Abstract
Bovine colonic crypt cells express CD77 molecules that potentially act as receptors for Shiga toxins (Stx). The implication of this finding for the intestinal colonization of cattle by human pathogenic Stx-producing Escherichia coli (STEC) remains undefined. We used flow cytometric and real-time PCR analyses of primary cultures of colonic crypt cells to evaluate cell viability, CD77 expression, and gene transcription in the presence and absence of purified Stx1. A subset of cultured epithelial cells had Stx receptors which were located mainly intracellularly, with a perinuclear distribution, and were resistant to Stx1-induced apoptosis and Stx1 effects on chemokine expression patterns. In contrast, a population of vimentin-positive cells, i.e., mesenchymal/nonepithelial cells that had high numbers of Stx receptors on their surface, was depleted from the cultures by Stx1. In situ, CD77+ cells were located in the lamina propria of the bovine colon by using immunofluorescence staining. A newly established vimentin-positive crypt cell line with high CD77 expression resisted the cytolethal effect of Stx1 but responded to Stx1 with a significant increase in interleukin-8 (IL-8), GRO-α, MCP-1, and RANTES mRNA. Combined stimulation with lipopolysaccharide and Stx1 increased IL-10 mRNA. Our results show that bovine colonic crypt cells of epithelial origin are resistant to both the cytotoxic and modulatory effects of Stx1. In contrast, some mucosal mesenchymal cells, preliminarily characterized as mucosal macrophages, are Stx1-responsive cells that may participate in the interaction of STEC with the bovine intestinal mucosa.
Some Shiga toxin-producing Escherichia coli (STEC) strains, enterohemorrhagic E. coli strains, are food-borne pathogens which evoke life-threatening diseases in humans (26). Cattle and other ruminants can shed STEC for long periods and are a major reservoir for zoonotic STEC (6, 61, 70, 72). Emerging human STEC infections make the reduction of STEC shedding by reservoir species a current challenge in veterinary public health.
Several lines of evidence indicate that STEC adherence to bovine intestinal epithelial cells is essential for long-term STEC colonization of ruminants. Within hours after oral infection, STEC O157:H7 can be detected throughout the gastrointestinal tract, including the rumen, of cattle (6, 18). As early as 4 days after inoculation, STEC strains colonize epithelial cells in the ileum, cecum, colon, rectum, and gall bladder in weaned calves (8, 59, 62). STEC O157:H7 strains principally colonize the rectoanal junction of weaned calves and older cattle (10, 33, 44), but O157:H7 colonization also can occur at other sites of the bovine intestinal tract (8, 23, 55). The ability of the majority of bovine STEC isolates to intimately attach to cells and rearrange the actin cytoskeleton (attaching-and-effacing [AE] lesions) (71) may facilitate adherence to the intestinal epithelium (5, 9, 44). Signature-tagged mutagenesis studies showed that factors not involved in AE lesion formation further support STEC colonization of the bovine intestinal epithelium (11, 68). The duration of STEC shedding correlates with epithelial cell turnover in the bovine intestine (35). Vaccination strategies directed against proteins involved in STEC adherence to the bovine intestinal mucosa have been successful in reducing STEC O157:H7 infection in cattle (49, 50, 52, 67).
Other STEC factors also may influence the duration of colonization. Recent studies suggest that STEC suppresses the bovine host's immune response, limits mucosal inflammation, and maintains intestinal homeostasis. Lymphostatin (2) and Shiga toxin 1 (Stx1) (39) block the proliferation of bovine lymphocytes in vitro. Stx1 alters the cytokine response of bovine intraepithelial lymphocytes (42), cells that are scattered within the epithelial layer and are affected in vivo by Stx1 from STEC strains that do not colonize next to organized lymphoid tissues (38). Some STEC O157:H7 strains exhibit a tropism for the follicle-associated epithelium of Peyer's patches in the bovine intestine (51) and may release modulating factors adjacent to induction sites of the immune response. Development of a cellular immune response against STEC antigens is significantly delayed in calves inoculated with Stx2-producing E. coli O157:H7 compared to that of calves inoculated with a nontoxigenic O157:H7 strain (22). Immune-modulating STEC factors are potential targets for future strategies aimed at reducing STEC shedding in cattle, but their mode of action in the bovine intestine is only partially understood.
Stx proteins are potent 1A:5B-structured cytotoxins with RNA N-glycosidase activity that inhibit protein synthesis of sensitive cells (13). Independent of cytolethal effects, Stx-induced expression of chemokines in human intestinal epithelial cells (66, 74) may be a key event in the pathogenesis of human STEC-related diseases (47). There is conflicting evidence about whether Stx are involved in STEC-epithelial cell interactions in cattle. Pruimboom-Brees et al. (54) did not detect Stx receptors in the bovine intestinal epithelium, but other researchers described the presence of Gb3/CD77 (a glycosphingolipid Stx receptor found on sensitive cells) and Stx binding sites in the bovine intestinal mucosa (20, 21, 57). Stx receptor expression was ascribed to proliferating epithelial cells at the bases of the crypts (21). However, Stx1 binding to these receptors in vitro is not cytolethal but leads to a rapid degradation of the toxin in the cellular lysosomal compartment (21). We hypothesized that bovine colonic epithelial cells are resistant to the cytotoxic, but not the modulatory, effects of Stx1. We used flow cytometry, fluorescence microscopy, and mRNA quantitation by real-time PCR to analyze Stx receptor distribution and the effects of Stx1 in a colonic crypt cell culture system.
MATERIALS AND METHODS
Collection of bovine tissues.
Intestinal specimens for the isolation of bovine colonic crypts were obtained from freshly slaughtered cattle (18 to 24 months old) of different breeds from a local abattoir (Giessen, Germany). Frozen bovine distal colon tissues were available from earlier infection studies at the National Animal Disease Center in Ames, IA, for the detection of CD14 and CD77 by immunofluorescence. These last tissue specimens had been collected from 4-month-old weaned calves at necropsy, snap-frozen in isopentane, and stored at −80°C. Because STEC pathogenicity for cattle is restricted to the immediate neonatal phase (7, 8, 10, 62), we considered both 4-month-old and older cattle representative of cattle prone to asymptomatic STEC colonization.
Isolation of bovine colonic crypts.
Crypts were isolated according to Föllmann et al. (15), with some modifications. Tissue samples, consisting of approximately 30-cm-long sections from the ascending colon of each animal, were extensively washed with isotonic NaCl solution (4°C) and cut open longitudinally. Mucus was removed by slightly scraping tissue with a glass slide. Specimens were transported to the laboratory in phosphate-buffered saline (PBS) supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, 2.5 μg/ml amphotericin, 4 mM l-glutamine (PAA Laboratories GmbH, Pasching, Austria), 2.5 μg/ml gentamicin (Biochrom AG, Berlin, Germany), and 0.2% glucose (Merck, Darmstadt, Germany). Mucosal tissue was separated from the lamina propria by scraping with a sterile glass slide, homogenized by a razor blade in Hank's buffered saline solution (HBSS; 4°C) and transferred to 50-ml tubes (Greiner, Frickenhausen, Germany). After centrifugation (5 min, 130 × g, 4°C) the mucus-containing layer was withdrawn together with the supernatant and discarded. The remaining pellet was washed twice and enzymatically digested in 60 ml of Dulbecco's modified Eagle's medium (DMEM; Invitrogen, Karlsruhe, Germany) plus 60 ml of HBSS, 150 U/ml collagenase I (Biochrom), 2 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 2.5 μg/ml gentamicin, and 2.5 μg/ml amphotericin. After incubation (45 min, 37°C, 8% CO2) with stirring (100 rpm), the solution was passed twice through 0.6-by-25-mm needles to further disintegrate cell clots. After a second digestion step (10 min) and subsequent centrifugation (7 min, 202 × g, 4°C), the remaining pellet was resuspended in a 2% d-sorbitol solution (in HBSS) and centrifuged (5 min, 50 × g, 4°C). The pellet was resuspended in 2% d-sorbitol. The procedure was repeated several times until the supernatant was clear. The isolated crypts (the pellet) were washed with HBSS (3 min, 65 × g, 4°C) and resuspended in DMEM.
Primary bovine colonic crypt cell cultures.
Crypts were seeded at a density of 350 to 400 crypts/cm2 (microscopic counts; 200 to 300 cells per crypt) in 25-cm2 culture flasks (Costar, Bethesda, United Kingdom) coated with rat tail collagen (2.8 μl/cm2 [CollagenR; Serva, Heidelberg, Germany], 1:10 in distilled water) and cultivated (37°C, 8% CO2). Crypt culture medium consisted of DMEM supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, 2.5 μg/ml gentamicin, 2.5 μg/ml amphotericin, 5 μg/ml bovine transferrin (Invitrogen), 10 μg/ml bovine insulin (Biochrom), 0.15 mM nonessential amino acids (Biochrom), 1 μg/ml hydrocortisone (Sigma-Aldrich, Steinheim, Germany), 30 ng/ml epidermal growth factor (AF Schützdeller GmbH, Germany), 4 mM l-glutamine, and 2.7 mg/ml glucose (Sigma-Aldrich). For the first 24 h, 10% fetal calf serum (FCS; Invitrogen) was added. Further cultivation was performed in medium containing the supplements named above with FCS at 2% only and an addition of 0.5% bovine pituitary extract (C.C.Pro GmbH, Neustadt, Germany). In some experiments, cells were cultured for 48 to 72 h in medium additionally supplemented with lipopolysaccharide (LPS) from E. coli O111:B4 (25 μg/ml; Sigma-Aldrich) or sodium butyrate (2 mM or 4 mM; Sigma-Aldrich). Flow cytometry analysis of cells harvested from representative cultures (n = 16) 96 h after seeding of the crypts showed that the cultures consisted of 93.82% ± 2.61% (mean ± standard deviation) cytokeratin-positive (i.e., epithelial) and 6.07% ± 4.16% vimentin-positive (mesenchymal) cells. These cultures are referred to as primary colonic crypt cell cultures.
