Abstract
Toxoplasma gondii is an obligate intracellular parasite that resides in the cytoplasm of its host in a unique membrane-bound vacuole known as the parasitophorous vacuole (PV). The membrane surrounding the parasite is remodeled by the dense granules, secretory organelles that release an array of proteins into the vacuole and to the PV membrane (PVM). Only a small portion of the protein constituents of the dense granules have been identified, and little is known regarding their roles in infection or how they are trafficked within the infected host cell. In this report, we identify a novel secreted dense granule protein, GRA14, and show that it is targeted to membranous structures within the vacuole known as the intravacuolar network and to the vacuolar membrane surrounding the parasite. We disrupted GRA14 and exploited the knockout strain to show that GRA14 can be transferred between vacuoles in a coinfection experiment with wild-type parasites. We also show that GRA14 has an unexpected topology in the PVM with its C terminus facing the host cytoplasm and its N terminus facing the vacuolar lumen. These findings have important implications both for the trafficking of GRA proteins to their ultimate destinations and for expectations of functional domains of GRA proteins at the host-parasite interface.
Capable of infecting essentially any warm-blooded vertebrate, Toxoplasma gondii is one of the most successful pathogens on the planet (20, 39). Toxoplasma infects nearly one-third of the human population and causes potentially fatal disease in immunocompromised individuals and congenitally infected neonates (20). This protozoan parasite also causes ocular disease in immunocompetent individuals who are either congenitally or postnatally infected (46). As an obligate intracellular parasite, Toxoplasma enters the host cell into a nonfusogenic vacuole (the parasitophorous vacuole [PV]), in which the parasite replicates in the cytoplasm of its host. The PV membrane (PVM) is porous to small molecules (less than 1,300 Da) but otherwise serves as a boundary between the host and parasite during its intracellular survival (36).
Toxoplasma invasion is mediated by a trio of specialized secretory organelles, named the micronemes, rhoptries, and dense granules, which contribute to the parasite's ability to initiate and sustain infection within its host. The first proteins secreted are from the micronemes, which release molecular adhesins that interact with the parasite's actin-myosin motor to provide the driving force for invasion (24). The rhoptries are then released and help to establish the nascent PV and modulate host cell processes (4). Lastly, proteins from the dense granules that are implicated in the remodeling and maintenance of the PV for intracellular survival are secreted (29).
The precise role of dense granule proteins (GRAs) in the T. gondii life cycle is still largely unknown. To date, two groups of GRA proteins have been identified. The first group contains proteins that lack homology to organisms other than closely related apicomplexan parasites, such as Neospora caninum. The second group contains proteins that have homologues in other eukaryotes, including NTPases and serine protease inhibitors (29). Following release from the dense granules, soluble GRA proteins are delivered to the lumen of the PV, whereas membrane-associated GRAs are trafficked to the PVM and/or the intravacuolar network (IVN), a structure that forms at the posterior end of the parasite and unfolds throughout the lumen of the vacuole (40). The IVN has been hypothesized to be involved in PV expansion and upkeep by increasing the surface area for exchange of nutrients between the parasite and its host cell (1). In addition, microscopy studies have suggested that the IVN serves as a mechanical support network for the parasite inside the PV (28). The dense granule proteins GRA2, GRA4, GRA6, and GRA9 all traffic exclusively to the IVN. GRA4 and GRA6 are likely anchored to the IVN via transmembrane domains, whereas GRA2 and GRA9 contain hydrophobic alpha helices that are proposed to facilitate IVN membrane interaction (29). At least two of these IVN-associating proteins, GRA2 and GRA6, are necessary for network biogenesis, as shown by gene knockout studies (30). In contrast, GRA3, GRA5, GRA7, and GRA8 traffic to the PVM, with GRA3 and GRA7 also partially trafficking to the IVN (29). These PVM-associated GRAs are predicted type I transmembrane proteins that span the PVM and thus can contact both the lumen of the PV and the host cytoplasm.
Transmembrane GRA proteins have unique biochemical properties that facilitate storage within the dense granules and release into the PV. Typically containing signal peptides, GRA proteins are first synthesized in the secretory pathway and then targeted to the dense granules, the default targeting pathway for T. gondii secretory proteins (23). Protein storage within the cores of the dense granules is believed to be achieved by the formation of high-molecular-weight GRA complexes, which explains how these proteins are able to mask their transmembrane domains and thus be stored internally in the organelle (6). Rather than classical vesicularly based trafficking, transmembrane GRA proteins are released as soluble proteins and then trafficked to the PVM and/or IVN by an unknown mechanism (26, 27).
To date, GRA5 is the only transmembrane dense granule protein for which topology in the PVM has been directly demonstrated (27). GRA5 is secreted and trafficked to the PVM, where its N terminus is exposed to the host cell cytosol and its C terminus to the PV lumen. Rhoptry proteins have also been shown to associate with the PVM, where ROP2's N terminus and ROP5's C terminus are exposed to the host cell cytosol (2, 15). While ROP2 family proteins (including ROP5) were initially thought to be transmembrane proteins, it now appears that they may instead be soluble proteins that are secreted into the host cell and subsequently attach to the cytoplasmic face of the PVM by an unknown mechanism (9, 15). GRA and ROP proteins that localize to the PVM have been directly implicated in interacting with their host cells. ROP2 has been shown to interact with host cell mitochondria (41), whereas GRA7 contributes to the delivery of host cell lysosomal compartments to the PV (10). Despite these examples, many fundamental questions regarding the precise roles of proteins associated with the PVM have yet to be answered.
In this study, we report the identification of a novel dense granule protein, GRA14, which is secreted into the vacuole and traffics to both the PVM and IVN. GRA14 colocalizes with other GRA proteins on PVM extensions, where GRA14-positive extensions were found to connect neighboring PVs. We disrupted the GRA14 gene and used the Δgra14 strain to demonstrate that GRA14 can be transferred between vacuoles during infection, a process we call intervacuolar transport. Moreover, we found that the topology of GRA14 in the PVM is opposite to that of GRA5, with its C terminus facing the host cytoplasm and its N terminus facing the vacuolar space. This finding indicates that the larger N-terminal domain of GRA14 functions in the lumen of the PV and provides a foundation for studying features of GRA proteins that dictate topology in the PVM.
MATERIALS AND METHODS
Host cell and parasite cultures.
RHΔhpt (parental) and modified strains of T. gondii were used to infect human foreskin fibroblasts (HFFs). The HFFs were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and 2 mM glutamine and maintained as previously described (4).
Antibodies.
The primary antibodies used in Western blot analysis and immunofluorescence assays (IFAs) were polyclonal anti-GRA3 (33), anti-GRA7 monoclonal antibody 12B6, anti-ROP7 monoclonal antibody 1B10, polyclonal anti-SAG1 (7), anti-ROP2/3/4 monoclonal antibody 4A7 (34), anti-ROP1 monoclonal antibody TG 49 (37), anti-hemagglutinin (HA) monoclonal antibody (Covance), and polyclonal anti-HA rabbit antibody (Invitrogen). Two of the monoclonal antibodies used here are newly described. 12B6 was generated from immunization with a mixed fraction of Toxoplasma organelles as described previously (12). 1B10 was generated from monoclonal antibodies prepared against purified rhoptries (18). The antibodies were used to immunoaffinity purify the proteins from Toxoplasma lysates, and the identities of the proteins were determined by mass spectrometry.
Generation of polyclonal antiserum to a recombinant portion of GRA14.
