and silver stained. (C) By analogy, Ddb1 and Ckn1 were coexpressed in insect cells and purified. Both complexes have the molecular masses expected for Ddb1/Wdr21 and Ddb1/Ckn1 heterodimers. Asterisks denote degraded Wdr21 or Ckn1.
Next, we coexpressed Ddb1, Wdr21, and Ckn1 in insect cells and examined ternary complexes. When Ddb1 was immunoprecipitated, we detected coimmunoprecipitation of both Wdr21 and Ckn1 (see Fig. S4A in the supplemental material). However, we did not detect coimmunoprecipitation of Ckn1, when Wdr21 was immunoprecipitated (see Fig. S4B in the supplemental material). Similarly, Wdr21 was not coimmunoprecipitated when Ckn1 was immunoprecipitated (see Fig. S4C in the supplemental material). Ddb1 was still coimmunoprecipitated in both cases. These data suggest that Ddb1, Wdr21, and Ckn1 do not form a ternary complex.
The WD40 repeat proteins associate with Ddb1 through a common motif, the DDB-box.
The observation that Ddb1, Wdr21, and Ckn1 do not form a ternary complex (see Fig. S4A, B, and C in the supplemental material) raised the possibility that Wdr21 and Ckn1 bind to the same surface on Ddb1, which in turn implied that Wdr21 and Ckn1 may share the same Ddb1-binding domain.
Wdr21 has conserved sequence on its N terminus, which is dissimilar to a WD40 repeat (see Fig. S1 in the supplemental material). We, therefore, expected at first that this N-terminal conserved sequence is involved in interaction with Ddb1, but deletion analysis showed that this sequence is dispensable for interaction (data not shown). Then, in the course of sequence analysis, we found that vertebrate WDR21 proteins share the same sequence as the previously identified DDB1-binding region of HBx (Fig. 3A) (see Fig. S5 in the supplemental material) (reference 3 and references therein). To examine whether this sequence is relevant for the interaction between DDB1 and WDR21, human DDB1 and WDR21 mutants were expressed in insect cells and immunoprecipitated. The WDR21Δ131-136 protein, which is deleted for the core of the HBx-WDR21 homologous region, did not interact with DDB1 (Fig. 3B). We also tested the impact of amino acid substitutions in this region on the interaction between DDB1 and WDR21. The WDR21-LGF134-136AAA protein did not interact with DDB1, while the WDR21-RKS130-132AAA protein did.
FIG. 3.
WDR21 has the same amino acid sequence as the DDB1-binding region of HBx. (A) Individual WDR21 proteins from vertebrates were searched with a profile hidden Markov model (11) generated from the DDB1-binding region of HBx shown in Fig. S5 in the supplemental material. The DDB1-binding sequence of HBx was found in vertebrate WDR21 proteins. The sequences of WDR21 proteins from Gallus gallus (Gg.WDR21), Tetraodone nigroviridis (Tn.WDR21), Xenopus laevis (Xl.WDR21), and Homo sapiens (Hs.WDR21) are shown. Gaps introduced to maximize alignment of the sequences are indicated by dashes. a.a., amino acids. (B) Human DDB1 and mutant forms of human WDR21 were coexpressed in insect cells, Myc-DDB1 was immunoprecipitated with an anti-Myc antibody, and coimmunoprecipitation of WDR21 was examined with an anti-HA antibody. WDR21Δ131-136 and WDR21-LGF134-136AAA fail to interact with DDB1. Twenty percent input is shown. In, input; B, bound.
Detailed analysis revealed that similar sequences are also present in other WD40 repeat proteins, and we named this sequence the DDB-box for devoted to DDB1 binding (Fig. 4A). The amino acid substitutions LGG90-92AAA in S. pombe Cdt2, which correspond to the alterations LGF134-136AAA in WDR21, abolished interaction between Ddb1 and Cdt2 (see Fig. S2A in the supplemental material). An S. pombe Iqwd1 N-terminal deletion mutant, which lacks the DDB-box region, failed to interact with Ddb1, and the Iqwd1-LDY11-13AAA protein, which has amino acid substitutions equivalent to those in the WDR21-LGF134-136AAA protein, also did not interact with Ddb1 (see Fig. S2B in the supplemental material). We also noted that N-terminal fragments of degraded Iqwd1 are also coimmunoprecipitated with Ddb1, indicating that relevant interaction domain is close to the N terminus. These data raise the possibility that the DDB-box motif in each protein constitutes a Ddb1-binding region.
FIG. 4.