Generation and cultivation of bovine colonic crypt mesenchymal cell lines.
Primary bovine colonic crypt cells grown for 96 h in collagen-coated petri dishes (diameter, 3.5 cm; Falcon), were lipofected with the pSV3neo plasmid carrying the SV40 large-T antigen (48). Four micrograms of plasmid and 20 μl of Superfect transfection reagent (Qiagen, Hilden, Germany) were mixed in 100 μl of DMEM, incubated for 10 min at 25°C and added to 1 ml of DMEM in each dish. After incubation (3 h, 37°C), supernatants were withdrawn, and cells were covered with 5 ml of fresh medium. After 48 h at 37°C, the selection of transformed cells was started by adding 40 μl of Geneticin solution (50 mg/ml; Sigma-Aldrich). Medium was changed every 48 to 72 h. When clonal proliferation became visible, cells were detached by trypsin (0.25% in HEPES buffer supplemented with 0.2% EDTA; PAA), seeded into fresh cell culture flasks (Falcon), and further propagated with RPMI 1640 medium containing 2 mM stabilized l-glutamine and 2.0 mg/ml NaHCO3 (PAN Biotech GmbH, Aidenbach, Germany), 100 U/ml penicillin, 100 μg/ml streptomycin, and 10% FCS.
Cytotoxicity assays.
The effect of Stx1 on the cellular metabolic activity was quantified by MTT (3-[4,5-dimethyl-2-thiazolyl]-2,5-diphenyl tetrazolium bromide, Sigma) reduction assay as described previously (39). Cells (2 × 104 per well) were seeded into microtiter plates and incubated (72 h, 37°C) with purified Stx1 (0.0002 to 2,000 CD50/ml; CD50 [50% cytotoxic dose] as determined on Vero cells, where 1 CD50 was calculated to be equivalent to 0.4 to 0.8 pg/ml of purified Stx1 [46]) without or after preincubation with purified mouse anti-StxB1 (immunoglobulin G1 [IgG1], clone 13C4; 1.5 μg/ml [63]). Methods for Stx1 and anti-Stx1 subunit B (StxB1) purification were previously published (39). Epithelial cell-specific apoptosis was quantified using an M30 CytoDEATH fluorescein isothiocyanate (FITC) test kit (Roche, Mannheim, Germany) and flow cytometry analysis.
Flow cytometry.
There is no indication that trypsin cleaves the Stx receptor Gb3/CD77, a glycosphingolipid, or that trypsin cleavage of proteinaceous Gb3/CD77 ligands on the surface of bovine cells unmasks Stx receptors and makes them more accessible for toxin binding. The only physiological Gb3/CD77 ligand detected so far in cattle is the type I interferon receptor protein (IFNAR) (17). However, the affinity of Stx1 for Gb3/CD77 is greater than the affinity of IFNAR for Gb3/CD77 (27). Thus, the use of trypsin was considered appropriate to generate cell suspensions for flow cytometry analysis of StxB1 binding to bovine colonic cells. Cells were detached by trypsinization, transferred to V-shaped microtiter plates (Greiner), and pelletized by centrifugation (150 × g, 7 min, 4°C). Detection of cell surface antigens and surface binding sites for the recombinant StxB1 (rStxB1) was performed with native cells as described previously (37, 60). Leukocyte antigens were detected using antibodies listed in Table 1. Binding of rStxB1 was detected by subsequent incubation with purified rStxB1 (30 μg/ml PBS [60]), anti-StxB1 clone 13C4 (45 μg/ml PBS), and anti-mouse IgG(γ) FITC (Medac, Hamburg, Germany). For detection of intracellular antigens, cells were fixed in paraformaldehyde (2% in PBS; 30 min, 25°C), washed with PBS, and permeabilized with digitonin (0.005% in PBS, 10 min, 25°C [Sigma-Aldrich]). Subsequently, cells were incubated with FITC-conjugated anti-pan cytokeratin antibody (clone C-11, 1:100 in PBS [Sigma-Aldrich]) or anti-vimentin antibody (clone 3B4, 1:13 in PBS [Dako, Hamburg, Germany]) and phycoerythrin-conjugated anti-mouse IgG antibody (1:50 in PBS [Sigma-Aldrich]). Finally, cells were washed twice and analyzed on an Epics Elite analyzer (Beckman-Coulter, Krefeld, Germany), acquiring 5,000 events per sample. Data were analyzed with FCS Express version 2 software (DeNovo software, Thornhill, Canada). Gates were defined according to the negative control (PBS) and secondary antibody control included in each test series, defining less than 2% of the cells as positive.
TABLE 1.
Antibodies used in this study
| Target antigen | Antibody
|
Working dilution | ||||
|---|---|---|---|---|---|---|
| Host | Isotype | Label | Clone | Sourcea | ||
| CD11a | Mouse | IgG2a | IL-A99 | DW | Nondiluted | |
| CD11b | Mouse | IgG1 | IL-A15 | JN | Nondiluted | |
| CD11c | Mouse | IgG1 | IL-A16 | JN | Nondiluted | |
| CD14 | Mouse | IgG1 | CC-G33 | DW | Nondiluted | |
| CD21 | Mouse | IgG2a | IL-A65 | JN | Nondiluted | |
| CD25 | Mouse | IgG1 | IL-A111 | JN | Nondiluted | |
| CD71 | Mouse | IgM | IL-A77 | JN | Nondiluted | |
| CD77 | Rat | IgM | 38.13 | Beckman-Coulter | 1:10 in PBS | |
| CD80 | Mouse | IgG1 | IL-A190 | DW | Nondiluted | |
| CD86 | Mouse | IgG1 | IL-A158 | DW | Nondiluted | |
| CD172a | Mouse | IgG1 | IL-A24 | JN | Nondiluted | |
| MHC-I | Mouse | IgG2a | IL-A88 | JN | Nondiluted | |
| MHC-II DQ | Mouse | IgG2a | CC158 | DW | Nondiluted | |
| MHC-II DR | Mouse | IgG1 | CC108 | DW | Nondiluted | |
| ACT-2 | Mouse | IgG1 | CACT26A | VMRD Inc. | 1:400 in PBS | |
| Vimentin | Mouse | IgG1 | 3B4 | Dako A/S | 1:13 in PBS | |
| Pan-cytokeratin | Mouse | IgG1 | FITC | C-11 | Sigma-Aldrich | 1:100 in PBS |
| Mouse IgG(γ) | FITC | Medac | 1:200 in PBS | |||
| Mouse IgG | Phycoerythrin | Sigma-Aldrich | 1:50 in PBS | |||
| Rat IgM(μ) | Phycoerythrin | Beckman-Coulter | 1:200 in PBS | |||
| Rat IgM | FITC | Dianova | 1:100 in PBS | |||
DW, Dirk Werling, Royal Veterinary College London, United Kingdom; JN, Jan Naessens, International Livestock Research Institute, Nairobi, Kenya.
Immunofluorescence staining for microscopy.
Cells grown in 12-well culture plates (Costar) were fixed and permeabilized as described for flow cytometry but omitting trypsinization. While being washed in PBS, the floors of the wells were punched out using a heated copper cutter and placed in PBS-prefilled 12-well plates for further processing. Labeling was carried out as described above, and samples were mounted on glass slides and dried overnight at 4°C. Immunofluorescence microscopy was performed using a Leica DMRB Laborlux 12 microscope with an analog camera (for conventional fluorescence microscopy) or a Leica DM IRBE microscope (for confocal fluorescence microscopy).
For immunofluorescence detection of CD14- and CD77-positive cells in situ, 5- to 6-μm sections of isopentane-flash-frozen bovine colon tissues were cut, fixed in cold ethanol (−20°C) for 10 s, and stored at −80°C until used. Slides were removed from −80°C storage and set directly 6 inches under a fluorescent light source for 18 to 20 h to quench nonspecific fluorescence, rehydrated (0.023 M PBS [pH 7.4], 30 min), and incubated in diaminobenzidine solution (HistoMark kit; Kirkegaard & Perry Laboratories, Gaithersburg, MD) for 10 min to block naturally fluorescent eosinophils (28). Sections were incubated for 30 min at room temperature with Image iT FX signal enhancer (Molecular Probes, Eugene, OR), washed with PBS with 0.125% Tween 20 (PBS-T), incubated for 18 h at 4°C with combined rat anti-human CD77 IgM (clone 38.13, 1:20 in PBS-T [Serotec, Raleigh, NC]) and mouse anti-M-M9 (CD14) IgG1 (clone CAM36A, 1:200 [VMRD, Pullman, WA]), washed in PBS-T, and incubated for 30 min (25°C) with Alexa Fluor 594-labeled goat anti-rat IgM and Alexa Fluor 488-labeled goat anti-mouse IgG1 ([Molecular Probes] 1:800 in PBS-T with 5% normal goat serum [Vector Laboratories, Burlingame, CA]). Rat IgM isotype control (1:20 [Cedarlane, Burlington, NC]) and mouse IgG1, clone W3/25, isotype control (1:500 [Serotec]) were used as negative control sera. Slides were examined by fluorescence microscopy (Nikon E800 microscope). Images were acquired using a digital camera (Spot) and MetaVue software.