The region of GRA14 encoding residues 36 to 282 was PCR amplified from a template of T. gondii strain RH genomic DNA with the forward primer CACCGCCAGTTTGGAGCAGACAATTTCC and the reverse primer CCACCACGTCCTCCGCAGCTT and subcloned into the pET161GW-D-TOPO vector, which adds a C-terminal His6 tag for purification (Invitrogen). Following sequencing, the plasmid was transformed into Escherichia coli BL21(DE3) cells and grown to an A600 of 0.6 to 0.8 before the bacteria were induced with 1 mM isopropyl-1-thio-d-galactopyranoside for 5 h. Recombinant GRA14 (residues 36 to 282) containing the His6 tag was purified using Ni-nitrilotriacetic acid-agarose chromatography under denaturing conditions and eluted using a low pH according to the manufacturer's guidelines (Qiagen). The resulting purified recombinant protein was dialyzed in phosphate-buffered saline (PBS), and ∼50 μg of protein was injected per immunization into BALB/c mice (Charles River) on a 21-day immunization schedule. The resulting mouse polyclonal antiserum was collected and tested by Western blot analysis and IFA.
Western blot analysis.
Parental- and modified-strain whole-cell lysates were separated by 12% or 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis as previously described (4). Samples were transferred to nitrocellulose overnight and probed with primary antibodies (see above). For all secondary-antibody incubations, horseradish peroxidase-conjugated goat anti-mouse or goat anti-rabbit antibodies (Chemicon Laboratories) were used at a 1:2,000 dilution. Following secondary incubation, an ECL detection kit was used for the detection of horseradish peroxidase activity (Amersham Biosciences).
IFA and fluorescence microscopy.
For IFAs, Toxoplasma strains were used to infect the coverslips of HFFs for 16 to 24 h. The coverslips were then fixed in either 3.5% formaldehyde/PBS for 15 min, 100% methanol for 3 min, or 100% acetone for 5 min. For formaldehyde-fixed coverslips, the formaldehyde was quenched in PBS plus 100 mM glycine for 5 min. The coverslips were washed in PBS and blocked in PBS/3% bovine serum albumin (BSA). For complete permeabilization of formaldehyde-fixed samples, cells were permeabilized in a solution containing PBS/3% BSA/0.1% Triton X-100 for 15 min. Samples were then incubated with primary antibody in PBS/3% BSA or PBS/3% BSA/0.1% Triton X-100 for 1 h. The samples were then washed five times in PBS and incubated with the secondary antibodies Alexa 488-conjugated goat anti-mouse and/or Alexa 594-conjugated goat anti-rabbit (Molecular Probes) diluted 1:2,000 in PBS/3% BSA. To detect PVM extensions, cells were incubated in 0.01% or 0.05% saponin for blocking and primary incubations. For the detection of evacuoles, cytochalasin D-treated parasites were prepared as previously described (19). Following secondary washes, the coverslips were mounted onto microscope slides with mounting medium (Vectashield), and the fluorescence was observed using a Zeiss upright light microscope (Zeiss Axio Imager Z1) with a 100× oil immersion objective. All images were rendered using the Zeiss Axiovision software and a Zeiss digital charge-coupled device camera (AxioCam MRm).
Selective permeabilization of infected host cells for topology determination.
For selective permeabilization conditions, infected cells were fixed in PBS/3.5% formaldehyde, blocked in PBS/3% BSA, and then treated with the detergent saponin ranging from 0.001% (which leaves the PVM intact) to 0.15% (which fully permeabilizes the PVM) in PBS/3% BSA and treated as described above for IFA conditions. For digitonin permeabilization conditions, infected host cells were treated as described previously (2).
GRA14 gene sequencing.
The entire GRA14 coding region (which codes for a 409-amino-acid protein) was PCR amplified from RH strain genomic DNA using the forward primer TCTAGAGGCGACACAAGAGAGTCCTTC and the reverse primer GCGGCCGCTTCGCTTGGTCTCTGGTAGCC, cloned into the TOPO 2.1 vector (Invitrogen), and sequenced.
Generation of GRA14-HA parasites.
To epitope tag GRA14, the entire GRA14 coding region plus 1.5 kb upstream of GRA14 were PCR amplified using the forward primer TCTAGAGGCGACACAAGAGAGTCCTTC and the reverse primer GCGGCCGCTTCGCTTGGTCTCTGGTAGCC and cloned into the pGRA-HA_HPT vector, which adds a C-terminal HA tag. The construct was linearized by NotI restriction digestion, and 30 μg of DNA was transfected by electroporation into T. gondii strain RHΔhpt. The transfected parasites were grown in medium containing 50 μg/ml mycophenolic acid (MPA) and 50 μg/ml xanthine, and selected parasites were cloned. To assess GRA14 targeting, complemented parasites were analyzed by IFA with rabbit polyclonal HA antibody and anti-GRA7 monoclonal antibody 12B6. To confirm that GRA14-HA was expressed at levels similar to those of the endogenous GRA14 protein, parental lysates and GRA14-HA lysates were subjected to Western blot analysis and probed with anti-GRA14 polyclonal antisera.
Deletion of the gene encoding GRA14.
For generation of a GRA14 deletion vector, upstream DNA regions (PCR amplified with the forward primer CCGGGGCGGCCGCTTGTGCGCTACAGAGGTGTTG and the reverse primer ACGTACTAGTGTGCTTAGCAGTGGGAACCTC) and downstream DNA regions (amplified with the forward primer GTGGGTACCCTTAGGTTTATATTC and the reverse primer CGCCGGTACCGCTGCAGATGTGTCAGAGCTT) flanking the sequence encoding GRA14 were amplified from T. gondii strain RH genomic DNA and subcloned into the pMini-GFP.hh vector (22). This vector contains the selectable marker hypoxanthine-xanthine-guanine phosphoribosyl transferase (HPT) gene and a green fluorescent protein (GFP) cassette located downstream for negative selection of heterologous recombinants. The 5′ flank was inserted using NotI and SpeI, and the 3′ flank was inserted using SmaI. The final construct, pGRA14 KO, was linearized by NotI digestion, and 30 μg of DNA was transfected by electroporation into T. gondii strain RHΔhpt. For selection of transformants, the transfected parasites were grown in medium containing 50 μg/ml MPA and 50 μg/ml xanthine. After 8 days of selection, the parasites were cloned by limiting dilution. GFP-negative parasites were then screened by IFA and confirmed by Western blot analysis using anti-GRA14 polyclonal antisera.
To remove the selectable marker HPT, the pGRA14 KO vector was digested with SpeI and RsrII to remove HPT, blunted by treatment with Klenow enzyme (New England Biotechnology), and recircularized by ligation. The resulting pHPT-KO vector was linearized by NotI digestion and transfected into ΔGRA14_HPT+ parasites. Selection for the absence of HPT was achieved using 200 μg/ml 6-thioxanthine (Sigma) for 4 weeks, after which the parasites were cloned by limiting dilution. The clones were then screened for the absence of GFP and tested for the inability to survive in medium containing 50 μg/ml MPA and 50 μg/ml xanthine.
Electron microscopy.
For immunoelectron microscopy, HFF monolayers were infected with T. gondii stably expressing GRA14-HA and allowed to grow for 36 h before fixation in 4% formaldehyde with 0.02% glutaraldehyde buffered in 100 mM HEPES, pH 7.2. Following fixation, samples were dehydrated in a graded ethanol series and embedded in LR White resin (EMS, Hatfield, PA) using a microwave-assisted protocol as previously described (47). Ultrathin sections (70 μm) were prepared using an Ultracut S ultramicrotome (Leica Microsystems, Deerfield, IL) and placed on specimen grids. The sections were quenched with PBS/0.15% glycine and blocked in PBS/1% BSA before serial incubation with the HA monoclonal antibody, an anti-mouse bridging antibody, and 10 nm protein A-gold (University of Utrecht, Utrecht, The Netherlands). The labeled sections were contrasted with 5% uranyl acetate and lead citrate before examination by transmission electron microscopy in a CM120 BioTwin (FEI, Hillsboro, OR) operating at 80 kV. Images were collected on negative film, digitally scanned, and prepared for publication with Adobe Photoshop Elements 4.0. Manipulations of the images (other than cropping and resizing) consisted of adjustments to the brightness and contrast applied uniformly to the entire image field.
Samples for transmission electron microscopy of RH and a Δgra14 strain were prepared and processed as previously described (43).