WD40 repeat proteins in the Ddb1 complex and HBx share a common protein motif. (A) Multiple-sequence alignment of the DDB-box was generated by sequence analysis with a hidden Markov model. The bracket indicates a region corresponding to amino acids 131 to 136 in human WDR21. Arrows denote highly conserved amino acids. HBx and its orthologs do not harbor a WD40 repeat. The CSA, Ckn1, IQWD1, Cdt2, WDR21, X, and HBx proteins of Homo sapiens (Hs.), Gallus gallus (Gg.), Xenopus laevis (Xl.), Danio rerio (Dr.), Tetraodone nigroviridis (Tn.), Schizosaccharomyces pombe (Sp.), Arabidopsis thaliana (At.), Oryza sativa (Os.), Caenorhabditis elegans (Ce.), woodchuck hepatitis B virus (WHV), ground squirrel hepatitis virus (GSHV), arctic ground squirrel hepatitis B virus (ASHV), Pan troglodytes (Pt.), and wooly monkey hepatitis B virus (WMHBV), clone WMHBV-2. Gaps introduced to maximize alignment of the sequences are indicated by dashes. (B to D) S. pombe Ddb1 and Ckn1 DDB-box mutants were coexpressed in S. pombe from pREP41/42N vectors, HH-Ddb1 was immunoprecipitated from whole-cell extract with an anti-HA antibody, and coimmunoprecipitation of MH-Ckn1 was examined with an anti-Myc antibody. Substitutions affecting conserved amino acids, namely, L5A, R8A, G11A, and F18A, weaken the interaction between Ddb1 and Ckn1. In, input; B, bound. (E) Simultaneous substitution of L5 and R8 with alanine completely abolishes interaction, and no evidence for coimmunoprecipitation was detected even when the blot was overexposed. Five percent input is shown for all panels.
Highly conserved amino acids in the DDB-box were tested by mutational analysis (Fig. 4A). Point mutations were introduced into S. pombe Ckn1, and mutant proteins were examined with pulldown assays from S. pombe whole-cell extracts. One reason we chose Ckn1 is that we could genetically test the impact of amino acids substitutions, as shown below. Substitutions of conserved residues, L5A, R8A, G11A, and F18A, strongly affected interaction between Ckn1 and Ddb1, while substitution of the less conserved residues L6 or E9 had a milder or undetectable effect (Fig. 4B, C, and D). Interaction was also affected by substitutions of W10, which is generally a hydrophobic residue, especially leucine, in DDB-box proteins except in CSA proteins from higher eukaryotes. Amino acid substitutions of this residue also affect interaction between DDB1 and HBx (27). Furthermore, interaction is also altered by substitutions of P14, whose nearby positions are frequently occupied by proline (Fig. 4D). Although Ckn1-L5A, Ckn1-R8A, and other single mutants still exhibited a residual interaction with Ddb1, the Ckn1-L5A/R8A mutant, in which both L5 and R8 are substituted, completely failed to interact with Ddb1 (Fig. 4E). The interaction of S. pombe Wdr21 with Ddb1 was abolished by the R73A substitution but only slightly affected by the L70A substitution (see Fig. S6 in the supplemental material). The arginine residue of HBx, which corresponds to R8 of Ckn1 and R73 of Wdr21, is also important for the interaction between DDB1 and HBx (27).
To confirm that DDB-box proteins share the same binding surface on DDB1 experimentally, we expressed DDB-box fragments of human WDR21, residues 93 to 161 or residues 114 to 161, which do not contain WD40 repeat at all, together with DDB1 in insect cells, and examined the coimmunoprecipitation. As shown in Fig. 5A, binding of DDB-box fragments to DDB1 was much weaker than full-length WDR21, but the interactions were still dependent on the wild-type DDB-box, namely, the LGF134-136AAA mutation abolished the binding to DDB1 (Fig. 5A), indicating that we certainly detected the physiological interactions between DDB-box fragments and DDB1. Furthermore, we coexpressed other DDB-box proteins and examined the binding of DDB-box fragment to DDB1. As shown in Fig. 5B, coexpression of DDB2, CSA, or WDR21 abolished coimmunoprecipitation of WDR21 (residues 93 to 161) with DDB1 (Fig. 5B), indicating that these proteins compete for binding to DDB1. These competitions show that WDR21 (residues 93 to 161) and DDB2 or CSA share the same binding surface on DDB1, suggesting that all of these proteins interact with DDB1 using common structure on each protein, the DDB-box.
FIG. 5.
DDB-box proteins compete for binding to DDB1. (A) Human DDB1 was coexpressed in insect cells with N-terminally HA-FLAG-tagged DDB-box fragments of human WDR21. Myc-DDB1 was immunoprecipitated with an anti-Myc antibody, and coimmunoprecipitation of each peptide was examined with an anti-HA antibody. Two percent input is shown. In, input; B, bound; a.a., amino acids. (B) Human DDB1 and WDR21 (residues 93 to 161) were coexpressed in insect cells with or without (−) DDB2, CSA or WDR21. Myc-DDB1 was immunoprecipitated, and coimmunoprecipitation of WDR21 (residues 93 to 161), DDB2, CSA, and WDR21 was examined with anti-HA antibody. Two percent input is shown.