Quantitation of mRNA species.
Primary colonic crypt epithelial cells grown to confluence in collagen-coated cell culture flasks within 96 h after seeding or confluent immortalized colonic crypt mesenchymal cells 48 h after passaging were incubated for 4 and 24 h (37°C) with purified Stx1 (200 CD50/ml). For comparison, cells were cultured with Stx1 after preincubation (30 min) with purified mouse anti-StxB1 (1.5 μg/ml). Cells were detached by trypsinization and washed twice (202 × g, 7 min, 20°C) with PBS. Cells were counted, and 1 × 106 cells were lysed in 600 μl of RLT buffer (RNeasy mini-kit; Qiagen, Hilden, Germany) with 2.5% β-mercaptoethanol (Amersham Biosciences, Buckinghamshire, United Kingdom) and stored at −70°C. The RNA isolation procedure and reverse transcription were performed according to the method described by Moussay et al. (42). PCR amplification was done on an automated fluorometer (ABI PRISM 5700 sequence detection system; Applied Biosystems) using 96-well optical plates. Sequences of primers and probes used to detect mRNA for the interleukin-8 (IL-8), GRO-α, MCP-1, RANTES, and TGF-β proteins have been published previously (31, 42).
Granulocyte migration assays.
A modification of the method described by Galligan and Coomber (16) was used as published (42). In brief, bovine granulocytes were obtained by density gradient centrifugation and lysis of erythrocytes. After bovine colonic crypt cells were cultured for 4 and 24 h in the absence or presence, respectively, of Stx1, supernatants (agonists) were obtained by centrifugation at 10,000 × g for 10 min and transferred into 12-well plates (i.e., the lower compartment of the migration chamber). The upper compartments of all migration chambers (12-mm Transwell clear culture inserts, 3-μm pores; Corning Costar, Germany) were placed in the wells, and each was filled with medium containing 5 × 105 granulocytes. After a 2-h incubation at 37°C, granulocytes from both compartments were harvested and counted by flow cytometry using 3-3′dioctadecyloxacarbocyanine perchlorate (DiO)-labeled BL-3 cells (ECACC catalog code 86062401) as counting particles. Spontaneous granulocyte migration, i.e., migration of granulocytes in chambers in which the lower compartment was filled with nonconditioned medium (freshly prepared medium not having been in contact with cells), was used as a reference (i.e., 100%).
Statistical analysis.
Data were analyzed by Student's t test and two-way analysis of variance (ANOVA) for normal distributed data and by the Mann-Whitney rank sum test for nonnormal distributed data (SigmaStat software, versions 2.03 and 3.11; SPSS Inc.). In all analyses, a significance level, α, of 0.05 was applied; levels of probability are indicated in the legends to the figures. Results were considered not significant if P was more than 0.05 and omitted from the figures.
RESULTS
Stx receptor expression by primary colonic crypt epithelial cells.
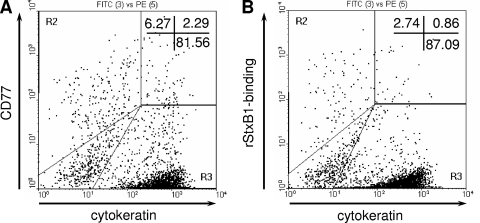
Flow cytometry analysis revealed that 5.3% ± 0.56% (n = 16 determinations/four independent experiments) of native bovine colonic crypt cells obtained from cattle, analyzed 96 h after crypts were seeded, expressed CD77 on the cell surface. After permeabilization, which allowed the antibody to access the interior of the cells, CD77 became detectable in 16% ± 10.8% of the cells (n = 24/eight experiments). The portion of cells that bound rStxB1 upon permeabilization was 22.5% ± 17.9%. The majority of CD77+ or rStxB1-positive cells within the cultures were not cytokeratin-positive cells. Analysis of double-labeled cells was complicated by a significant variation in cellular autofluorescence, but, according to gates set relative to that of the control samples prepared without primary reagents, only a minor portion of CD77+ and rStxB1-binding cells could be assigned to the cytokeratin-positive epithelial cell population (Fig. 1).
FIG. 1.
Detection of CD77 antigens (A) and binding sites for rStxB1 (B) in primary bovine colonic crypt epithelial cells. Monolayers of cells grown from seeded colonic crypts within 96 h of culture were detached by trypsinization, fixed, permeabilized, and incubated with anti-CD77 and rStxB1, respectively. Flow cytometry analysis of the cells with gates set relative to control samples without primary reagents revealed that anti-CD77 and rStxB1 bound to a small portion of cytokeratin-positive cells. Dot plots are representative of 12 determinations from five independent experiments. Percentages of positive cells are shown in the upper right corner of the plots. PE, phycoerythrin.
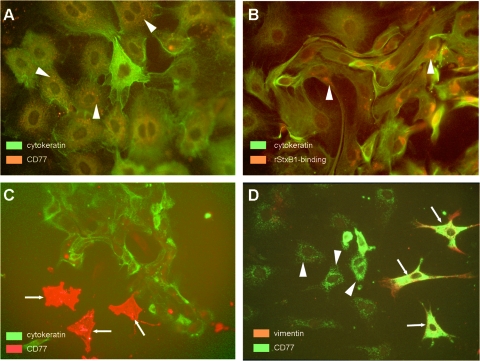
Immunofluorescence microscopy analysis was used to identify cells of epithelial origin in colonic crypt cultures. Epithelial cells were identified by anti-cytokeratin immunostaining after fixation and permeabilization (Fig. 2A). A subset representing approximately 15% of these large, mono- or binucleated cells stained weakly with anti-CD77. The signal was distributed in a spot-like manner within the cellular body surrounding the nucleus. A similar perinuclear signal distribution was observed in binding studies with purified rStxB1 (Fig. 2B). Neither CD77 nor rStxB1 binding sites were detected on the surface of colonic crypt epithelial cells by conventional fluorescence microscopy or by confocal laser scanning microscopy (data not shown).
FIG. 2.
Localization of CD77 antigens and binding sites for rStxB1 in primary bovine colonic crypt epithelial and mesenchymal cells. Monolayers of cells grown from seeded colonic crypts within 96 to 120 h of culture were fixed, permeabilized, and overlaid with anti-CD77 and rStxB1, respectively. By using conventional fluorescence microscopy, we detected CD77 in epithelial cells (cytokeratin-positive) in a spot-like distribution surrounding the nucleus (A, arrowheads). Epithelial cells also harbored perinuclear rStxB1 binding sites (B, arrowheads). (C) A small population of cytokeratin-negative cells strongly bound anti-CD77 (arrows). (D) Double labeling with anti-vimentin revealed that the latter cells (arrows) were of mesenchymal origin (i.e., vimentin-positive), while cells with a perinuclear CD77 localization were vimentin-negative (arrowheads). Figures depict labeled cells from selected regions and are not representative of all cells in culture. Original magnification was ×400.
Supplementation of cell culture medium with LPS (25 μg/ml) or different concentrations of sodium butyrate (2 to 4 mM), the latter of which is known as a transcriptional regulator of differentiation genes in many cell types, did not significantly affect the percentage of CD77+ cells in colonic crypt cell cultures (25.9% ± 8.8%, 28% ± 8.8%, 23.7% ± 5.7%, and 24.3% ± 6.7% in the medium control and after cultivation with LPS, 2 mM butyrate, and 4 mM butyrate, respectively; n = 4 independent experiments; P > 0.05, Student's t test) as determined by flow cytometry analysis.
Functional analysis of primary colonic crypt cells cultured in the presence of Stx1.
Colonic crypt cells were passaged and plated with purified Stx1 (0.0002 to 2,000 CD50/ml, quantified on Vero cells). After further incubation for 72 h, the cellular metabolic activity of Stx-treated cells (determined with an MTT reduction assay) was not different from that of control cultures (P > 0.05 for the impact of Stx1 concentration; two-way ANOVA). Stx1 with or without costimulation by LPS had no significant effect on epithelial cell-specific apoptosis (by M30 CytoDEATH test; data not shown).