Generation of a Δgra14/GFP+ parasite line.
To generate Δgra14 parasites expressing GFP, 30 μg of NotI-digested pGRA_GFP vector (25) to which HPT had been added was transfected into Δgra14 strain parasites. The transfected parasites were selected using MPA-and-xanthine medium and cloned as described above. To confirm the expression of GFP, the parasite clones were analyzed by fluorescence microscopy.
Intervacuolar-transport coinfection assay.
To assess GRA14 intervacuolar transport, equal numbers of Δgra14/GFP+ and RHΔhpt parasite lines were mixed and then used to infect HFF monolayers for 16 to 24 h and subjected to IFAs as described above.
Triton X-114 phase partitioning.
To assess whether GRA14 partitions with membrane or soluble fractions, 4 × 107 parasites were subjected to Triton X-114 phase partitioning (38). Extracellular parasites were pelleted at 1,000 × g, washed in PBS, and lysed in 1% Triton X-114/10 mM Tris (pH 7.5)/5 mM NaCl for 30 min at 4°C. Following a low-speed spin at 2,500 × g to remove insoluble material, the lysates were partitioned as previously described (38).
Carbonate extraction of extracellular T. gondii.
To assess the solubility of GRA14 inside the dense granules, extracellular parasites were fractionated as previously described (27, 40). The cell pellets were resuspended in sodium carbonate (pH 11.5) with protease inhibitors (Roche) and sonicated using three 30-s pulses. The lysates were then placed on ice for 30 min and cleared by low-speed centrifugation at 2,500 × g. The low-speed supernatant was then fractionated by centrifugation at 150,000 × g for 1.5 h, and the resulting high-speed supernatant and pellet were analyzed by Western blot analysis.
Cell fractionation and carbonate extraction of secreted GRA14 from intracellular T. gondii parasites.
To assess the solubility of secreted GRA14 on vacuolar membranes, infected cells were fractionated as previously described (27, 40). The cells were first infected with either T. gondii strain GRA14-HA or RH for 24 h and then scraped and passed through a 27-gauge needle. The lysates were cleared at 2,500 × g, and the resulting low-speed supernatant was fractionated by centrifugation at 150,000 × g for 1.5 h. The high-speed pellet was then resuspended in sodium carbonate (pH 11.5), incubated on ice for 30 min, and centrifuged at 150,000 × g for 1 h. The resulting supernatant and pellet were then analyzed by Western blot analysis.
Nucleotide sequence accession number.
The sequence of the GRA14 coding region was submitted to GenBank and given accession number FJ015061.
RESULTS
Identification of a novel dense granule protein, GRA14.
Our proteomic analysis of the T. gondii rhoptries identified 38 novel proteins. Rhoptry localization for 11 of these was verified by antibody production or epitope tagging (5). In this proteomic study, we reported that our rhoptry fractions also contained a small amount of highly expressed dense granule proteins, including NTPase, GRA3, and GRA7. We suspected that one of the novel proteins identified, gene model 49.m03169 (Fig. 1A), might be a dense granule protein because only five peptides were identified in the proteomic analysis, yet a large number of corresponding expressed sequence tags (>100) indicated that it is a highly expressed gene (3). Computational analysis of 49.m03169 revealed that the protein lacks homology to known proteins and contains a predicted signal peptide and transmembrane domain, indicating that it is a type I transmembrane protein originating from one of the secretory organelles (16, 44). This initial analysis suggested that the secreted protein would reside on the PVM and/or IVN following release from the rhoptries or dense granules.
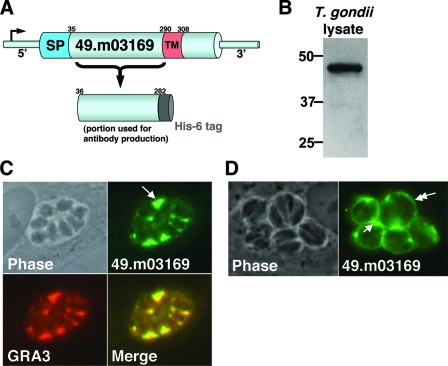
FIG. 1.
Identification of a novel dense granule protein, GRA14. (A) Schematic of gene model 49.m03169, which depicts a predicted signal peptide (SP) and transmembrane domain (TM) within the protein's primary sequence. Also indicated are the residues in the N-terminal region used to generate a His6 recombinant protein for polyconal antiserum production. (B) 49.m03169 antiserum detects a band in Toxoplasma cell lysates at 47 kDa by Western blot analysis. (C) IFA with 49.m03169 antiserum showing strong immunoreactivity in the PV (arrow) and colocalization with GRA3, indicating that 49.m03169 is a novel dense granule protein (GRA14). (D) IFA of infected cells fixed with acetone showing GRA14 on the rim of the PVM (double arrow) and in the PV (single arrow), indicating that GRA14 partially traffics to the PVM.
To resolve whether the protein encoded by gene model 49.m03169 localized to the rhoptries or was a dense granule contaminant of our fraction, residues 36 to 282 were expressed as a His6 fusion in E. coli, purified by Ni-agarose chromatography, and used for antibody production (Fig. 1A). The resulting polyclonal antibody was used to probe a Western blot of Toxoplasma total cell lysate and detected a band at 47 kDa (Fig. 1B), whereas the predicted mass of the protein lacking its signal peptide is 41 kDa. We suspect that this difference in migration was at least partially due to a feature(s) in the protein's primary sequence, as the recombinant protein expressed in E. coli also showed aberrant migration by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (data not shown). To address subcellular localization, 49.m03169 antiserum was used for IFAs where strong immunoreactivity in the PV was found, indicating that 49.m03169 is a dense granule protein (Fig. 1C). To confirm dense granule localization, we costained with an anti-GRA3 antibody and found completely overlapping staining patterns. These results indicate that 49.m03169 is a dense granule protein, which we therefore named dense granule protein 14 (GRA14). The staining within the PV and the presence of a predicted transmembrane domain indicate that GRA14 localizes to the IVN (Fig. 1C). To address whether GRA14 is also localized to the PVM, we fixed infected cells with acetone, a treatment that removes lipids and improves the detection of proteins on the PVM, and found that GRA14 also localizes to the PVM (Fig. 1D). These data indicate that GRA14, like GRA3 and GRA7, is released from the dense granules and traffics to both the PVM and IVN.
Epitope-tagged GRA14 traffics to the PV.
To study GRA14 trafficking and topology, we engineered a version of GRA14 containing a C-terminal HA tag. The GRA14-HA expression construct contained the GRA14 promoter (included in a 1.5-kb region of DNA upstream of GRA14), the entire GRA14 coding sequence, a C-terminal HA tag, the GRA2 3′ untranslated region, and the selectable marker HPT (diagrammed in Fig. 2A). Following transfection of the linearized construct into RHΔhpt parasites and selection with MPA and xanthine, several stable clones were tested for HA expression by Western blot analysis and IFA. Similar to endogenous GRA14, GRA14-HA trafficked to the PV and colocalized with the dense granule protein GRA7 (Fig. 2B).
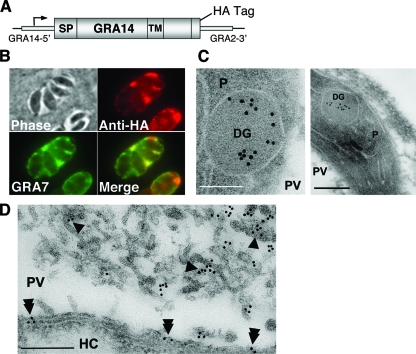
FIG. 2.