The DDB-box is also found in not all DDB1-binding WD40 repeat proteins reported by others but in a subset of the proteins reported by others (1, 16, 17, 21), and based on secondary structure prediction, it shows homology with the DDB1-binding region of the SV5 V protein (see Fig. S7 in the supplemental material). In DDB2, the DDB-box is located in the N-terminal helical region (see Fig. S7 in the supplemental material). It was shown that deletion of amino acid residues 68 to 79 of human DDB2, which encompasses the DDB-box, abolishes binding to DDB1 (21). The competitive binding of DDB2 and DDB-box peptides to DDB1 also indicates that the DDB-box exists in DDB2 (Fig. 5B). In DDB2, the arginine residue, which corresponds to R8 of Ckn1, is replaced by histidine, glutamine, asparagine, and serine depending on the species (see Fig. S7 in the supplemental material). In WDR21, this position is also occupied by asparagine, serine, and leucine in addition to arginine (Fig. 4A) (see Fig. S7 in the supplemental material). Thus, this arginine residue can be substituted in some proteins.
Ddb1 and Ckn1 are involved in transcription-coupled repair.
To investigate the functional importance of the interaction between Ddb1 and WD40 repeat proteins, we disrupted ddb1+ and other genes. None of the WD40 repeat proteins is essential for cell viability (data not shown) (44). In addition to NER, S. pombe has another excision repair pathway, UV-damaged DNA endonuclease (UVDE)-dependent excision repair (UVER) (43). Here, we tested the uvde+ disruptant in UV survival and strand-specific repair assays.
Disruption of ckn1+ resulted in increased UV sensitivity in the Δuvde background, but in contrast, disruption of wdr21+ did not affect UV survival in either the wild-type or Δuvde background (Fig. 6A). The Δrad13 strain lacks a Rad2/XPG homolog and is completely defective in NER, and the Δrad13 Δuvde strain showed extremely high UV sensitivity (Fig. 6A). In the Δuvde strain, TCR of CPDs on the transcribed strand by NER was observed, but we did not detect CPD removal from nontranscribed strands under our conditions (Fig. 6D), because UVER is highly efficient and GGR is only a minor component of NER in S. pombe (42). We also did not observe a clear decrease in CPD removal from nontranscribed strands in double mutants, such as the Δddb1 Δuvde strain (data not shown). The Δckn1 Δuvde strain was partially defective in the removal of CPDs from transcribed strands; on the other hand, the Δwdr21 Δuvde strain showed the same repair proficiency as the Δuvde strain did (Fig. 6D).
FIG. 6.
ddb1+ and ckn1+ are involved in rhp26+-dependent TCR. (A) UV survival curves of S. pombe strains were obtained as described in Materials and Methods. Disruption of ckn1+ induces an increase in UV sensitivity in the Δuvde background but not in the wild-type background. Disruption of wdr21+ does not affect UV survival in either background. (B) Disruption of ddb1+ or rhp26+ causes an increase in UV sensitivity in the Δuvde background. (C) The Δddb1 Δckn1 Δuvde and Δckn1 Δrhp26 Δuvde strains have the same UV survival curves as the Δddb1 Δuvde and Δrhp26 Δuvde strains, respectively. (D) CPD removal was measured by a strand-specific repair assay as described in Materials and Methods. Disruption of ckn1+ decreases the efficiency of CPD removal from transcribed strands in the Δuvde background, but preferential repair of transcribed stands is still observed. In contrast, the Δwdr21 Δuvde strain is as proficient at CPD removal as the Δuvde strain is. CPD removal from nontranscribed strands is not apparent under our conditions. TS, transcribed strand; NTS, nontranscribed strand. (E) Like the Δckn1 Δuvde strain, the Δrhp26 Δuvde, Δpcu4 Δuvde, and Δddb1 Δuvde strains are partially deficient in CPD removal from transcribed strands. (F) Disruption of rhp26+, pcu4+, or ddb1+ does not affect CPD removal from transcribed strands in the Δckn1 Δuvde background.