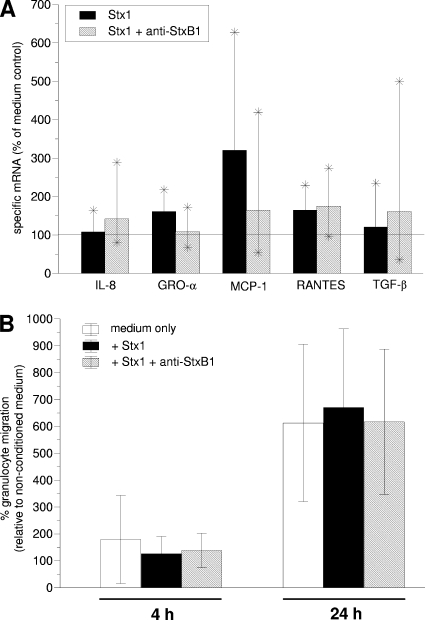
Stx1 did not significantly alter the amount of mRNA for IL-8, GRO-α, MCP-1, RANTES, and the epithelial cell differentiation factor TGF-β (Fig. 3A). Extending the incubation time to 24 h did not result in Stx1 having a significant effect on the quantities of specific mRNA (data not shown). Stx1 also did not alter the release of granulocyte chemoattractants: within 24 h, colonic crypt cells released detectable amounts of granulocyte migration-inducing substances into the culture supernatant, but the quantities did not differ between those of Stx1-treated and nontreated cultures (Fig. 3B).
FIG. 3.
Relative quantities of gene transcripts harbored by bovine colonic crypt cells upon cultivation in the presence of purified Stx1 (A) and migratory activity of bovine granulocytes toward supernatants derived from these cultures (B). Cells grown from seeded colonic crypts within 96 h were further incubated for 4 h with Stx1 (200 CD50/ml, as determined on Vero cells) without or after preincubation with anti-StxB1 (1.5 μg/ml) (A). Subsequently, mRNA was reverse transcribed and quantified by real-time PCR. Transcription of the GAPDH housekeeping gene was used for normalization of the samples. Cells incubated with medium were used as a control (i.e., 100%; indicated by the horizontal line). Data are the mean values of six independent experiments; minima and maxima are indicated by asterisks at the end of the vertical lines. Analysis by the Mann-Whitney rank sum test did not detect any statistically significant effect of Stx1. The release of granulocyte chemoattractants in these cultures was determined with a filter-based migration assay (B). Bovine granulocytes were allowed to migrate for 2 h at 37°C toward the lower compartment containing the agonists: supernatants of untreated colonic crypt cell cultures (i.e., medium only), supernatants of cultures incubated with Stx1 without (+Stx1) or after preincubation with anti-StxB1 (+ Stx1 +anti-StxB1). Granulocytes were then harvested and counted. Results are expressed relative to the spontaneous granulocyte migration occurring in chambers, in which the lower compartment was filled with nonconditioned (i.e., fresh) medium (100%). Data are means and standard deviations of five to six independent migration assays with supernatants of colonic crypt cell cultures conditioned for 4 and 24 h.
Identification of CD77+ mesenchymal cells in the bovine colonic mucosa.
Conventional fluorescence microscopy and confocal laser scanning microscopy examination of colonic crypt cell cultures obtained from 18- to 24-month-old cattle revealed the presence of a small population of cells (approximately 5% of the monolayer) that intensely expressed CD77 (Fig. 2C+D) and rStxB1 binding sites (data not shown) on the cell surface. The cells were cytokeratin negative (Fig. 2C) but vimentin positive (Fig. 2D). Flow cytometry analyses showed that these cells were Stx1 sensitive (i.e., supplementation of the culture medium with Stx1 led to a reduction in the number of CD77+ and vimentin-positive but cytokeratin-negative cells; data not shown).
Immunohistologic staining of frozen tissues from 4-month-old calves was used to confirm the presence of CD77+ cells of mesenchymal origin in the bovine colon in situ. An infrequent presence of CD77+ cells was found in subbasilar lymphoid aggregates within the lamina propria, and occasionally, scattered individual CD77+ cells were found in the lamina propria not associated with lymphoid aggregates. Smooth myocytes within the tunica muscularis were occasionally CD77+. Immunohistologic staining identified the frequent CD14+ cells within the epithelium of mucosal crypts, as well as the surface epithelium. Individual CD14+ cells were found within the lamina propria, particularly focused in lymphoid aggregates. CD14+ cells were also found within the connective tissue between smooth muscle bundles in the tunica muscularis. Cells staining positive with both anti-CD77 and anti-CD14 were not seen in the epithelium of the mucosal crypts or surface epithelium but were detected in low numbers in the lamina propria between the colonic crypts (Fig. 4).
FIG. 4.
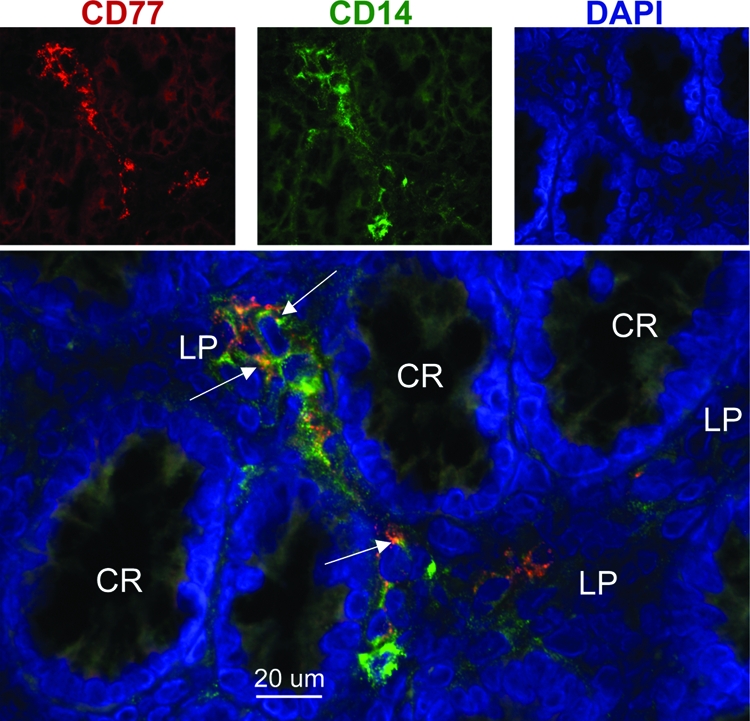
Immunodetection of CD14- and CD77-expressing cells in the bovine colonic mucosa. Frozen sections of distal colon tissue from a 4-month-old calf were fixed and immunostained with antibodies against CD14 (green) and CD77 (red). Nuclei were stained with 4′,6′-diamidino-2-phenylindole (DAPI; blue). Fluorescence images were captured in black and white and then pseudocolored (upper row of images). Arrows in the lower merged image identify examples of the colocalization of CD14 and CD77 in the lamina propria (LP) between the colonic crypts (CR).
Generation and characterization of colonic crypt mesenchymal cell lines.
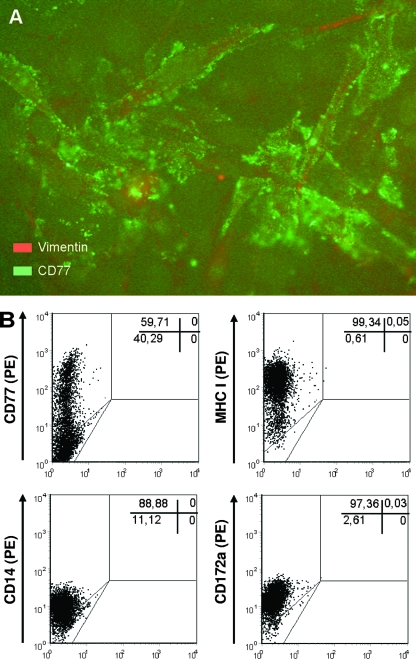
We established and evaluated 10 vimentin-positive cell clones from bovine colonic crypt cell cultures that were used to identify and characterize colonic crypt mesenchymal cells that are potentially sensitive to Stx1. Cells of all clones stained positive with anti-CD77 after permeabilization. Nonpermeabilized cells of one line (designated clone 12) exhibited a marked CD77 expression on the cell surface (Fig. 5A and B) and coexpressed major histocompatibility complex class I (MHC-I), CD14, and CD172a (Fig. 5B) but lacked MHC-II DQ, MHC-II DR, CD11a, CD11b, CD11c, CD21, CD25, CD71, CD80, CD86, and ACT-2 (data not shown).
FIG. 5.
Phenotype of bovine colonic crypt mesenchymal cell line clone 12. Conventional fluorescence microscopy of the immortalized cell line upon permeabilization revealed an abundant CD77 expression with a spot-like pattern (A). Original magnification was ×400. Flow cytometry analysis (B) of native, nonpermeabilized cells revealed that CD77 expression on the cell surface was restricted to a major subpopulation of the cells at the time of labeling, while virtually all cells expressed MHC-I, CD14, and CD172a. Dot plots are representative of double determinations from one to three independent experiments. Percentages of positive cells are given in the upper right corners of the plots. PE, phycoerythrin.
Effects of Stx1 on immortalized colonic crypt mesenchymal cells.
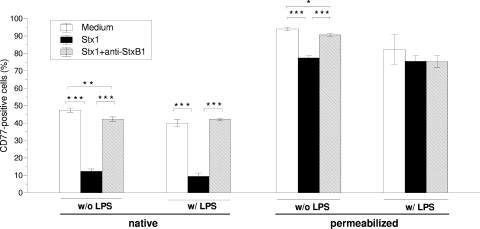
Incubation of clone 12 cells with 200 verocytotoxic CD50 of Stx1/ml did not alter the cellular metabolic activity in comparison to that of cells cultured with Stx1 plus anti-StxB1 (P > 0.05 for the impact of Stx1 concentration; two-way ANOVA). Despite this, Stx1 markedly lowered the percentage of cells with CD77 on the cell surface (Fig. 6). Permeabilization of cells from Stx1-treated and -nontreated cultures prior to labeling with anti-CD77 generally increased the numbers of CD77-positive cells. This applied particularly to Stx1-treated cultures, thereby markedly reducing the effect of Stx1 on the percentage of CD77-positive cells. The findings are consistent with an Stx1-induced redistribution of CD77 from the cell surface to intracellular compartments. Supplementation of the medium with LPS slightly reduced the percentage of CD77+ cells without influencing the effect of Stx1 on the surface versus intracellular expression of CD77.