Epitope-tagged GRA14 traffics to the PV and localizes to the IVN and PVM. (A) Diagram of the GRA14-HA construct showing that expression is driven from the endogenous promoter and indicating the position of the HA epitope tag at the extreme C terminus of the protein. A heterologous GRA2 3′ untranslated region was used, and the selectable marker HPT is not shown. (B) IFA of infected cells (with a clonal parasite line stably expressing GRA14-HA) demonstrating that GRA14-HA traffics correctly to the PV and colocalizes with GRA7. (C and D) Immunoelectron microscopy was used to examine cells infected by GRA14-HA parasites. P, parasite; DG, dense granule; HC, host cell. (C) GRA14-HA labeling was found inside the cores of the dense granules. Two different parasite images are shown (scale bars, 100 nm in the first image and 200 nm in the second image). (D) GRA14-HA labeling was also found on the IVN (single arrowhead) and on the PVM (double arrowhead) (scale bar, 200 nm).
GRA14-HA is detected in the dense granules and on the IVN and PVM by immunoelectron microscopy.
To more precisely study GRA14's localization, we used immunoelectron microscopy to examine cells infected by GRA14-HA parasites. As shown for other transmembrane GRA proteins, GRA14 was found inside the cores of the dense granules, confirming localization to this compartment within the parasite (Fig. 2C). The most abundant GRA14 labeling was found inside the PV on the IVN, where GRA14-HA appeared to localize throughout the network (Fig. 2D). Consistent with our IFA data, labeling was also found on the PVM (Fig. 2D). Taken together, these data confirm that GRA14 localization is similar to that of GRA3 and GRA7, which are found on both the PVM and IVN.
Targeted disruption of the gene encoding GRA14.
To better understand the function of GRA14, we disrupted the GRA14 gene by homologous recombination in RHΔhpt parental-strain parasites (13). The GRA14 knockout plasmid utilizes the selectable marker HPT, surrounded by ∼4 kb of GRA14 5′ flanking and 3 kb of GRA14 3′ flanking regions. In addition, the construct contains a downstream GFP marker to select for homologous recombinants (22) (Fig. 3A, GRA14 KO plasmid). The knockout plasmid was transfected into RHΔhpt strain parasites, selected for HPT with MPA and xanthine, and cloned by limiting dilution. Five GFP-negative clones were tested by IFA for GRA14 immunoreactivity (Fig. 3B), four of which showed no staining by IFA. One of these was selected and tested for GRA14 expression by Western blot analysis (Fig. 3C), which showed the lack of GRA14 expression and confirmed the successful generation of a Δgra14_ HPT+ strain.
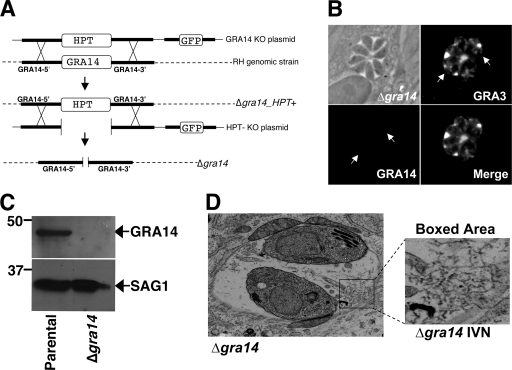
FIG. 3.
Targeted disruption of the gene encoding GRA14. (A) Schematic depicting the GRA14 knockout strategy. Using homologous recombination, the GRA14 coding region was replaced with the selectable marker HPT. After the generation of ΔGRA14_HPT+ clonal parasites, the HPT marker was removed by a second round of homologous recombination to generate the Δgra14strain. (B) IFA of cells infected with Δgra14 parasites does not show staining with GRA14 polyclonal antiserum (the arrows indicate the positions of the vacuole) but does show GRA3 staining in the PV (positive control). (C) Western blot analysis of parental and Δgra14 parasite lysates showing that GRA14 is not present in Δgra14 lysates. SAG1 was used as a loading control. (D) Using transmission electron microscopy, Δgra14 parasites were analyzed for ultrastructural defects in the PV. No gross changes in the IVN structure (boxed area), PVM morphology, or host organelle association were observed.
To exclude possible effects of the HPT marker and to enable complementation using HPT, we transfected Δgra14_HPT+ parasites with a second plasmid that contained the GRA14 flanks but lacked the selectable marker HPT (Fig. 3A, HPT- KO plasmid). The transfected parasites were selected against HPT with 6-thioxanthine and cloned by limiting dilution. The resulting Δgra14_HPT− line (hereafter referred to as Δgra14) was tested for growth under positive selection for HPT, where no growth was observed.
GRA14 is not essential for growth in vitro or virulence in vivo in RH strain parasites.
We found that Δgra14 parasites do not display a severe growth defect in vitro. To assess more subtle growth differences in Δgra14 parasites versus the parental strain, we used a competition growth assay (18) and found that the parental strain and the Δgra14 strain parasites grew with approximately the same kinetics (data not shown). As GRA14 localizes to both the PVM and the IVN, we examined whether Δgra14 parasites displayed any ultrastructural defects in the PV by transmission electron microscopy (Fig. 3D). In contrast to Δgra2 and Δgra6 strains, which show a scrambled phenotype in the IVN (30), we found no apparent defects in the IVN in Δgra14 parasites. Similarly, no noticeable changes in PVM morphology or host organelle association were observed. Lastly, we assessed in vivo virulence by injecting mice with either the Δgra14 strain or the RHΔhpt parental strain (one to four parasites) and found that the two strains killed the mice with approximately the same kinetics (data not shown).
GRA14 is detected on PVM extensions connecting neighboring vacuoles and colocalizes with PVM-associating GRA proteins.
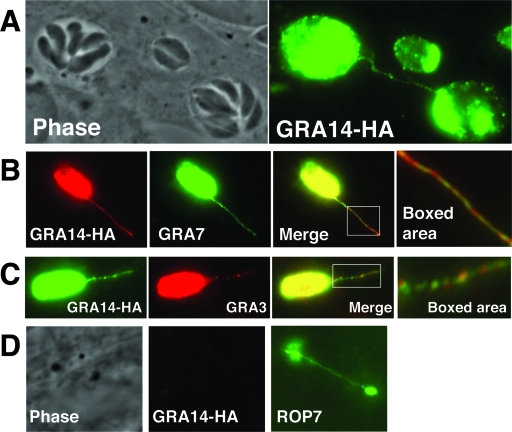
The PV contains membranous strand-like extensions (called PVM extensions) that are decorated with GRA proteins that extrude into the host cell cytoplasm (14). The composition and function of these extensions is not understood, but secreted proteins that localize to PVM extensions include GRA3 and GRA7 (14, 21). In GRA14 localization experiments, we also observed immunoreactivity on PVM extensions using GRA14 antibodies or HA antibodies in the GRA14-HA strain (Fig. 4A). These GRA14-positive extensions colocalized with the known PVM markers GRA7 (Fig. 4B) and GRA3 (Fig. 4C). GRA14 did not completely overlap with either GRA3 or GRA7 on these extensions, as several areas on GRA14-positive strands contained distinct GRA14 puncta (Fig. 4B and C, boxed area). As these extensions are reminiscent of Toxoplasma evacuoles (19), we investigated whether GRA14 is found on evacuoles using cytochalasin D-arrested parasites in early-invasion assays. Using ROP7 (a rhoptry marker) as a control for evacuole detection, we found that GRA14 is not present in evacuoles (Fig. 4D), which suggests that PVM extensions are a feature of the developing or mature vacuole. In addition, GRA14 is not necessary for the formation of PVM extensions, as Δgra14 parasites have similar numbers of PVM extensions, as assessed by GRA7 staining (data not shown). Occasionally, we observed neighboring vacuoles that were connected by GRA14-positive extensions (Fig. 4A), raising the question of whether GRA proteins are transferred between vacuoles in infected cells.
FIG. 4.
GRA14 is detected on PVM extensions connecting neighboring vacuoles and colocalizes with PVM-associating GRA proteins. (A) Cells were infected with GRA14-HA parasites for 24 h and then analyzed by IFA. GRA14-HA immunoreactivty was found on PVM extensions that connected neighboring vacuoles. (B) GRA14-HA colocalizes with GRA7 on PVM extensions but does not appear to perfectly overlap, as distinct GRA14 and GRA7 puncta were observed (boxed area). (C) GRA14-HA-positive PVM extensions also colocalized with GRA3 and, like GRA7, did not perfectly overlap with GRA14 (boxed area). (D) GRA14-HA is not detected in evacuoles generated by cytochalasin D-arrested parasites. Evacuoles are seen with ROP7 antibody staining (positive control), but not with GRA14-HA staining.
