Abstract
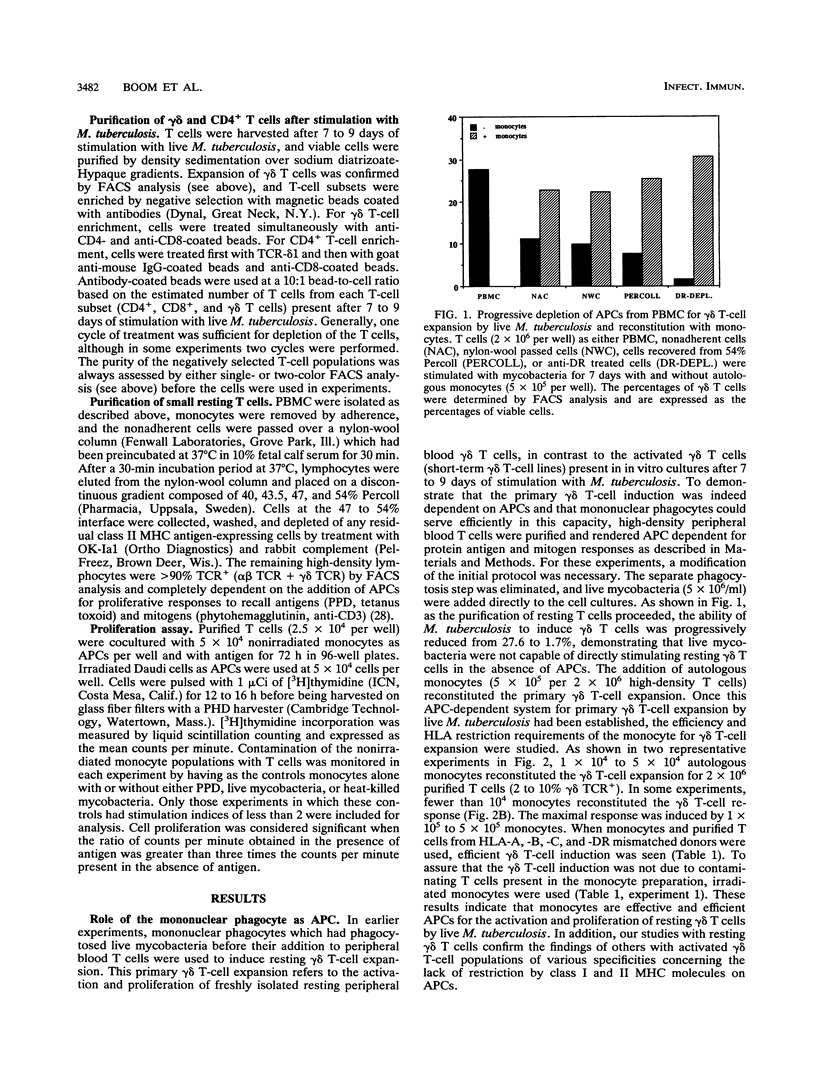
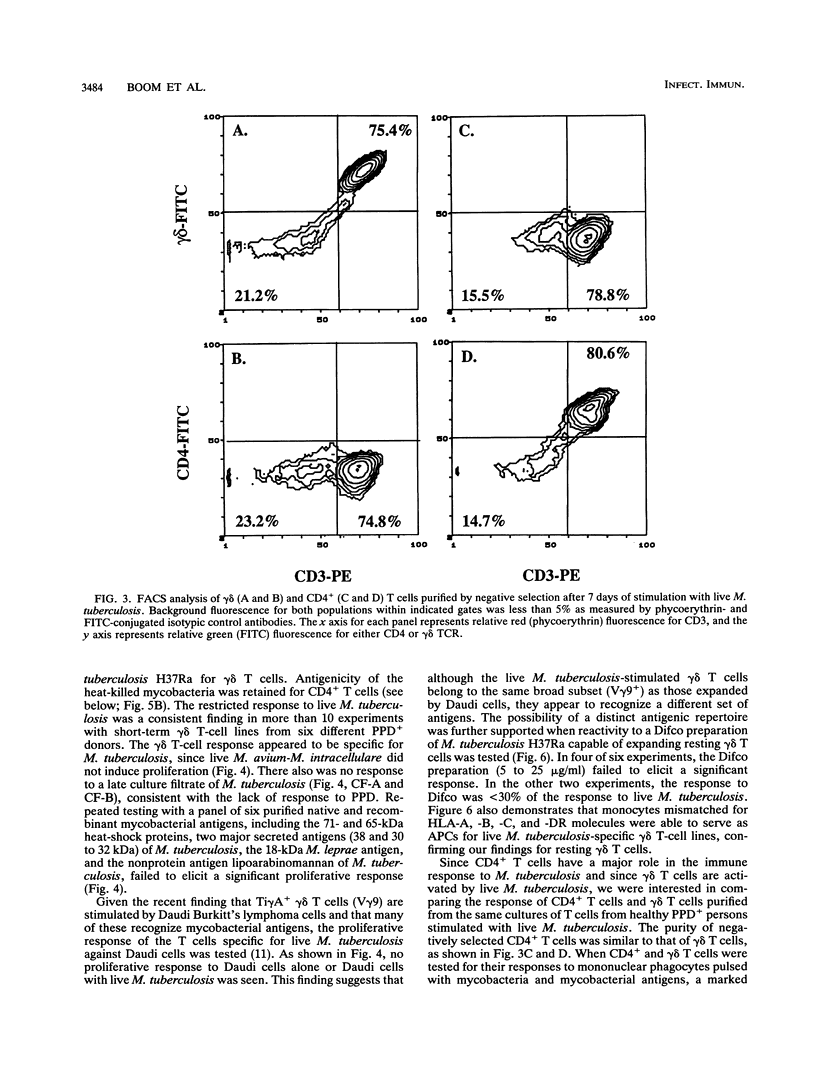
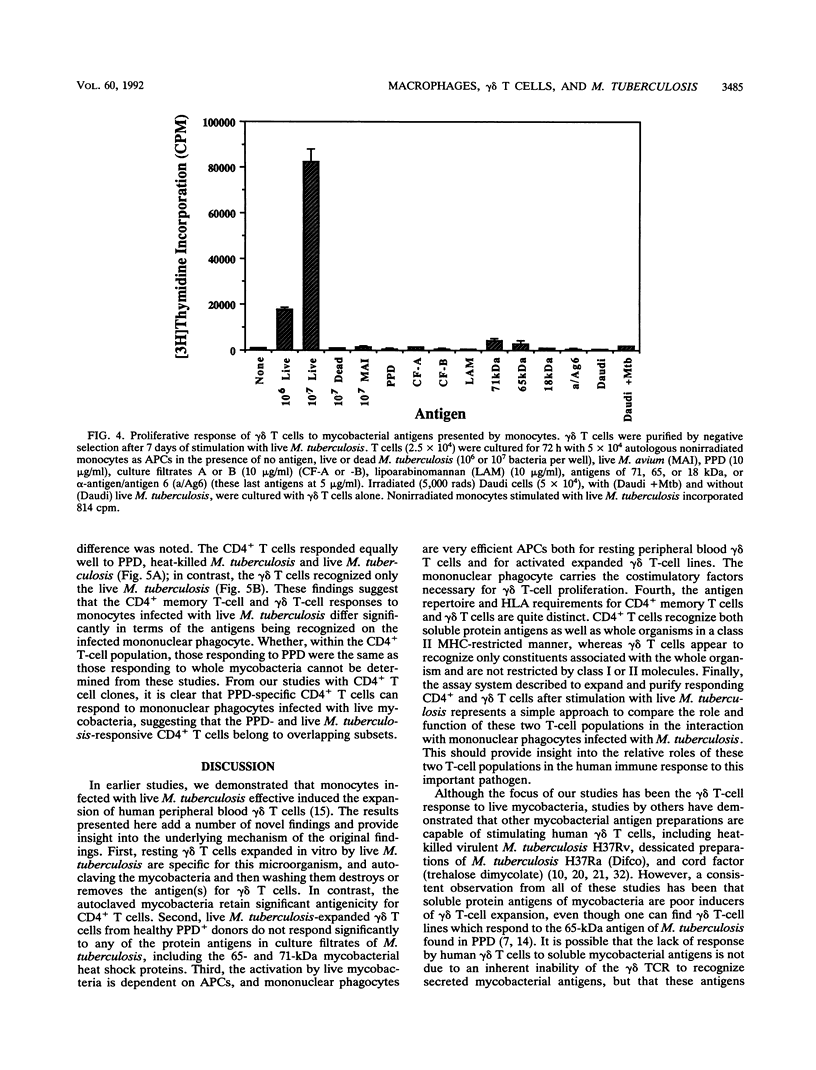
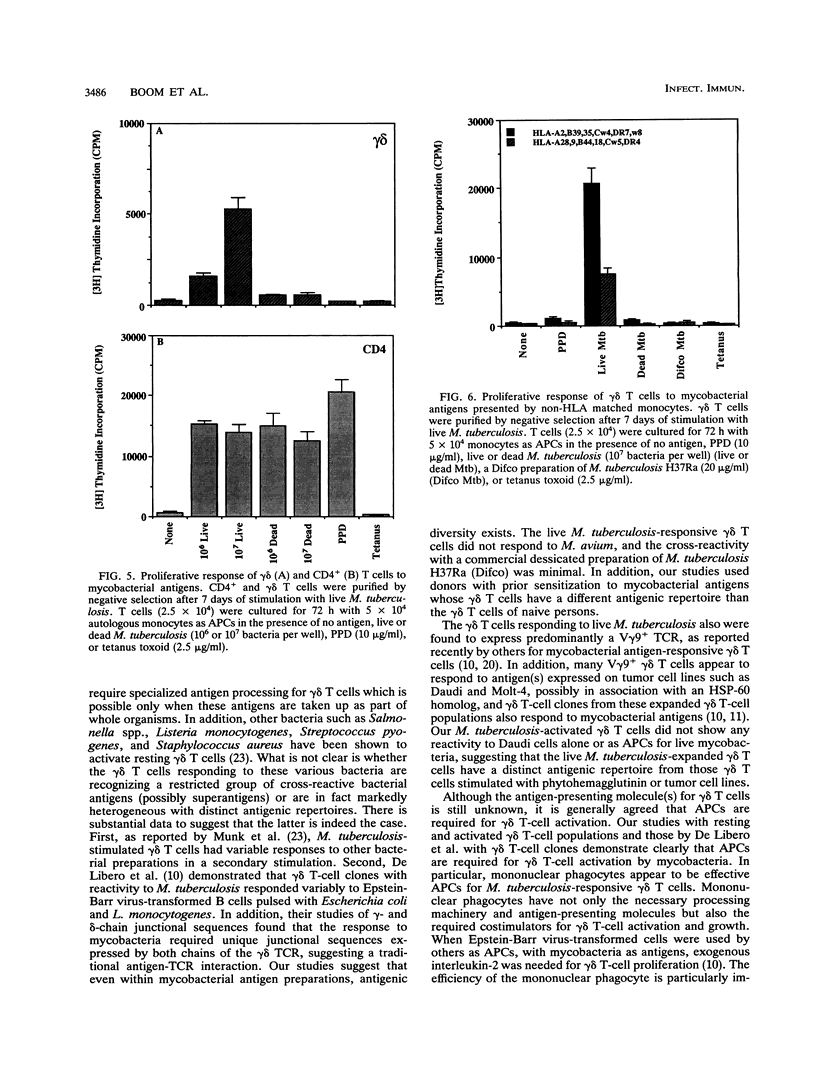
gamma delta T cells, both human and murine, have been found to be highly responsive to mycobacterial antigens. However, the role and function of gamma delta T cells in the immune response to Mycobacterium tuberculosis remain largely unknown. In earlier studies, we demonstrated that monocytes infected with live M. tuberculosis were particularly effective inducers of human peripheral blood gamma delta T cells. The present studies were performed to further characterize the interaction between human mononuclear phagocytes, gamma delta T cells, and live M. tuberculosis, in comparison with CD4+ T cells. First, we found that resting gamma delta T cells expanded in vitro by live M. tuberculosis were specific for M. tuberculosis, and that heat killing and washing the mycobacteria removed the antigen(s) for gamma delta T cells. In contrast, the heat-killed mycobacteria