FIG. 6.
Effect of Stx1 on CD77 expression by bovine colonic crypt mesenchymal cell line clone 12. Cells were incubated for 72 h with Stx1 (200 CD50/ml, as determined with Vero cells) without or after preincubation with anti-StxB1 (1.5 μg/ml). CD77 was quantified by flow cytometry after labeling of native cells (left) and of permeabilized cells (right). Student's t test detected P ≤ 0.05 (*), P ≤ 0.01 (**), and P ≤ 0.001 (***).
Addition of Stx and LPS to clone 12 cells significantly increased the quantities of mRNA specific for IL-8, GRO-α, MCP-1, RANTES, and IL-10 relative to that of the LPS control. Stx1 induced this effect within 4 h (data not shown), but the effect was more prominent within 24 h (Fig. 7). Stx1, with or without LPS, did not alter the quantities of TGF-β- and IL-12-specific mRNAs.
FIG. 7.
Effect of Stx1 on the relative quantities of gene transcripts harbored by bovine colonic crypt cell line clone 12. Cells were incubated for 24 h with Stx1 (200 CD50/ml, as determined with Vero cells) without or after preincubation with anti-StxB1 (1.5 μg/ml). Subsequently, mRNA was quantified as described in the legend to Fig. 3. Data are the means of three independent experiments without (A) or with (B) additional supplementation of the culture medium with LPS. Minima and maxima are indicated by asterisks at the end of the vertical lines. In the absence of LPS, the IL-10 and IL-12 mRNA was detectable only in one of three cultures. Student's t test detected P ≤ 0.05 (*), P ≤ 0.01 (**), and P ≤ 0.001 (***). Bold asterisks above horizontal brackets indicate significant differences between Stx1- and Stx1 + anti-StxB1-treated cultures.
DISCUSSION
Epithelial cells are the first host cells exposed to Stx during infections of cattle with STEC. Cells in the depths of bovine colonic crypts express Stx receptors (20), but Stx are not enterotoxic in cattle (54) as they are in rabbits (25, 40). To determine if epithelial cells in the bovine intestine are responsive to Stx, we identified and characterized Stx receptor-expressing cells in bovine colonic crypt cultures. A small subset of primary epithelial cells harbored Stx receptors, apparently with a predominantly intracellular location. Epithelial cells resisted the cytolethal effect of Stx1 and also did not respond to the toxin by releasing immune mediators. Some cultured nonepithelial cells also had Stx receptors, as did the nonepithelial cells we localized adjacent to colonic crypts in situ. One subset of Stx receptor-positive nonepithelial cells was preliminarily identified as mucosal macrophages and responded to low concentrations of Stx1 by altering their chemokine and cytokine expression patterns in vitro. Identification of cells with various receptor expression patterns and Stx responsiveness will help elucidate the roles of different types of colonic cells during STEC colonization of the bovine intestine.
Using overlay assays of frozen sections of bovine intestinal tissues, other investigators have detected binding of Stx1 or anti-CD77 antibodies to the apical surface of cells in the crypt region of the small and large intestines (20, 21, 57). Crypts are composed of mixed populations of cells including immature proliferating cells, a subset of which migrates out of the crypts and differentiates, acquiring the enzymatic and morphological characteristics of mature surface epithelium. It has been suggested that the observed lack of Stx1 binding to surface epithelium in the bovine colon corresponds to a loss of Stx receptors as the cells differentiate (20). Hoey et al. (21) reported that Stx1 did not bind to monolayers of colonic crypt cells in vitro but that Stx receptors localized specifically to the remaining crypt fragments, which consisted of cells expressing proliferation markers but were not further specified by the authors. We did not assess the proliferation rate of the cells analyzed herein. Advanced functional analyses (P. S. Bridger, M. Mohr, I. Stamm, J. Fröhlich, S. Birkner, W. Föllmann, H. Metcalfe, D. Werling, G. Baljer, and C. Menge, unpublished data) revealed that cells with epithelial cell morphology retained their ability to polarize, as indicated by the expression of tight junction marker ZO-1 when grown on collagenized 3-μm-pore filters. Low lactase and sucrase enzymatic activities of colonic crypt cultures, as used for this study, are evidence that these cells were immature and representative of epithelial cells in the bovine colonic crypts. By using flow cytometry analysis, we detected a small number of cytokeratin-positive cultured colonic epithelial cells that coexpressed CD77 and bound rStxB1. Using conventional and confocal microscopy, however, we did not find CD77 and rStxB1 binding sites, either on the surface of primary bovine colonic epithelial cell monolayers or associated with crypt fragments that had remained in the cultures. A weak reaction with anti-CD77 was restricted to intracellular sites and a subset of epithelial cells. It has been speculated that some human leukocytes harbor Stx receptors that are different from those of CD77 (64), but this does not appear to be the case with bovine colonic cells. Although the antibody against CD77 used in our study does not recognize all isoforms of Gb3/CD77 that may serve as Stx receptors (3), the similarities between the anti-CD77 binding and cellular labeling patterns and those of rStxB1 appear to rule out the existence of alternate Stx binding sites on cultured bovine colonic epithelial cells. The lack of detectable Stx receptors on the surface of cultured epithelial cells also cannot be explained by a dependency on a very early immature state or on polarization of the cells, as anti-CD77-labeling patterns were similar to that of polarized cells on filter inserts and cells grown on plastic surfaces (data not shown).
The lack of Stx receptors on the cell surface of cultured epithelial cells was corroborated by the absence of significant anti-CD77 staining in the epithelial layers of nonpermeabilized bovine colonic tissues examined immunohistologically in this study. Weak anti-CD77 staining of crypt mucosal epithelial cells, similar to that shown for the cultured epithelial cells (Fig. 2), was noted in some samples but was interpreted as variable background staining and was not characterized further. Previous discrepant findings about whether bovine epithelium expresses Stx receptors (20, 54) have been attributed to methodological constraints (20). The method applied herein for harvesting and processing tissue specimens from the bovine gut best maintains the structure of the intestinal mucosa (32). The clearly detectable binding of anti-CD77 to cells of the lamina propria in this study confirms the suitability of this method. The exact lineage assignment of crypt cells and the biochemical character of the structure that allows Stx1, but not Stx2, to bind to bovine crypts in tissue sections in some instances (57) have not been determined.
The results of our study imply that the majority of Stx receptors on bovine colonic crypt epithelial cells are inaccessible to luminal Stx. In experimentally and naturally infected cattle, STEC organisms principally colonize absorptive cells of the luminal epithelium in the bovine colon and not epithelial cells in the depth of the crypts (7, 43). Stx is not enterotoxic in calves (54), and epithelial lesions during bovine STEC infections can be ascribed to the ability of the bacteria to cause AE lesions (9). Our findings extend evidence that multiple factors contribute to the extensive resistance of intestinal epithelial cells to the cytolethal effect of Stx in situ during STEC infections of cattle. These factors may include low concentrations of Stx in the crypts, a lack of bovine epithelial Stx2 receptors (57), extensive absence of Stx1 receptors from the surface of crypt epithelial cells (as in this study), and eventually a specific endocytotic pathway possessed by the remaining cells that still may endocytose some amounts of the toxin (21).
We did not detect a cytolethal effect of Stx on bovine colonic epithelial cells, but it is possible that the cells respond to Stx by other means. Granulocyte migration assays showed that incubation of cultured bovine colonocytes with 200 verocytotoxic CD50/ml Stx1 (equivalent to concentrations in the lower nanogram range [46, 57]) had no effect on the release of granulocyte chemoattractants. Treatment also left the quantities of selected chemokine mRNAs unaltered. In human intestinal epithelial cell lines, 10 ng/ml Stx1 increased the amount of IL-8 protein (65) and 1 ng/ml increased the amount of IL-8 mRNA (74). Concentrations of Stx1 similar to those in the present study induced a 40-fold increase in the amount of IL-4 mRNA in bovine intraepithelial lymphocytes (42). We therefore interpret our findings as sufficient evidence to conclude that bovine colonic crypt epithelial cells are largely resistant to Stx also in terms of conferring proinflammatory signals.
Surprisingly, we identified novel mesenchymal/nonepithelial Stx target cells in the crypt area of the bovine colonic mucosa. A subset of cytokeratin-negative, vimentin-positive cells with fibroblast-like morphology in primary bovine colonic crypt cell cultures intensely expressed CD77 on the surface and were sensitive to modulation by Stx1. To our knowledge, this is the first report of immunohistochemical identification of mesenchymal CD77+ cells scattered within the colonic lamina propria of cattle. To evaluate the origin and the significance of these Stx1 target cells, we generated several clones by the immortalization of primary cells. One of these clones, designated clone 12, resembled mesenchymal cells in the primary cultures as it expressed high levels of CD77 on the cell surface. However, only a small subset of CD77+ cells within the lamina propria of the bovine distal colon coexpressed CD14, as did clone 12. Thus, clone 12 is representative of only one of several CD77+ mesenchymal cell types in the bovine colonic mucosa that are potentially sensitive to Stx in situ.