Intervacuolar GRA14 transport between neighboring vacuoles.
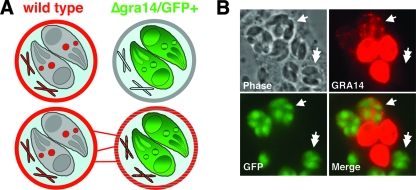
The observation of PVM extensions connecting neighboring vacuoles (Fig. 4A) led us to hypothesize that Toxoplasma is capable of transferring protein between neighboring PVs, either by connections made by vacuolar extensions or by some unidentified mechanism. To address this, we engineered Δgra14 parasites to express GFP (25). The resulting Δgra14/GFP+ parasites were used in a coinfection experiment with RHΔhpt parasites. We then assessed whether GRA14 could be transferred from vacuoles formed by wild-type parasites to those formed by Δgra14/GFP+ parasites (schematic in Fig. 5A). Conversely, if this process were not occurring, then Δgra14/GFP+ vacuoles would be completely devoid of GRA14 staining.
FIG. 5.
GRA14 is transferred to neighboring vacuoles. (A) Cartoon depicting two possible fates of GRA14 in a coinfection with wild-type and Δgra14/GFP+ parasites. (Top) A situation where GRA14 is not transferred to neighboring Δgra14/GFP+ vacuoles. (Bottom) Intervacuolar GRA14 transfer by either PVM extensions or another mechanism. (B) GRA14 signal (red) was seen in neighboring vacuoles formed by Δgra14/GFP+ parasites (single arrows), where intervacuolar transfer occurred in ∼5 to 10% of Δgra14/GFP+ vacuoles. Vacuoles formed by Δgra14/GFP+ parasites that were not in direct contact with wild-type vacuoles did not show intervacuolar protein transport (double arrows).
Using the coinfection assay described above, we found that wild-type PVs do indeed transfer GRA14 to neighboring vacuoles formed by Δgra14/GFP+ parasites (Fig. 5B). Intervacuolar transfer occurred in ∼5 to 10% of Δgra14/GFP+ vacuoles and, as expected, was variable depending on the multiplicity of infection. We also imaged several Δgra14/GFP+ vacuoles that were not in direct contact with wild-type vacuoles; these vacuoles showed no GRA14 signal (Fig. 5B). We were unable to detect GRA14 signal in Δgra14/GFP+ vacuoles that were connected to wild-type vacuoles via PVM extensions. However, this was likely due to the inability to detect small amounts of GRA14 transferred by these extensions. Moreover, the vacuole size (ranging from 2 to 8 parasites per vacuole) did not appear to affect either the ability of wild-type parasites to transfer GRA14 or that of Δgra14/GFP+ vacuoles to receive GRA14, suggesting that this process occurs throughout the lytic cycle of the intracellular tachyzoite T. gondii. These findings demonstrate that Toxoplasma has the ability to transfer a component(s) of the PV to neighboring parasite vacuoles. To our knowledge, this is the first demonstration of GRA protein transfer between two PVs, a process we now refer to as intervacuolar transport.
GRA14 behaves as an integral membrane protein by biochemical fractionation.
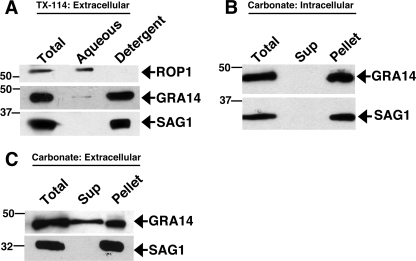
As GRA14 contains a predicted transmembrane domain and is detected on the PVM and IVN, we investigated whether GRA14 behaves as an integral membrane protein by TX-114 phase partitioning (38) (Fig. 6A). We found that GRA14 is tightly associated with membranes, as the vast majority of the protein is found in the TX-114 detergent phase, along with the GPI-anchored surface antigen SAG1, whereas the soluble rhoptry protein ROP1 is found exclusively in the aqueous fraction. We next investigated whether secreted GRA14 behaves as an integral membrane protein by carbonate extraction, a method which solubilizes proteins loosely associated with membranes. Using a fractionation method that enriches for secreted GRA proteins on vacuolar membranes (40), we found that secreted GRA14 is also membrane associated in carbonate (pH 11.5), displaying a fractionation pattern identical to that seen for the membrane-anchored SAG1 (Fig. 6B). Taken together, these results demonstrate that GRA14 is a transmembrane protein anchored in the PVM and IVN.
FIG. 6.
GRA14 behaves as an integral membrane protein by biochemical fractionation. (A) Triton X-114 phase partitioning showing that GRA14 is found preferentially in the detergent (membrane) fraction with the GPI-anchored SAG1, whereas the soluble marker ROP1 is found in the aqueous (soluble) phase. (B) Cell fractionation and carbonate extraction of 24-h-infected cells revealed that secreted GRA14 is tightly associated with membranes, along with the GPI-anchored SAG1. Sup, supernatant. (C) Carbonate extraction of extracellular parasites revealed that a portion of GRA14 partitions with the soluble fraction, whereas the membrane protein SAG1 is completely membrane associated. The GRA14-HA strain was used for panels A and C, and RH strain parasites were used for panel B.
As GRA14 appears to be stored internally in the dense granules (Fig. 2C), we also used carbonate extraction of extracellular parasites to investigate whether GRA14 is membrane associated within the organelle. We found that a fraction of stored GRA14 is solubilized by carbonate treatment (Fig. 6C). These results show that within the dense granules, a fraction of GRA14 is not tightly associated with membranes. Similar fractionation patterns are seen with the transmembrane proteins GRA4, GRA5, and GRA6, which are stored in both soluble and insoluble forms within the dense granules (26, 27).
GRA14 is inserted into the PVM as an integral membrane protein with its C terminus facing the host cell cytoplasm.
The majority of GRA proteins identified to date appear to be classic type I transmembrane proteins that contain a signal peptide and a transmembrane domain located in the C-terminal third of the protein. The topology in the PVM has been shown directly for GRA5, in which the N-terminal region faces the host cytoplasm and the C-terminal region faces the PV lumen (27). The similar structures of other predicted type I transmembrane GRA proteins suggest a uniform topology. To determine the topology of GRA14 in the PVM, we used the detergent saponin to selectively permeabilize the host plasma membrane, but not the PVM. Using sensitive antibodies against the parasite surface antigen SAG1 (to test whether the PVM was permeabilized), we found that saponin concentrations in the range of 0.001% to 0.005% were able to selectively permeabilize the host cell plasma membrane, but not the PVM. The GRA14-HA strain enabled us to assess GRA14 topology by probing for the N-terminal region of GRA14 with our antibodies against recombinant protein and the C-terminal region with antibodies against the HA tag.