The Δddb1 mutant was previously reported to show UV sensitivity, which is dependent on an aberrant regulation of ribonucleotide reductase (6), but the Δddb1 mutation did not confer a significant increase in UV sensitivity in the wild-type background under our conditions (see Fig. S8A in the supplemental material). It has been reported that ribonucleotide reductase-dependent UV sensitivity of the Δddb1 strain is more pronounced in cells in the logarithmic growth phase (5). We, therefore, performed the UV survival assay with stationary-phase cells anticipating that the influence of ribonucleotide reductase is eliminated and that repair-dependent UV sensitivity can be monitored. The disruption of ddb1+ in the Δuvde background resulted in a moderate but significant decrease in UV survival, even in stationary phase (Fig. 6B), and disruption of ckn1+ did not confer any effect on UV survival in the Δddb1 Δuvde background (Fig. 6C). Disruption of rhp26+ decreased the UV survival of the Δuvde strain (Fig. 6B) (42) but not of the Δckn1 Δuvde strain (Fig. 6C). These results were confirmed in logarithmic-growth-phase cells, giving a clearer difference between the Δuvde and Δddb1 Δuvde or Δrhp26 Δuvde strains (see Fig. S8B and C in the supplemental material) (42). In the Δuvde background, CPD removal from transcribed strands was decreased as a result of disruption of ddb1+, pcu4+, or rhp26+ (Fig. 6E), although disruption of ddb1+, pcu4+, or rhp26+ did not affect CPD removal in the Δckn1 Δuvde strain (Fig. 6F). These data suggest that ckn1+, ddb1+, pcu4+, and rhp26+ comprise an epistasis group in UV tolerance and that they are involved in the TCR pathway of NER. rhp41+ is a Rad4/XPC homolog in S. pombe and is essential for TCR (13). The Δckn1 Δrhp41 Δuvde, Δddb1 Δrhp41 Δuvde, and Δckn1 Δddb1 Δrhp41 Δuvde strains showed the same UV survival as the Δrhp41 Δuvde strain did (see Fig. S8D in the supplemental material), further confirming the above results.
The interaction between Ckn1 and Ddb1 is essential for TCR.
Amino acid substitutions were introduced into chromosomal ckn1+, and the resulting strains were tested for UV survival and in a strand-specific repair assay. The ckn1-L5A/R8A Δuvde and ckn1-L6A Δuvde strains showed the same UV survival curves as the Δckn1 Δuvde and Δuvde strains did, respectively (Fig. 7A). With respect to UV survival, CPD removal from transcribed strands in the ckn1-L5A/R8A Δuvde strain was decreased to the same extent as in the Δckn1 Δuvde strain, and the ckn1-L6A Δuvde strain was as proficient as the Δuvde strain in CPD removal (Fig. 7B). These data indicate that the interaction between Ckn1 and Ddb1 is essential for rhp26+-dependent TCR. The L5A and R8A single-amino-acid substitutions only mildly affected both UV survival and CPD removal (data not shown).
FIG. 7.
The ckn1+ DDB-box mutant strain shows defects in TCR. (A) The ckn1-L5A/R8A Δuvde strain shows the same UV sensitivity as the Δckn1 Δuvde strain does, while the ckn1-L6A Δuvde strain shows the same UV survival curve as the Δuvde strain does. (B) In the ckn1-L5A/R8A Δuvde strain, CPD removal from transcribed strands decreases to the same extent as in the Δckn1 Δuvde strain, but in the ckn1-L6A Δuvde strain, CPD removal is as efficient as in the Δuvde strain. TS, transcribed strand; NTS, nontranscribed strand.
Rhp26 is ubiquitinated following UV irradiation in a manner independent of ckn1+.
Finally, we examined the substrate of Ddb1/Pcu4/Ckn1 ubiquitin ligase. We tried to detect ubiquitination of Rpb1 in S. pombe but failed despite extensive effort. Recently, CSB was reported to be ubiquitinated by the CSA complex (15); thus, we examined ubiquitination of Rhp26. When the rhp26-Myc strain was UV irradiated, the slower-migrating form of Rhp26 was detected on an SDS-polyacrylamide gel (see Fig. S9A in the supplemental material). When ubiquitinated proteins were immunoprecipitated from extracts obtained from UV-irradiated cells, Rhp26 was detected as ladder-like bands, a characteristic of polyubiquitinated proteins (see Fig. S9B in the supplemental material). Although a small amount of the nonubiquitinated form of Rhp26 was immunoprecipitated in Fig. S9B in the supplemental material, the top bands, namely, ubiquitinated forms of Rhp26 were not detected in the absence of ectopic expression of HA-ubiquitin (data not shown). These results show that Rhp26 is ubiquitinated following UV irradiation in S. pombe. However, the slower-migrating form of Rhp26 was still observed in the Δckn1 strain (see Fig. S9 in the supplemental material), suggesting that the ubiquitination of Rhp26 is independent of ckn1+.
DISCUSSION
Recent studies have outlined multiple roles for DDB1/cullin 4A E3s in many cellular processes (32), but the role of DDB1 in NER, in which it was originally isolated as UV-DDB, was unknown until a report by Sugasawa et al. describing its involvement in GGR (35). Here, we provide data about the role of Ddb1 in NER with respect to functions in TCR.