Several criteria led us to consider that clone 12 may have descended from (i) fibroblasts, (ii) myofibroblasts, (iii) dendritic cells (DCs), or (iv) monocytes/macrophages. (i) Clone 12 cells have a spindle-shaped morphology resembling that of fibroblasts. Tissue fibroblasts descend from the primary mesenchyme or may develop as blood-derived fibrocytes from CD14+ monocytes (1). Like clone 12 cells, blood-derived fibrocytes express CD172a, lack MHC-II expression (1), and produce IL-10 (4). Unlike clone 12 cells, however, blood-derived fibroblasts cease to express CD14 upon cultivation (1). (ii) The spatial distribution of CD77+ cells in the lamina propria of the bovine colon was comparable to the binding patterns of Stx1 and Stx2 to pericryptic fibroblasts (synonymous with myofibroblasts) in human intestinal tissue (57). Myofibroblasts are specialized fibroblasts that share phenotypical properties of fibroblasts and smooth muscle cells, are located directly below the epithelium (53), and have been implicated in Stx-induced damage of the human colonic mucosa (57). (iii) Clone 12 cells share similarities with DCs, including expression of CD172a. Bovine CD172a is expressed principally by granulocytes, monocytes, macrophages, and a subpopulation of afferent lymph veiled cells and by cells with DC morphology in lymphoid tissues, skin, and intestinal epithelium (12, 19, 36). Contact between mucosal DCs and epithelial cells induces the expression of tight junction proteins, enabling the DCs to open the epithelial cell junctions without affecting the integrity of the monolayer and to extrude dendrites into the gut lumen (56). Such intraepithelially located DCs may have been isolated during the crypt preparation. (iv) Several lines of evidence favor the notion that clone 12 cells descended from macrophages. Werling et al. (69) observed that stimulation with Toll-like receptor agonists (e.g., LPS) induces different reaction patterns in bovine macrophages and DCs: while DCs predominantly respond by transcribing IL-12, macrophages typically respond with IL-10 transcription. Cells of clone 12 responded, independently of Stx1, to LPS stimulation with increased IL-12 transcription (data not shown), implying their relationship to DCs. However, clone 12 cells lacked CD11c, a major marker for DCs (45), but expressed CD14, typical for bovine macrophages (24). Resident macrophages are devoid of CD14, but macrophages derived from blood monocytes are CD14+ (58). Macrophages experiencing LPS are characterized by an IL-10high and IL-12low phenotype and support type II immune responses (41). Morphological features, markers, and cytokine expression profiles, and the fact that Stx1, in conjunction with LPS, induces a shift toward increased IL-10 transcription, led us to propose that the predecessors of clone 12 cells belong to type II intestinal macrophages.
Initial attempts to purify mesenchymal cells from the crypt preparations resulted in heterogenous cultures of primary cells, with variable CD172a expression, which were not suitable for further functional analyses. Transfection with the pSVneo3 plasmid carrying the simian virus 40 (SV40) large-T antigen under the control of the SV40 promoter allowed us to select for cells originating from a single progenitor cell by limiting dilution analysis. Since the level of Stx receptor expression correlates with the state of malignant degeneration of cells, data obtained with immortalized cells must be interpreted with some caution. Normal human colonic epithelial cells lack CD77, but the Stx receptor is highly expressed by metastatic colon cancer (29). Normal bovine B cells resist the cytolethal effect of the toxin (39), but bovine B cells transformed by infection with a retrovirus (e.g., bovine leukemia virus) are highly susceptible to the apoptosis-inducing effect of Stx1 (14, 39). Likewise, retroviral transfection of Madin-Darby canine kidney cells with the human multidrug resistance efflux pump (MDR1) increased expression of Stx receptors and cell sensitivity to Stx1 (30). Though the authors of the last report discuss the possibility that the retroviral infection per se has selected a subpopulation of Stx-sensitive cells, the effect could be attributed mainly to the activity of the efflux pump. Clone 12 cells, utilized as a tool in this study, closely resembled a subset of primary cells in the initial bovine colonic crypt cultures with regard to cellular distribution of CD77 and rStxB1 binding sites and resisted the cytolethal effects of Stx1. The detection of CD77+ mesenchymal cells in bovine colonic crypt tissues in situ (this study) together with the observation that primary bovine monocytes coexpress CD172a and CD77 (C. Menge, personal observation) strongly supports the conclusion that CD77 expression is an inherent feature of the progenitor cell of clone 12 and was not induced de novo by transfection with retroviral genes.
Despite the fact that the progenitor cells of the different mesenchymal/nonepithelial cells adjacent to bovine colonic crypts are yet to be fully defined, the data presented here point to the possibility that various Stx-responsive cells in the lamina propria are involved in the interaction between STEC and the bovine intestine. Intestinal antigen sampling cells have gained considerable interest in recent years because of their implication in the induction of oral tolerance to commensal bacteria (34, 73). Studying the interaction of STEC and bovine intestinal lamina propria cells with and without Stx receptors may help to elucidate how STEC realizes a commensal-like lifestyle in cattle and facilitate the development of effective strategies to reduce shedding of this zoonotic pathogen by ruminants.
Acknowledgments
We thank Bryan K. Wheeler for excellent technical assistance with immunohistology. We acknowledge Dirk Werling, Royal Veterinary College, London, United Kingdom, for provision of antibodies and helpful suggestions for the characterization of the colonic crypt mesenchymal cell line clone 12 and Anja Taubert and Carlos Hermosilla, Institut für Parasitologie, Justus-Liebig-Universität, Giessen, for helpful discussions.
This work was supported by grants from the Deutsche Forschungsgemeinschaft to M.M., E.S., and C.M. (Sonderforschungsbereich 535).
Editor: S. R. Blanke
Footnotes
Published ahead of print on 2 September 2008.
REFERENCES
- 1.Abe, R., S. C. Donnelly, T. Peng, R. Bucala, and C. N. Metz. 2001. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J. Immunol. 1667556-7562. [DOI] [PubMed] [Google Scholar]
- 2.Abu-Median, A. B., P. M. van Diemen, F. Dziva, I. Vlisidou, T. S. Wallis, and M. P. Stevens. 2006. Functional analysis of lymphostatin homologues in enterohaemorrhagic Escherichia coli. FEMS Microbiol. Lett. 25843-49. [DOI] [PubMed] [Google Scholar]
- 3.Chark, D., A. Nutikka, N. Trusevych, J. Kuzmina, and C. Lingwood. 2004. Differential carbohydrate epitope recognition of globotriaosyl ceramide by verotoxins and a monoclonal antibody. Eur. J. Biochem. 271405-417. [DOI] [PubMed] [Google Scholar]
- 4.Chesney, J., and R. Bucala. 1997. Peripheral blood fibrocytes: novel fibroblast-like cells that present antigen and mediate tissue repair. Biochem. Soc. Trans. 25520-524. [DOI] [PubMed] [Google Scholar]
- 5.Cornick, N. A., S. L. Booher, and H. W. Moon. 2002. Intimin facilitates colonization by Escherichia coli O157:H7 in adult ruminants. Infect. Immun. 702704-2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cray, W. C., Jr., and H. W. Moon. 1995. Experimental infection of calves and adult cattle with Escherichia coli O157:H7. Appl. Environ. Microbiol. 61:1586-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dean-Nystrom, E. A., B. T. Bosworth, W. C. Cray, Jr., and H. W. Moon. 1997. Pathogenicity of Escherichia coli O157:H7 in the intestines of neonatal calves. Infect. Immun. 65:1842-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dean-Nystrom, E. A., B. T. Bosworth, and H. W. Moon. 1999. Pathogenesis of Escherichia coli O157:H7 in weaned calves. Adv. Exp. Med. Biol. 473173-177. [DOI] [PubMed] [Google Scholar]
- 9.Dean-Nystrom, E. A., B. T. Bosworth, H. W. Moon, and A. D. O'Brien. 1998. Escherichia coli O157:H7 requires intimin for enteropathogenicity in calves. Infect. Immun. 66:4560-4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dean-Nystrom, E. A., W. C. Stoffregen, B. T. Bosworth, H. W. Moon, and J. F. Pohlenz. Early attachment sites for shigatoxigenic Escherichia coli O157:H7 in experimentally inoculated weaned calves. Appl. Environ. Microbiol., in press. [DOI] [PMC free article] [PubMed]