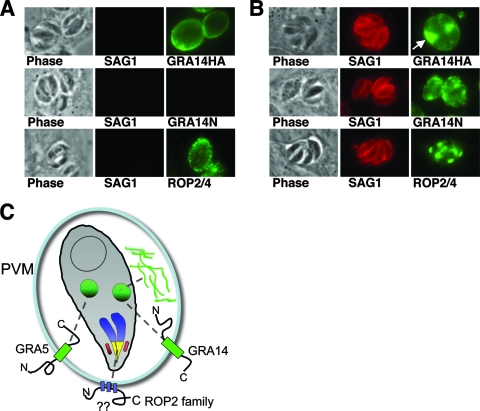
Although the levels of permeabilization across infected coverslips varied, we routinely found SAG1-negative vacuoles that stained in a rim-like pattern at the PVM with the HA antibody, indicating that the GRA14 C terminus was exposed to the host cell cytosol (Fig. 7A). In contrast, we never detected the N-terminal region of GRA14 unless the vacuole was permeabilized and SAG1 staining was also observed (Fig. 7A and B). In these permeabilized vacuoles, internal staining within the PV was also seen (Fig. 7B). Similarly, in separate experiments simultaneously probing for the GRA14 C terminus with HA antibodies and the N terminus with GRA14 antibodies, the N-terminal region could be detected only when the C-terminal region was observed, whereas the C-terminal region could be detected in the absence of N-terminal signal (data not shown). To confirm the specificity of this assay, we also stained saponin-permeabilized cells with antibodies directed against the PVM-associating protein ROP2 (2) and found vacuoles that were ROP2 positive and SAG1 negative (Fig. 7A). The topology of GRA14 in the PVM is not influenced by the HA tag, as endogenous GRA14 can be detected with the N-terminal antibody in wild-type parasites only when SAG1 is also detected (data not shown). In addition, we confirmed this topology using the detergent digitonin, under conditions previously described (not shown) (2). These results demonstrate that GRA14 is anchored in the PVM with its C terminus facing the host cell cytosol and its N terminus facing the lumen of the PV (Fig. 7C).
FIG. 7.
GRA14 is inserted into the PVM as an integral membrane protein with its C terminus exposed to the host cell cytoplasm. (A) Differential permeabilization conditions with 0.001% saponin were used in IFAs of cells infected with GRA14-HA parasites. All cells were stained with SAG1 (to control for permeabilization of the PVM) and stained with either anti-HA or anti-GRA14 antiserum or anti-ROP2. PVs stained with the HA antibody (showing a rim-like pattern) were SAG1 negative, indicating that the GRA14 C terminus is facing the host cell cytosol. GRA14 antiserum (N-terminal) staining was never observed in cells that were also SAG1 negative. ROP2 staining, which showed immunoreactivity on the outside of the PVM (in SAG1-negative vacuoles), was used as a positive control. (B) Infected cells were fully permeabilized in 0.15% saponin and stained with the same antibodies as in panel A. All PVs were SAG1 positive and either HA, GRA14N, or ROP2 positive. The arrow indicates internal staining within the PV. (C) Cartoon showing the topology of the characterized transmembrane-containing GRA proteins and PVM-associating ROP2 family proteins. The question marks represent the uncertainty of the topology of ROP2 family members at the PVM.
DISCUSSION
Proteins secreted from the dense granules are thought to be responsible for remodeling and maintenance of the PV. The majority of characterized GRA proteins lack homology to known proteins and have similar structures consisting of a signal peptide at the N terminus and a single transmembrane domain located in the C-terminal portion of the protein. As the functions of most GRA proteins in the Toxoplasma life cycle are still largely unknown, the discovery of new dense granule proteins promises to offer clues to how these proteins contribute to T. gondii survival in host cells. In this study, we identified a novel dense granule protein, GRA14, which traffics to both the IVN and the PVM. GRA14 localization to the IVN and on the PVM was shown by IFA and confirmed using immunoelectron microscopy. This dual targeting is also seen with the dense granule proteins GRA3 and GRA7, both of which colocalize with GRA14 in the PV (29). Specific signals for targeting to the IVN or PVM have not been identified, but such signals are likely to exist, as some GRA proteins are found exclusively in one compartment or the other (e.g., GRA8 is detected only on the PVM and GRA2 is found only on the IVN) (8, 45).
We have disrupted the GRA14 gene and have seen no apparent phenotype in vitro as assessed by examining growth or PV ultrastructure. This may be due to the growth conditions under which the knockout was tested (e.g., medium conditions and host cell type) or may suggest the existence of a parasite protein with a redundant function. BLAST analysis of the Toxoplasma genome revealed no obvious candidates that might fulfill GRA14's function in its absence. Several other GRA proteins have been disrupted, including GRA2, GRA3, GRA5, GRA6, and GRA7 (10, 11, 30-32). Although none of these GRAs is essential, deletion of GRA2 or GRA6 causes morphological defects in the IVN, deletion of GRA7 results in a growth defect in vitro under limiting nutrient conditions, and the Δgra2 strain is reduced in virulence in vivo. The absence of a phenotype for the Δgra14 strain in vivo may be a result of the extremely high virulence of the RH strain in which the knockout was performed (35). Disruption of GRA14 in less virulent and more physiologically relevant strains will allow assessment of more subtle roles in virulence or alterations in cyst formation or tissue tropism.
We have shown that GRA14 partially colocalizes with the PVM-associating proteins GRA3 and GRA7 on PVM extensions. GRA14 does not appear to perfectly overlap with either of these proteins, which suggests that vesicles comprising these extensions may contain different compositions of GRA proteins. While GRA proteins are believed to be secreted as high-molecular-weight complexes, preliminary immunoprecipitations of GRA14 have not yielded coprecipitating GRA proteins. This is likely due to the stringency of the immunoprecipitations, as these complexes have been detected only in lysates lacking detergents (6).
We have shown that GRA14-positive extensions can connect neighboring PVs, similar to those seen with GRA3 (14). This led us to directly address whether Toxoplasma possesses the ability to transfer vacuolar membrane proteins, such as GRA14, to neighboring PVs. Using a mixing experiment with a Δgra14/GFP+ strain and wild-type strain parasites, we have demonstrated that Toxoplasma can transfer GRA14 between vacuoles. Although transfer was seen only in vacuoles that were in close contact, it seems likely that transport is also occurring in vacuoles connected by PVM extensions but that the levels of transferred protein are below the sensitivity of our detection.
The physiological relevance of Toxoplasma intervacuolar protein transport remains to be elucidated. The fusion of PV-related structures with mature vacuoles has been demonstrated with Toxoplasma evacuoles. Evacuoles are generated by a burst of rhoptry secretion during invasion, and they are able to fuse with nascent or fully formed vacuoles (19). Although evacuoles may contain some constitutively released dense granule proteins, we were unable to detect the presence of GRA14 in these structures. This indicates that intervacuolar protein transport is not related to the process by which evacuoles fuse with preexisting PVs. Furthermore, the amount of GRA14 transferred and the close proximity of the vacuoles we see here indicate that fully formed, closely apposed vacuoles are able to transfer GRA proteins throughout the lytic cycle. Transfer between Toxoplasma PVs is likely to depend on the unique composition of the PVM, as GRA proteins are not readily detected in other cellular compartments outside of the vacuole. A further indication that intervacuolar transfer is limited to Toxoplasma-derived vacuoles is suggested by Coxiella burnetii coinfection experiments, in which GRA3 has been shown to be restricted to Toxoplasma vacuoles and not transferred to those formed by Coxiella (42).
The topology of transmembrane GRA proteins within the PVM dictates whether the protein's functional domain(s) will be exposed to the lumen of the PV or to the host cytosol. We demonstrated here that GRA14 exposes its C terminus to the host cell cytosol and its N terminus to the vacuole lumen, contrary to the topology shown for GRA5. The inverted topology of GRA14 demonstrates that GRA proteins do not adopt a uniform topology in the PVM and that the larger N-terminal domain of GRA14 (∼250 amino acids) functions in the lumen of the PV, whereas the smaller C-terminal portion (∼98 amino acids) is able to access the host cytosol. Both domains may be functional in their respective compartments, or one domain may serve in trafficking or topology while the other is responsible for the function of the protein. Though our carbonate extraction, TX-114 phase partitioning, and topology studies strongly indicate that GRA14 is indeed a transmembrane protein, we cannot exclude the possibility that GRA14 is a globular protein that is somehow secreted to the cytoplasmic face of the PVM with its N-terminal epitopes exposed only in the presence of detergent. This situation has recently been proposed for ROP2 family proteins, which are likely secreted to the outer face of the PVM (15), but GRA14 has a stronger predicted transmembrane domain and appears more similar to the large number of known transmembrane GRA proteins.