The residual preferential repair observed in the Δrhp26 Δuvde strain (Fig. 6E) represents the rhp26+-independent TCR pathway, as is the case in S. cerevisiae (25), because the preferential repair of transcribed strands is completely abolished in strains like the Δrhp41 Δuvde and Δrhp23 Δuvde mutants (13, 30). The rhp26+, ddb1+, and pcu4+ genes exhibit epistasis with ckn1+ with respect to UV survival and CPD removal (Fig. 6C and F) (see Fig. S8B and C in the supplemental material), meaning that ckn1+, ddb1+, and pcu4+ comprise an rhp26+-dependent TCR pathway. Although S. cerevisiae also has two TCR pathways, rhp26+-dependent TCR in S. pombe seems to be more closely related to the mammalian mode of TCR than to that of S. cerevisiae, as indicated by the following points. First, S. cerevisiae Rad28, which was reported as a CSA homolog, is dispensable for TCR (4). Second, a DDB1 sequence homolog has not been identified. Finally, Rsp5, the only E3 which ubiquitinates Rpb1 in S. cerevisiae, is also dispensable for TCR (31). In the course of UV survival assays, we noticed that the UV survival of the Δddb1 Δuvde and Δrhp26 Δuvde mutants were not similar (Fig. 6B). It has been reported that the Δddb1 mutant shows higher sensitivity to UV, especially in the low-dose range (45), and that this is due to the regulatory role of ddb1+ in ribonucleotide reductase activity (6). Thus, the difference in the survival curves of the Δddb1 Δuvde and Δrhp26 Δuvde mutants after UV irradiation could be attributed to the fact that ddb1+ is involved in the regulation of ribonucleotide reductase activity in addition to its involvement in TCR, while rhp26+ is involved only in TCR. Curiously, our data using cells in the logarithmic growth phase did not show such a difference in the survival curves of the Δddb1 Δuvde and Δrhp26 Δuvde mutants after UV irradiation (see Fig. S5B in the supplemental material). We must assume that the contribution of ddb1+ to the regulation of ribonucleotide reductase activity is different in stationary- and log-phase cell cultures. This will be an interesting point to pursue in the future.
The ckn1+-L5A/R8A Δuvde strain is completely deficient in rhp26+-dependent TCR (Fig. 7B), which means that the function of ckn1+ in TCR is fully dependent on Ddb1/Pcu4 E3 in vivo, underscoring the hypothesis that Ckn1 acts as an adaptor protein for Ddb1/Pcu4 E3. This interpretation is consistent with a previous observation that TCR is delayed by knocking down CSN5, a core subunit of the COP9/signalosome that promotes cullin-RING ubiquitin ligase activity in vivo by stabilizing substrate-specific adaptors (14, 41). These observations indicate that ubiquitination of some substrate proteins by Ddb1/Pcu4/Ckn1 E3 is critical for the rhp26+-dependent, or mammalian, mode of TCR. Currently, the most probable substrate protein of Ddb1/Pcu4/Ckn1 E3 is RNA polymerase II (8), but we failed to detect ubiquitination of Rpb1 in S. pombe, and Rhp26 is ubiquitinated upon UV irradiation independent of ckn1+(see Fig. S9 in the supplemental material). Thus, it is still possible that Ddb1/Pcu4/Ckn1 E3 recognizes other substrate proteins important for TCR.
While the F-box, SOCS-box, and BTB domains are related (33), the DDB-box does not appear to be related to any of these motifs. This is reasonable because the molecular structure of DDB1 is quite different from that of other cullin-RING E3 subunits (26). Human CSA and IQWD1 show sequence similarity up to about 30 amino acids; thus, the DDB-box is potentially of the same length. A subdomain of WD40 repeats, the WDXR motif, has been reported as a common DDB1-binding motif (1, 16, 17, 21). DDB1 has a large pocket composed of two β-propellers, which holds the N-terminal helical region of the SV5 V protein and represents the main interaction surface (26). However, it is unlikely that the WDXR motif on the β-propeller structure is in contact with this double-propeller pocket on DDB1, as has also been pointed out by Angers et al. (1). In contrast, like other common motifs for cullin-RING E3s, the DDB-box is located outside the WD40 repeats and thus should extend from the β-propeller fold. Secondary structure predictions of HBx and other DDB-box proteins suggest the presence of α-helices around the N terminus of the alignment shown in Fig. 4 and in Fig. S7 in the supplemental material and loop structures in the following region (27) (data not shown), a configuration that completely matches the structure of the DDB1-binding region of the SV5 V protein (26). Furthermore, the SV5 V protein and HBx have been suggested to share the same DDB1-binding surface (24). Thus, we propose that the DDB-box is embedded in the double-propeller pocket on DDB1 in the same manner as the N-terminal helical region of the V protein. It is noteworthy that the interaction between the DDB-box and DDB1 is potentially independent of the β-propeller structure of DDB-box proteins, because HBx is not a WD40 repeat protein and the DDB-box itself can associate with DDB1 (Fig. 5A). Currently, the relative roles of the DDB-box and the WDXR motif in bridging DDB1 association are not known; however, the crystal structure of DDB1-SV5 V protein complex gives us some clues. In addition to the N-terminal helical domain, the viral V protein is fastened to the surface of the β-propeller of DDB1 through the C-terminal globular zinc finger domain, which should correspond to the β-propeller domain in DDB1-binding WD40 repeat proteins (26). In the same way, in DDB1-binding WD40 repeat proteins, the DDB-box regions should be inserted into the double-propeller pocket on DDB1, while the β-propeller domain of DDB-box proteins would be packed against the surface of the β-propeller of DDB1, probably through the WDXR motif. A similar model was also illustrated for DDB1-DDB2 interactions recently (23), and our and others' models can explain the apparent discrepancy that both the DDB-box and the WDXR motif are essential for DDB1 binding. Such interaction utilizes a large protein surface for complex formation and ensures a tight interaction between DDB1 and substrate-specific adaptors.