- 11.Dziva, F., P. M. van Diemen, M. P. Stevens, A. J. Smith, and T. S. Wallis. 2004. Identification of Escherichia coli O157: H7 genes influencing colonization of the bovine gastrointestinal tract using signature-tagged mutagenesis. Microbiology 1503631-3645. [DOI] [PubMed] [Google Scholar]
- 12.Ellis, J. A., W. C. Davis, N. D. MacHugh, D. L. Emery, A. Kaushal, and W. I. Morrison. 1988. Differentiation antigens on bovine mononuclear phagocytes identified by monoclonal antibodies. Vet. Immunol. Immunopathol. 19325-340. [DOI] [PubMed] [Google Scholar]
- 13.Endo, Y., K. Tsurugi, T. Yutsudo, Y. Takeda, T. Ogasawara, and K. Igarashi. 1988. Site of action of a Vero toxin (VT2) from Escherichia coli O157:H7 and of Shiga toxin on eukaryotic ribosomes. RNA N-glycosidase activity of the toxins. Eur. J. Biochem. 17145-50. [DOI] [PubMed] [Google Scholar]
- 14.Ferens, W. A., L. J. Grauke, and C. J. Hovde. 2004. Shiga toxin 1 targets bovine leukemia virus-expressing cells. Infect. Immun. 721837-1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Follmann, W., S. Weber, and S. Birkner. 2000. Primary cell cultures of bovine colon epithelium: isolation and cell culture of colonocytes. Toxicol. In Vitro 14435-445. [DOI] [PubMed] [Google Scholar]
- 16.Galligan, C. L., and B. L. Coomber. 2000. Effects of human IL-8 isoforms on bovine neutrophil function in vitro. Vet. Immunol. Immunopathol. 7471-85. [DOI] [PubMed] [Google Scholar]
- 17.Ghislain, J., C. A. Lingwood, and E. N. Fish. 1994. Evidence for glycosphingolipid modification of the type 1 IFN receptor. J. Immunol. 1533655-3663. [PubMed] [Google Scholar]
- 18.Grauke, L. J., I. T. Kudva, J. W. Yoon, C. W. Hunt, C. J. Williams, and C. J. Hovde. 2002. Gastrointestinal tract location of Escherichia coli O157:H7 in ruminants. Appl. Environ. Microbiol. 682269-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hall, G. A., P. Sopp, and C. J. Howard. 1993. An investigation of temporary workshop clusters reacting with cells of the mononuclear phagocytic system. Vet. Immunol. Immunopathol. 39225-236. [DOI] [PubMed] [Google Scholar]
- 20.Hoey, D. E., C. Currie, R. W. Else, A. Nutikka, C. A. Lingwood, D. L. Gally, and D. G. Smith. 2002. Expression of receptors for verotoxin 1 from Escherichia coli O157 on bovine intestinal epithelium. J. Med. Microbiol. 51143-149. [DOI] [PubMed] [Google Scholar]
- 21.Hoey, D. E., L. Sharp, C. Currie, C. A. Lingwood, D. L. Gally, and D. G. Smith. 2003. Verotoxin 1 binding to intestinal crypt epithelial cells results in localization to lysosomes and abrogation of toxicity. Cell. Microbiol. 585-97. [DOI] [PubMed] [Google Scholar]
- 22.Hoffman, M. A., C. Menge, T. A. Casey, W. Laegreid, B. T. Bosworth, and E. A. Dean-Nystrom. 2006. Bovine immune response to Shiga-toxigenic Escherichia coli O157:H7. Clin. Vaccine Immunol. 131322-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeong, K. C., M. Y. Kang, C. Heimke, J. A. Shere, I. Erol, and C. W. Kaspar. 2007. Isolation of Escherichia coli O157:H7 from the gall bladder of inoculated and naturally-infected cattle. Vet. Microbiol. 119339-345. [DOI] [PubMed] [Google Scholar]
- 24.Jungi, T. W., H. Sager, H. Adler, M. Brcic, and H. Pfister. 1997. Serum factors, cell membrane CD14, and β2 integrins are not required for activation of bovine macrophages by lipopolysaccharide. Infect. Immun. 653577-3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kandel, G., A. Donohue-Rolfe, M. Donowitz, and G. T. Keusch. 1989. Pathogenesis of Shigella diarrhea. XVI. Selective targeting of Shiga toxin to villus cells of rabbit jejunum explains the effect of the toxin on intestinal electrolyte transport. J. Clin. Investig. 841509-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karmali, M. A. 1989. Infection by verocytotoxin-producing Escherichia coli. Clin. Microbiol. Rev. 215-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khine, A. A., and C. A. Lingwood. 2000. Functional significance of globotriaosyl ceramide in interferon-alpha(2)/type 1 interferon receptor-mediated antiviral activity. J. Cell Physiol. 18297-108. [DOI] [PubMed] [Google Scholar]
- 28.Kingston, D., and J. R. Pearson. 1981. The use of the peroxidase reaction to obliterate staining of eosinophils by fluorescein-labelled conjugates. J. Immunol. Methods 44191-198. [DOI] [PubMed] [Google Scholar]
- 29.Kovbasnjuk, O., R. Mourtazina, B. Baibakov, T. Wang, C. Elowsky, M. A. Choti, A. Kane, and M. Donowitz. 2005. The glycosphingolipid globotriaosylceramide in the metastatic transformation of colon cancer. Proc. Natl. Acad. Sci. USA 10219087-19092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lala, P., S. Ito, and C. A. Lingwood. 2000. Retroviral transfection of Madin-Darby canine kidney cells with human MDR1 results in a major increase in globotriaosylceramide and 10(5)- to 10(6)-fold increased cell sensitivity to verocytotoxin. Role of p-glycoprotein in glycolipid synthesis. J. Biol. Chem. 2756246-6251. [DOI] [PubMed] [Google Scholar]
- 31.Leutenegger, C. M., A. M. Alluwaimi, W. L. Smith, L. Perani, and J. S. Cullor. 2000. Quantitation of bovine cytokine mRNA in milk cells of healthy cattle by real-time TaqMan polymerase chain reaction. Vet. Immunol. Immunopathol. 77275-287. [DOI] [PubMed] [Google Scholar]
- 32.Liebler, E. M., J. Waschbusch, J. F. Pohlenz, V. Moennig, and B. Liess. 1991. Distribution of antigen of noncytopathogenic and cytopathogenic bovine virus diarrhea virus biotypes in the intestinal tract of calves following experimental production of mucosal disease. Arch. Virol. Suppl. 3109-124. [DOI] [PubMed] [Google Scholar]
- 33.Lim, J. Y., J. Li, H. Sheng, T. E. Besser, K. Potter, and C. J. Hovde. 2007. Escherichia coli O157:H7 colonization at the rectoanal junction of long-duration culture-positive cattle. Appl. Environ. Microbiol. 731380-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Macpherson, A. J., and T. Uhr. 2004. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science 3031662-1665. [DOI] [PubMed] [Google Scholar]
- 35.Magnuson, B. A., M. Davis, S. Hubele, P. R. Austin, I. T. Kudva, C. J. Williams, C. W. Hunt, and C. J. Hovde. 2000. Ruminant gastrointestinal cell proliferation and clearance of Escherichia coli O157:H7. Infect. Immun. 683808-3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McKeever, D. J., N. D. MacHugh, B. M. Goddeeris, E. Awino, and W. I. Morrison. 1991. Bovine afferent lymph veiled cells differ from blood monocytes in phenotype and accessory function. J. Immunol. 1473703-3709. [PubMed] [Google Scholar]
- 37.Menge, C. 2003. Protocols to study effects of Shiga toxin on mononuclear leukocytes. Methods Mol. Med. 73275-289. [DOI] [PubMed] [Google Scholar]
- 38.Menge, C., I. Stamm, P. M. Van Diemen, P. Sopp, G. Baljer, T. S. Wallis, and M. P. Stevens. 2004. Phenotypic and functional characterization of intraepithelial lymphocytes in a bovine ligated intestinal loop model of enterohaemorrhagic Escherichia coli infection. J. Med. Microbiol. 53573-579. [DOI] [PubMed] [Google Scholar]
- 39.Menge, C., L. H. Wieler, T. Schlapp, and G. Baljer. 1999. Shiga toxin 1 from Escherichia coli blocks activation and proliferation of bovine lymphocyte subpopulations in vitro. Infect. Immun. 672209-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mobassaleh, M., A. Donohue-Rolfe, M. Jacewicz, R. J. Grand, and G. T. Keusch. 1988. Pathogenesis of shigella diarrhea: evidence for a developmentally regulated glycolipid receptor for shigella toxin involved in the fluid secretory response of rabbit small intestine. J. Infect. Dis. 1571023-1031. [DOI] [PubMed] [Google Scholar]
- 41.Mosser, D. M. 2003. The many faces of macrophage activation. J. Leukoc. Biol. 73209-212. [DOI] [PubMed] [Google Scholar]
- 42.Moussay, E., I. Stamm, A. Taubert, G. Baljer, and C. Menge. 2006. Escherichia coli Shiga toxin 1 enhances il-4 transcripts in bovine ileal intraepithelial lymphocytes. Vet. Immunol. Immunopathol. 113367-382. [DOI] [PubMed] [Google Scholar]