The inverted topology of GRA14 may provide clues to how transmembrane GRA proteins are trafficked and inserted into the PVM. If trafficking to the PVM occurs by vesicularly based transport, one might expect all type I transmembrane proteins to adopt the same topology in the PVM as GRA14 (in which vesicles originating from the parasite plasma membrane would fuse with the PVM and invert the protein). Although this model for the trafficking of GRA proteins to the PVM cannot be ruled out, the GRA5 topology and current data regarding GRA protein storage and trafficking do not support it. As we show here for GRA14, and similar to other GRA proteins, storage of transmembrane GRAs appears to be internal, within the dense core of the organelle (Fig. 2C), potentially in oligomeric protein complexes that mask their transmembrane domains (6). Released GRAs are then believed to undergo a conformational change that leads to the exposure of their transmembrane domains and, possibly with the help of vacuolar components (either protein or lipid), leads to protein insertion into the PVM (9). GRA proteins would then be expected to insert into the PVM in an orientation that is based upon the physicochemical properties of the protein. In the case of GRA5, it has recently been shown that the N-terminal region is responsible for proper trafficking to the dense granules and insertion into the PVM (17). Whether this is true for GRA14 and whether the topology of GRA14 or GRA5 is more common among GRA proteins remain to be determined. Regardless, the studies described here open new possibilities for GRA protein function at the PVM and provide a framework for dissecting determinants of GRA protein topology.
Acknowledgments
We thank Debbie Guerrero and Siva Wu for assistance with electron microscopy, Jean Francois Dubremetz for GRA3 and ROP2/3/4 antibodies, Joe Schwartzman for ROP1 antibodies, and John Boothroyd for SAG1 antibodies. We also thank Mike Drummond for his assistance in identifying monoclonal antibodies and the Bradley laboratory for helpful discussions of the manuscript.
This work was supported by a Mason Scholar URSP award to M.E.R., a Microbial Pathogenesis Training Grant (T32-AI07323) to J.M.T., a shared facilities grant from the NIH (5 P-30 DC006276-03) to P.W., and an NIH grant (1R01AI064616) to P.J.B.
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 2 September 2008.
REFERENCES
- 1.Adjogble, K. D., C. Mercier, J. F. Dubremetz, C. Hucke, C. R. Mackenzie, M. F. Cesbron-Delauw, and W. Daubener. 2004. GRA9, a new Toxoplasma gondii dense granule protein associated with the intravacuolar network of tubular membranes. Int. J. Parasitol. 341255-1264. [DOI] [PubMed] [Google Scholar]
- 2.Beckers, C. J., J. F. Dubremetz, O. Mercereau-Puijalon, and K. A. Joiner. 1994. The Toxoplasma gondii rhoptry protein ROP 2 is inserted into the parasitophorous vacuole membrane, surrounding the intracellular parasite, and is exposed to the host cell cytoplasm. J. Cell Biol. 127947-961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boguski, M. S., T. M. Lowe, and C. M. Tolstoshev. 1993. dbEST-database for “expressed sequence tags.” Nat. Genet. 4332-333. [DOI] [PubMed] [Google Scholar]
- 4.Bradley, P. J., and L. D. Sibley. 2007. Rhoptries: an arsenal of secreted virulence factors. Curr. Opin. Microbiol. 10582-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradley, P. J., C. Ward, S. J. Cheng, D. L. Alexander, S. Coller, G. H. Coombs, J. D. Dunn, D. J. Ferguson, S. J. Sanderson, J. M. Wastling, and J. C. Boothroyd. 2005. Proteomic analysis of rhoptry organelles reveals many novel constituents for host-parasite interactions in Toxoplasma gondii. J. Biol. Chem. 28034245-34258. [DOI] [PubMed] [Google Scholar]
- 6.Braun, L., L. Travier, S. Kieffer, K. Musset, J. Garin, C. Mercier, and M. F. Cesbron-Delauw. 2008. Purification of Toxoplasma dense granule proteins reveals that they are in complexes throughout the secretory pathway. Mol. Biochem. Parasitol. 15713-21. [DOI] [PubMed] [Google Scholar]
- 7.Burg, J. L., D. Perelman, L. H. Kasper, P. L. Ware, and J. C. Boothroyd. 1988. Molecular analysis of the gene encoding the major surface antigen of Toxoplasma gondii. J. Immunol. 1413584-3591. [PubMed] [Google Scholar]
- 8.Carey, K. L., C. G. Donahue, and G. E. Ward. 2000. Identification and molecular characterization of GRA8, a novel, proline-rich, dense granule protein of Toxoplasma gondii. Mol. Biochem. Parasitol. 10525-37. [DOI] [PubMed] [Google Scholar]
- 9.Cesbron-Delauw, M. F., C. Gendrin, L. Travier, P. Ruffiot, and C. Mercier. 2008. Apicomplexa in mammalian cells: trafficking to the parasitophorous vacuole. Traffic 9657-664. [DOI] [PubMed] [Google Scholar]
- 10.Coppens, I., J. D. Dunn, J. D. Romano, M. Pypaert, H. Zhang, J. C. Boothroyd, and K. A. Joiner. 2006. Toxoplasma gondii sequesters lysosomes from mammalian hosts in the vacuolar space. Cell 125261-274. [DOI] [PubMed] [Google Scholar]
- 11.Craver, M. P., and L. J. Knoll. 2007. Increased efficiency of homologous recombination in Toxoplasma gondii dense granule protein 3 demonstrates that GRA3 is not necessary in cell culture but does contribute to virulence. Mol. Biochem. Parasitol. 153149-157. [DOI] [PubMed] [Google Scholar]
- 12.DeRocher, A., I. Coppens, A. Karnataki, L. A. Gilbert, M. E. Rome, J. E. Feagin, P. J. Bradley, and M. Parsons. 2008. A thioredoxin family protein of the apicoplast periphery identifies abundant candidate transport vesicles in Toxoplasma. Eukaryot. Cell 71518-1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donald, R. G., and D. S. Roos. 1998. Gene knock-outs and allelic replacements in Toxoplasma gondii: HXGPRT as a selectable marker for hit-and-run mutagenesis. Mol. Biochem. Parasitol. 91295-305. [DOI] [PubMed] [Google Scholar]
- 14.Dubremetz, J. F., A. Achbarou, D. Bermudes, and K. A. Joiner. 1993. Kinetics and pattern of organelle exocytosis during Toxoplasma gondii/host-cell interaction. Parasitol. Res. 79402-408. [DOI] [PubMed] [Google Scholar]
- 15.El Hajj, H., M. Lebrun, M. N. Fourmaux, H. Vial, and J. F. Dubremetz. 2007. Inverted topology of the Toxoplasma gondii ROP5 rhoptry protein provides new insights into the association of the ROP2 protein family with the parasitophorous vacuole membrane. Cell Microbiol. 954-64. [DOI] [PubMed] [Google Scholar]
- 16.Emanuelsson, O., S. Brunak, G. von Heijne, and H. Nielsen. 2007. Locating proteins in the cell using TargetP, SignalP and related tools. Nat. Protoc. 2953-971. [DOI] [PubMed] [Google Scholar]
- 17.Gendrin, C., C. Mercier, L. Braun, K. Musset, J. F. Dubremetz, and M. F. Cesbron-Delauw. Toxoplasma gondii uses unusual sorting mechanisms to deliver transmembrane proteins into the host-cell vacuole. Traffic, in press. [DOI] [PubMed]