Substrate-specific adaptors are composed of specific motifs and a protein-protein interaction domain. For DDB1/cullin 4A E3s, most substrate-specific adaptors reported so far are WD40 repeat proteins, but proteins with other protein-protein interaction domains are recognized by DDB1/cullin 4A. For example, the SV5 V protein has a zinc finger in its substrate recognition domain. The WDXR motif is part of the WD40 repeat, and adaptor proteins without the repeat are defined by the presence of the DDB-box. Exploration of the DDB-box or DDB-box/WDXR motif proteins by bioinformatics and proteomics approaches should link many cellular processes to DDB1/cullin 4A E3s and reveal various novel regulatory pathways regulated by the ubiquitin-proteasome system.
Supplementary Material
Acknowledgments
We thank Mitsuhiro Yanagida, Yukinobu Nakaseko, Akira Yasui, and Shinji Yasuhira for providing yeast strains and vectors; Fumi Amano and Masayuki Yokoi for helpful discussions, technical support, and/or reading the manuscript prior to submission; and Yoshiaki Ohkuma, Chikahide Masutani, and other current and previous laboratory members for helpful comments. We are grateful to Agilent Technologies for their generous loan of Agilent series 1100 LC/MSD trap SL for our use and thank Naoto Shimizu (Agilent Technologies) for MS operation.
This work was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan and by the Solution Oriented Research for Science and Technology (SORST) program of the Japan Science and Technology Agency. Y.F. was supported by the Junior Research Associate program of RIKEN.
Footnotes
Published ahead of print on 15 September 2008.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Angers, S., T. Li, X. Yi, M. J. MacCoss, R. T. Moon, and N. Zheng. 2006. Molecular architecture and assembly of the DDB1-CUL4A ubiquitin ligase machinery. Nature 443590-593. [DOI] [PubMed] [Google Scholar]
- 2.Beaudenon, S. L., M. R. Huacani, G. Wang, D. P. McDonnell, and J. H. Huibregtse. 1999. Rsp5 ubiquitin-protein ligase mediates DNA damage-induced degradation of the large subunit of RNA polymerase II in Saccharomyces cerevisiae. Mol. Cell. Biol. 196972-6979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergametti, F., J. Bianchi, and C. Transy. 2002. Interaction of hepatitis B virus X protein with damaged DNA-binding protein p127: structural analysis and identification of antagonists. J. Biomed. Sci. 9706-715. [DOI] [PubMed] [Google Scholar]
- 4.Bhatia, P. K., R. A. Verhage, J. Brouwer, and E. C. Friedberg. 1996. Molecular cloning and characterization of Saccharomyces cerevisiae RAD28, the yeast homolog of the human Cockayne syndrome A (CSA) gene. J. Bacteriol. 1785977-5988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bondar, T., E. V. Mirkin, D. S. Ucker, W. E. Walden, S. M. Mirkin, and P. Raychaudhuri. 2003. Schizosaccharomyces pombe Ddb1 is functionally linked to the replication checkpoint pathway. J. Biol. Chem. 27837006-37014. [DOI] [PubMed] [Google Scholar]
- 6.Bondar, T., A. Ponomarev, and P. Raychaudhuri. 2004. Ddb1 is required for the proteolysis of the Schizosaccharomyces pombe replication inhibitor Spd1 during S phase and after DNA damage. J. Biol. Chem. 2799937-9943. [DOI] [PubMed] [Google Scholar]
- 7.Bouchard, M. J., and R. J. Schneider. 2004. The enigmatic X gene of hepatitis B virus. J. Virol. 7812725-12734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bregman, D. B., R. Halaban, A. J. van Gool, K. A. Henning, E. C. Friedberg, and S. L. Warren. 1996. UV-induced ubiquitination of RNA polymerase II: a novel modification deficient in Cockayne syndrome cells. Proc. Natl. Acad. Sci. USA 9311586-11590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cardozo, T., and M. Pagano. 2004. The SCF ubiquitin ligase: insights into a molecular machine. Nat. Rev. Mol. Cell Biol. 5739-751. [DOI] [PubMed] [Google Scholar]
- 10.de Laat, W. L., N. G. J. Jaspers, and J. H. J. Hoeijmakers. 1999. Molecular mechanism of nucleotide excision repair. Genes Dev. 13768-785. [DOI] [PubMed] [Google Scholar]
- 11.Eddy, S. R. 1998. Profile hidden Markov models. Bioinformatics 14755-763. [DOI] [PubMed] [Google Scholar]
- 12.Friedberg, E. C., G. C. Walker, W. Siede, R. D. Wood, R. A. Schultz, and T. Ellenberger. 2005. DNA repair and mutagenesis, 2nd ed. ASM Press, Washington, DC.