- 43.Moxley, R. A., and D. H. Francis. 1986. Natural and experimental infection with an attaching and effacing strain of Escherichia coli in calves. Infect. Immun. 53339-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Naylor, S. W., J. C. Low, T. E. Besser, A. Mahajan, G. J. Gunn, M. C. Pearce, I. J. McKendrick, D. G. Smith, and D. L. Gally. 2003. Lymphoid follicle-dense mucosa at the terminal rectum is the principal site of colonization of enterohemorrhagic Escherichia coli O157:H7 in the bovine host. Infect. Immun. 711505-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Niedergang, F., A. Didierlaurent, J. P. Kraehenbuhl, and J. C. Sirard. 2004. Dendritic cells: the host Achilles’ heel for mucosal pathogens? Trends Microbiol. 1279-88. [DOI] [PubMed] [Google Scholar]
- 46.Olsnes, S., R. Reisbig, and K. Eiklid. 1981. Subunit structure of Shigella cytotoxin. J. Biol. Chem. 2568732-8738. [PubMed] [Google Scholar]
- 47.Paton, J. C., and A. W. Paton. 1998. Pathogenesis and diagnosis of Shiga toxin-producing Escherichia coli infections. Clin. Microbiol. Rev. 11450-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pauly, T., K. Elbers, M. Konig, T. Lengsfeld, A. Saalmuller, and H. J. Thiel. 1995. Classical swine fever virus-specific cytotoxic T lymphocytes and identification of a T cell epitope. J. Gen. Virol. 763039-3049. [DOI] [PubMed] [Google Scholar]
- 49.Peterson, R. E., T. J. Klopfenstein, R. A. Moxley, G. E. Erickson, S. Hinkley, G. Bretschneider, E. M. Berberov, D. Rogan, and D. R. Smith. 2007. Effect of a vaccine product containing type III secreted proteins on the probability of Escherichia coli O157:H7 fecal shedding and mucosal colonization in feedlot cattle. J. Food Prot. 702568-2577. [DOI] [PubMed] [Google Scholar]
- 50.Peterson, R. E., T. J. Klopfenstein, R. A. Moxley, G. E. Erickson, S. Hinkley, D. Rogan, and D. R. Smith. 2007. Efficacy of dose regimen and observation of herd immunity from a vaccine against Escherichia coli O157:H7 for feedlot cattle. J. Food Prot. 702561-2567. [DOI] [PubMed] [Google Scholar]
- 51.Phillips, A. D., and G. Frankel. 2000. Intimin-mediated tissue specificity in enteropathogenic Escherichia coli interaction with human intestinal organ cultures. J. Infect. Dis. 1811496-1500. [DOI] [PubMed] [Google Scholar]
- 52.Potter, A. A., S. Klashinsky, Y. Li, E. Frey, H. Townsend, D. Rogan, G. Erickson, S. Hinkley, T. Klopfenstein, R. A. Moxley, D. R. Smith, and B. B. Finlay. 2004. Decreased shedding of Escherichia coli O157:H7 by cattle following vaccination with type III secreted proteins. Vaccine 22362-369. [DOI] [PubMed] [Google Scholar]
- 53.Powell, D. W., P. A. Adegboyega, J. F. Di Mari, and R. C. Mifflin. 2005. Epithelial cells and their neighbors. I. Role of intestinal myofibroblasts in development, repair, and cancer. Am. J. Physiol. Gastrointest. Liver Physiol. 289G2-7. [DOI] [PubMed] [Google Scholar]
- 54.Pruimboom-Brees, I. M., T. W. Morgan, M. R. Ackermann, E. D. Nystrom, J. E. Samuel, N. A. Cornick, and H. W. Moon. 2000. Cattle lack vascular receptors for Escherichia coli O157:H7 Shiga toxins. Proc. Natl. Acad. Sci. USA 9710325-10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reinstein, S., J. T. Fox, X. Shi, and T. G. Nagaraja. 2007. Prevalence of Escherichia coli O157:H7 in gallbladders of beef cattle. Appl. Environ. Microbiol. 731002-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rescigno, M., M. Urbano, B. Valzasina, M. Francolini, G. Rotta, R. Bonasio, F. Granucci, J. P. Kraehenbuhl, and P. Ricciardi-Castagnoli. 2001. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat. Immunol. 2361-367. [DOI] [PubMed] [Google Scholar]
- 57.Schuller, S., G. Frankel, and A. D. Phillips. 2004. Interaction of Shiga toxin from Escherichia coli with human intestinal epithelial cell lines and explants: Stx2 induces epithelial damage in organ culture. Cell. Microbiol. 6289-301. [DOI] [PubMed] [Google Scholar]
- 58.Smith, P. D., E. N. Janoff, M. Mosteller-Barnum, M. Merger, J. M. Orenstein, J. F. Kearney, and M. F. Graham. 1997. Isolation and purification of CD14-negative mucosal macrophages from normal human small intestine. J. Immunol. Methods 2021-11. [DOI] [PubMed] [Google Scholar]
- 59.Snider, T. A., A. J. Fabich, K. E. Washburn, W. P. Sims, J. L. Blair, P. S. Cohen, T. Conway, and K. D. Clinkenbeard. 2006. Evaluation of a model for Escherichia coli O157:H7 colonization in streptomycin-treated adult cattle. Am. J. Vet. Res. 671914-1920. [DOI] [PubMed] [Google Scholar]
- 60.Stamm, I., M. Wuhrer, R. Geyer, G. Baljer, and C. Menge. 2002. Bovine lymphocytes express functional receptors for Escherichia coli Shiga toxin 1. Microb. Pathog. 33251-264. [DOI] [PubMed] [Google Scholar]
- 61.Stevens, M. P., P. M. van Diemen, F. Dziva, P. W. Jones, and T. S. Wallis. 2002. Options for the control of enterohaemorrhagic Escherichia coli in ruminants. Microbiology 1483767-3778. [DOI] [PubMed] [Google Scholar]
- 62.Stoffregen, W. C., J. F. Pohlenz, and E. A. Dean-Nystrom. 2004. Escherichia coli O157:H7 in the gallbladders of experimentally infected calves. J. Vet. Diagn. Investig. 1679-83. [DOI] [PubMed] [Google Scholar]
- 63.Strockbine, N. A., L. R. Marques, R. K. Holmes, and A. D. O'Brien. 1985. Characterization of monoclonal antibodies against Shiga-like toxin from Escherichia coli. Infect. Immun. 50695-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.te Loo, D. M., L. A. Monnens, T. J. van Der Velden, M. A. Vermeer, F. Preyers, P. N. Demacker, L. P. van Den Heuvel, and V. W. van Hinsbergh. 2000. Binding and transfer of verocytotoxin by polymorphonuclear leukocytes in hemolytic uremic syndrome. Blood 953396-3402. [PubMed] [Google Scholar]
- 65.Thorpe, C. M., B. P. Hurley, L. L. Lincicome, M. S. Jacewicz, G. T. Keusch, and D. W. Acheson. 1999. Shiga toxins stimulate secretion of interleukin-8 from intestinal epithelial cells. Infect. Immun. 675985-5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thorpe, C. M., W. E. Smith, B. P. Hurley, and D. W. Acheson. 2001. Shiga toxins induce, superinduce, and stabilize a variety of C-X-C chemokine mRNAs in intestinal epithelial cells, resulting in increased chemokine expression. Infect. Immun. 696140-6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van Diemen, P. M., F. Dziva, A. Abu-Median, T. S. Wallis, H. van den Bosch, G. Dougan, N. Chanter, G. Frankel, and M. P. Stevens. 2007. Subunit vaccines based on intimin and Efa-1 polypeptides induce humoral immunity in cattle but do not protect against intestinal colonisation by enterohaemorrhagic Escherichia coli O157:H7 or O26:H. Vet. Immunol. Immunopathol. 11647-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van Diemen, P. M., F. Dziva, M. P. Stevens, and T. S. Wallis. 2005. Identification of enterohemorrhagic Escherichia coli O26:H− genes required for intestinal colonization in calves. Infect. Immun. 731735-1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Werling, D., J. C. Hope, C. J. Howard, and T. W. Jungi. 2004. Differential production of cytokines, reactive oxygen and nitrogen by bovine macrophages and dendritic cells stimulated with Toll-like receptor agonists. Immunology 11141-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Widiasih, D. A., N. Ido, K. Omoe, S. Sugii, and K. Shinagawa. 2004. Duration and magnitude of faecal shedding of Shiga toxin-producing Escherichia coli from naturally infected cattle. Epidemiol. Infect. 13267-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wieler, L. H., A. Schwanitz, E. Vieler, B. Busse, H. Steinruck, J. B. Kaper, and G. Baljer. 1998. Virulence properties of Shiga toxin-producing Escherichia coli (STEC) strains of serogroup O118, a major group of STEC pathogens in calves. J. Clin. Microbiol. 361604-1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wieler, L. H., G. Sobjinski, T. Schlapp, K. Failing, R. Weiss, C. Menge, and G. Baljer. 2007. Longitudinal prevalence study of diarrheagenic Escherichia coli in dairy calves. Berl. Munch. Tierarztl. Wochenschr. 120296-306. [PubMed] [Google Scholar]
- 73.Worbs, T., U. Bode, S. Yan, M. W. Hoffmann, G. Hintzen, G. Bernhardt, R. Forster, and O. Pabst. 2006. Oral tolerance originates in the intestinal immune system and relies on antigen carriage by dendritic cells. J. Exp. Med. 203519-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yamasaki, C., Y. Natori, X. T. Zeng, M. Ohmura, S. Yamasaki, and Y. Takeda. 1999. Induction of cytokines in a human colon epithelial cell line by Shiga toxin 1 (Stx1) and Stx2 but not by nontoxic mutant Stx1 which lacks N-glycosidase activity. FEBS Lett. 442231-234. [DOI] [PubMed] [Google Scholar]