- 18.Gilbert, L. A., S. Ravindran, J. M. Turetzky, J. C. Boothroyd, and P. J. Bradley. 2007. Toxoplasma gondii targets a protein phosphatase 2C to the nuclei of infected host cells. Eukaryot. Cell 673-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hakansson, S., A. J. Charron, and L. D. Sibley. 2001. Toxoplasma evacuoles: a two-step process of secretion and fusion forms the parasitophorous vacuole. EMBO J. 203132-3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hill, D. E., S. Chirukandoth, and J. P. Dubey. 2005. Biology and epidemiology of Toxoplasma gondii in man and animals. Anim. Health Res. Rev. 641-61. [DOI] [PubMed] [Google Scholar]
- 21.Jacobs, D., J. F. Dubremetz, A. Loyens, F. Bosman, and E. Saman. 1998. Identification and heterologous expression of a new dense granule protein (GRA7) from Toxoplasma gondii. Mol. Biochem. Parasitol. 91237-249. [DOI] [PubMed] [Google Scholar]
- 22.Karasov, A. O., J. C. Boothroyd, and G. Arrizabalaga. 2005. Identification and disruption of a rhoptry-localized homologue of sodium hydrogen exchangers in Toxoplasma gondii. Int. J. Parasitol. 35285-291. [DOI] [PubMed] [Google Scholar]
- 23.Karsten, V., R. S. Hegde, A. P. Sinai, M. Yang, and K. A. Joiner. 2004. Transmembrane domain modulates sorting of membrane proteins in Toxoplasma gondii. J. Biol. Chem. 27926052-26057. [DOI] [PubMed] [Google Scholar]
- 24.Keeley, A., and D. Soldati. 2004. The glideosome: a molecular machine powering motility and host-cell invasion by Apicomplexa. Trends Cell Biol. 14528-532. [DOI] [PubMed] [Google Scholar]
- 25.Kim, K., M. S. Eaton, W. Schubert, S. Wu, and J. Tang. 2001. Optimized expression of green fluorescent protein in Toxoplasma gondii using thermostable green fluorescent protein mutants. Mol. Biochem. Parasitol. 113309-313. [DOI] [PubMed] [Google Scholar]
- 26.Labruyere, E., M. Lingnau, C. Mercier, and L. D. Sibley. 1999. Differential membrane targeting of the secretory proteins GRA4 and GRA6 within the parasitophorous vacuole formed by Toxoplasma gondii. Mol. Biochem. Parasitol. 102311-324. [DOI] [PubMed] [Google Scholar]
- 27.Lecordier, L., C. Mercier, L. D. Sibley, and M. F. Cesbron-Delauw. 1999. Transmembrane insertion of the Toxoplasma gondii GRA5 protein occurs after soluble secretion into the host cell. Mol. Biol. Cell 101277-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Magno, R. C., L. Lemgruber, R. C. Vommaro, W. De Souza, and M. Attias. 2005. Intravacuolar network may act as a mechanical support for Toxoplasma gondii inside the parasitophorous vacuole. Microsc. Res. Tech. 6745-52. [DOI] [PubMed] [Google Scholar]
- 29.Mercier, C., K. D. Adjogble, W. Daubener, and M. F. Delauw. 2005. Dense granules: are they key organelles to help understand the parasitophorous vacuole of all apicomplexa parasites? Int. J. Parasitol. 35829-849. [DOI] [PubMed] [Google Scholar]
- 30.Mercier, C., J. F. Dubremetz, B. Rauscher, L. Lecordier, L. D. Sibley, and M. F. Cesbron-Delauw. 2002. Biogenesis of nanotubular network in Toxoplasma parasitophorous vacuole induced by parasite proteins. Mol. Biol. Cell 132397-2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mercier, C., D. K. Howe, D. Mordue, M. Lingnau, and L. D. Sibley. 1998. Targeted disruption of the GRA2 locus in Toxoplasma gondii decreases acute virulence in mice. Infect. Immun. 664176-4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mercier, C., B. Rauscher, L. Lecordier, D. Deslee, J. F. Dubremetz, and M. F. Cesbron-Delauw. 2001. Lack of expression of the dense granule protein GRA5 does not affect the development of Toxoplasma tachyzoites. Mol. Biochem. Parasitol. 116247-251. [DOI] [PubMed] [Google Scholar]
- 33.Ossorio, P. N., J. F. Dubremetz, and K. A. Joiner. 1994. A soluble secretory protein of the intracellular parasite Toxoplasma gondii associates with the parasitophorous vacuole membrane through hydrophobic interactions. J. Biol. Chem. 26915350-15357. [PubMed] [Google Scholar]
- 34.Sadak, A., Z. Taghy, B. Fortier, and J. F. Dubremetz. 1988. Characterization of a family of rhoptry proteins of Toxoplasma gondii. Mol. Biochem. Parasitol. 29203-211. [DOI] [PubMed] [Google Scholar]
- 35.Saeij, J. P., J. P. Boyle, S. Coller, S. Taylor, L. D. Sibley, E. T. Brooke-Powell, J. W. Ajioka, and J. C. Boothroyd. 2006. Polymorphic secreted kinases are key virulence factors in toxoplasmosis. Science 3141780-1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwab, J. C., C. J. Beckers, and K. A. Joiner. 1994. The parasitophorous vacuole membrane surrounding intracellular Toxoplasma gondii functions as a molecular sieve. Proc. Natl. Acad. Sci. USA 91509-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwartzman, J. D., and E. C. Krug. 1989. Toxoplasma gondii: characterization of monoclonal antibodies that recognize rhoptries. Exp. Parasitol. 6874-82. [DOI] [PubMed] [Google Scholar]
- 38.Seeber, F., J. F. Dubremetz, and J. C. Boothroyd. 1998. Analysis of Toxoplasma gondii stably transfected with a transmembrane variant of its major surface protein, SAG1. J. Cell Sci. 11123-29. [DOI] [PubMed] [Google Scholar]
- 39.Sibley, L. D. 2003. Toxoplasma gondii: perfecting an intracellular life style. Traffic 4581-586. [DOI] [PubMed] [Google Scholar]
- 40.Sibley, L. D., I. R. Niesman, S. F. Parmley, and M. F. Cesbron-Delauw. 1995. Regulated secretion of multi-lamellar vesicles leads to formation of a tubulo-vesicular network in host-cell vacuoles occupied by Toxoplasma gondii. J. Cell Sci. 1081669-1677. [DOI] [PubMed] [Google Scholar]
- 41.Sinai, A. P., and K. A. Joiner. 2001. The Toxoplasma gondii protein ROP2 mediates host organelle association with the parasitophorous vacuole membrane. J. Cell Biol. 15495-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sinai, A. P., S. Paul, M. Rabinovitch, G. Kaplan, and K. A. Joiner. 2000. Coinfection of fibroblasts with Coxiella burnetti and Toxoplasma gondii: to each their own. Microbes Infect. 2727-736. [DOI] [PubMed] [Google Scholar]
- 43.Sinai, A. P., P. Webster, and K. A. Joiner. 1997. Association of host cell endoplasmic reticulum and mitochondria with the Toxoplasma gondii parasitophorous vacuole membrane: a high affinity interaction. J. Cell Sci. 1102117-2128. [DOI] [PubMed] [Google Scholar]
- 44.Sonnhammer, E. L., G. von Heijne, and A. Krogh. 1998. A hidden Markov model for predicting transmembrane helices in protein sequences. Proc. Int. Conf. Intell. Syst. Mol. Biol. 6175-182. [PubMed] [Google Scholar]
- 45.Travier, L., R. Mondragon, J. F. Dubremetz, K. Musset, M. Mondragon, S. Gonzalez, M. F. Cesbron-Delauw, and C. Mercier. 2008. Functional domains of the Toxoplasma GRA2 protein in the formation of the membranous nanotubular network of the parasitophorous vacuole. Int. J. Parasitol. 38757-773. [DOI] [PubMed] [Google Scholar]
- 46.Vallochi, A. L., M. V. Nakamura, D. Schlesinger, M. C. Martins, C. Silveira, R. Belfort, Jr., and L. V. Rizzo. 2002. Ocular toxoplasmosis: more than just what meets the eye. Scand. J. Immunol. 55:324-328. [DOI] [PubMed] [Google Scholar]
- 47.Webster, P. 2007. Microwave-assisted processing and embedding for transmission electron microscopy. Methods Mol. Biol. 36947-65. [DOI] [PubMed] [Google Scholar]