- 13.Fukumoto, Y., H. Hiyama, M. Yokoi, Y. Nakaseko, M. Yanagida, and F. Hanaoka. 2002. Two budding yeast RAD4 homologs in fission yeast play different roles in the repair of UV-induced DNA damage. DNA Repair 1833-845. [DOI] [PubMed] [Google Scholar]
- 14.Groisman, R., J. Polanowska, I. Kuraoka, J. Sawada, M. Saijo, R. Drapkin, A. F. Kisselev, K. Tanaka, and Y. Nakatani. 2003. The ubiquitin ligase activity in the DDB2 and CSA complexes is differentially regulated by the COP9 signalosome in response to DNA damage. Cell 113357-367. [DOI] [PubMed] [Google Scholar]
- 15.Groisman, R., I. Kuraoka, O. Chevallier, N. Gaye, T. Magnaldo, K. Tanaka, A. F. Kisselev, A. Harel-Bellan, and Y. Nakatani. 2006. CSA-dependent degradation of CSB by the ubiquitin-proteasome pathway establishes a link between complementation factors of the Cockayne syndrome. Genes Dev. 201429-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He, Y. J., C. M. McCall, J. Hu, Y. Zeng, and Y. Xiong. 2006. DDB1 functions as a linker to recruit receptor WD40 proteins to CUL4-ROC1 ubiquitin ligases. Genes Dev. 202949-2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higa, L. A., M. Wu, T. Ye, R. Kobayashi, H. Sun, and H. Zhang. 2006. CUL4-DDB1 ubiquitin ligase interacts with multiple WD40-repeat proteins and regulates histone methylation. Nat. Cell Biol. 81277-1283. [DOI] [PubMed] [Google Scholar]
- 18.Holmberg, C., O. Fleck, H. A. Hansen, C. Liu, R. Slaaby, A. M. Carr, and O. Nielsen. 2005. Ddb1 controls genome stability and meiosis in fission yeast. Genes Dev. 19853-862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reference deleted.
- 20.Huibregtse, J. M., J. C. Yang, and S. L. Beaudenon. 1997. The large subunit of RNA polymerase II is a substrate of the Rsp5 ubiquitin-protein ligase. Proc. Natl. Acad. Sci. USA 943656-3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin, J., E. E. Arias, J. Chen, J. W. Harper, and J. C. Walter. 2006. A family of diverse Cul4-Ddb1-interacting proteins includes Cdt2, which is required for S phase destruction of the replication factor Cdt1. Mol. Cell 23709-721. [DOI] [PubMed] [Google Scholar]
- 22.Kuznetsova, A. V., J. Meller, P. O. Schnell, J. A. Nash, M. L. Ignacak, Y. Sanchez, J. W. Conaway, R. C. Conaway, and M. F. Czyzyk-Krzeska. 2003. von Hipple-Lindau protein binds hyperphosphorylated large subunit of RNA polymerase II through a proline hydroxylation motif and targets it for ubiquitination. Proc. Natl. Acad. Sci. USA 1002706-2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee, J., and P. Zhou. 2007. DCAFs, the missing link of the CUL4-DDB1 ubiquitin ligase. Mol. Cell 26775-780. [DOI] [PubMed] [Google Scholar]
- 24.Leupin, O., S. Bontron, and M. Strubin. 2003. Hepatitis B virus X protein and simian virus 5 V protein exhibit similar UV-DDB1 binding properties to mediate distinct activities. J. Virol. 776274-6283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li, S., and M. J. Smerdon. 2002. Rpb4 and Rpb9 mediate subpathways of transcription-coupled DNA repair in Saccharomyces cerevisiae. EMBO J. 215921-5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li, T., X. Chen, K. C. Garbutt, P. Zhou, and N. Zheng. 2006. Structure of DDB1 in complex with a paramyxovirus V protein: viral hijack of a propeller cluster in ubiquitin ligase. Cell 124105-117. [DOI] [PubMed] [Google Scholar]
- 27.Lin-Marq, N., S. Bontron, O. Leupin, and M. Strubin. 2001. Hepatitis B virus X protein interferes with cell viability through interaction with the p127-kDa UV-damaged DNA-binding protein. Virology 287266-274. [DOI] [PubMed] [Google Scholar]
- 28.Liu, C., M. Poitelea, A. Watson, S. H. Yoshida, C. Shimoda, C. Holmberg, O. Nielsen, and A. M. Carr. 2005. Transactivation of Schizosaccharomyces pombe cdt2+ stimulates a Pcu4-Ddb1-CSN ubiquitin ligase. EMBO J. 243940-3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu, C., K. A. Powell, K. Mundt, L. Wu, A. M. Carr, and T. Caspari. 2003. Cop9/signalosome subunits and Pcu4 regulate ribonucleotide reductase by both checkpoint-dependent and -independent mechanisms. Genes Dev. 171130-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lombaerts, M., J. I. Goeloe, H. den Dulk, J. A. Brandsma, and J. Brouwer. 2000. Identification and characterization of the rhp23+ DNA repair gene in Schizosaccharomyces pombe. Biochem. Biophys. Res. Commun. 268210-215. [DOI] [PubMed] [Google Scholar]
- 31.Lommel, L., M. E. Bucheli, and K. S. Sweder. 2000. Transcription-coupled repair in yeast is independent from ubiquitylation of RNA pol II: implications for Cockayne's syndrome. Proc. Natl. Acad. Sci. USA 979088-9092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31a.Moreno, S., A. Klar, and P. Nurse. 1991. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 194795-823. [DOI] [PubMed] [Google Scholar]
- 32.Petroski, M. D., and R. J. Deshaies. 2005. Function and regulation of cullin-RING ubiquitin ligases. Nat. Rev. Mol. Cell Biol. 69-20. [DOI] [PubMed] [Google Scholar]
- 33.Pintard, L., A. Willems, and M. Peter. 2004. Cullin-based ubiquitin ligases: Cul3-BTB complexes join the family. EMBO J. 231681-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rigaut, G., A. Shevchenko, B. Rutz, M. Wilm, M. Mann, and B. Séraphin. 1999. A generic protein purification method for protein complex characterization and proteome exploration. Nat. Biotechnol. 171030-1032. [DOI] [PubMed] [Google Scholar]
- 35.Sugasawa, K., Y. Okuda, M. Saijo, R. Nishi, N. Matsuda, G. Chu, T. Mori, S. Iwai, K. Tanaka, K. Tanaka, and F. Hanaoka. 2005. UV-induced ubiquitylation of XPC protein mediated by UV-DDB-ubiquitin ligase complex. Cell 121387-400. [DOI] [PubMed] [Google Scholar]
- 36.Svejstrup, J. Q. 2002. Mechanisms of transcription-coupled DNA repair. Nat. Rev. Mol. Cell Biol. 321-29. [DOI] [PubMed] [Google Scholar]
- 37.Svejstrup, J. Q. 2003. Rescue of arrested RNA polymerase II complexes. J. Cell Sci. 116447-451. [DOI] [PubMed] [Google Scholar]
- 38.Tsai, T. C., Y. L. Lee, W. C. Hsiao, Y. P. Tsao, and S. L. Chen. 2005. NRIP, a novel nuclear receptor interaction protein, enhances the transcriptional activity of nuclear receptors. J. Biol. Chem. 28020000-20009. [DOI] [PubMed] [Google Scholar]
- 39.Ulane, C. M., and C. M. Horvath. 2002. Paramyxoviruses SV5 and HPIV2 assemble STAT protein ubiquitin ligase complexes from cellular components. Virology 304160-166. [DOI] [PubMed] [Google Scholar]
- 40.van Gool, A. J., R. Verhage, S. M. A. Swagemakers, P. van de Putte, J. Brouwer, C. Troelstra, D. Bootsma, and J. H. J. Hoeijmakers. 1994. RAD26, the functional S. cerevisiae homolog of the Cockayne syndrome B gene ERCC6. EMBO J. 135361-5369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wee, S., R. K. Geyer, T. Toda, and D. A. Wolf. 2005. CSN facilitates Cullin-RING ubiquitin ligase function by counteracting autocatalytic adapter instability. Nat. Cell Biol. 7387-391. [DOI] [PubMed] [Google Scholar]
- 42.Yasuhira, S., M. Morimyo, and A. Yasui. 1999. Transcription dependence and the roles of two excision repair pathways for UV damage in fission yeast Schizosaccharomyces pombe. J. Biol. Chem. 27426822-26827. [DOI] [PubMed] [Google Scholar]
- 43.Yasui, A., and S. J. McCready. 1998. Alternative repair pathways for UV-induced DNA damage. BioEssays 20291-297. [DOI] [PubMed] [Google Scholar]
- 44.Yoshida, S., H. Al-Amodi, T. Nakamura, C. J. McInerny, and C. Shimoda. 2003. The Schizosaccharomyces pombe cdt2+ gene, a target of G1-S phase-specific transcription factor complex DSC1, is required for mitotic and premeiotic DNA replication. Genetics 164881-893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zolezzi, F., J. Fuss, S. Uzawa, and S. Linn. 2002. Characterization of a Schizosaccharomyces pombe strain deleted for a sequence homologue of the human damaged DNA binding 1 (DDB1) gene. J. Biol. Chem. 27741183-41191. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.