retained significant antigenicity for CD4+ T cells. Second, live M. tuberculosis-expanded gamma delta T cells from healthy tuberculin-positive donors did not respond significantly to the antigens in M. tuberculosis culture filtrate, including the 65- and 71-kDa mycobacterial heat shock proteins. Third, the activation of gamma delta T cells by live mycobacteria was dependent on antigen-presenting cells, and mononuclear phagocytes were found to be very efficient antigen-presenting cells both for resting peripheral blood gamma delta T cells and for activated expanded gamma delta T cells. The mononuclear phagocyte carried the necessary costimulatory factors necessary for gamma delta T-cell proliferation. Fourth, the antigen repertoire and HLA requirements for CD4+ memory T cells and those for gamma delta T cells appear to be quite distinct from each other. CD4+ T cells recognized both soluble protein antigens and whole organisms in a class II major histocompatibility complex-restricted manner, whereas gamma delta T cells appeared to recognize only constituents associated with the whole organism and were not restricted by class I or class II major histocompatibility complex molecules. Finally, the assay system described to expand and purify responding CD4+ and gamma delta T cells after stimulation with live M. tuberculosis represented a simple approach to the direct comparison of these two T-cell populations in the interaction with mononuclear phagocytes infected with M. tuberculosis. Such studies provide insight not only into the relative roles of human CD4+ and gamma delta T cells in the human immune response to intracellular bacterial pathogens such as M. tuberculosis but also into the basic biologic role of human gamma delta T cells in antimicrobial immunity.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen A. B., Hansen E. B. Structure and mapping of antigenic domains of protein antigen b, a 38,000-molecular-weight protein of Mycobacterium tuberculosis. Infect Immun. 1989 Aug;57(8):2481–2488. doi: 10.1128/iai.57.8.2481-2488.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustin A., Kubo R. T., Sim G. K. Resident pulmonary lymphocytes expressing the gamma/delta T-cell receptor. Nature. 1989 Jul 20;340(6230):239–241. doi: 10.1038/340239a0. [DOI] [PubMed] [Google Scholar]
- Band H., Hochstenbach F., McLean J., Hata S., Krangel M. S., Brenner M. B. Immunochemical proof that a novel rearranging gene encodes the T cell receptor delta subunit. Science. 1987 Oct 30;238(4827):682–684. doi: 10.1126/science.3672118. [DOI] [PubMed] [Google Scholar]
- Boom W. H., Wallis R. S., Chervenak K. A. Human Mycobacterium tuberculosis-reactive CD4+ T-cell clones: heterogeneity in antigen recognition, cytokine production, and cytotoxicity for mononuclear phagocytes. Infect Immun. 1991 Aug;59(8):2737–2743. doi: 10.1128/iai.59.8.2737-2743.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth R. J., Harris D. P., Love J. M., Watson J. D. Antigenic proteins of Mycobacterium leprae. Complete sequence of the gene for the 18-kDa protein. J Immunol. 1988 Jan 15;140(2):597–601. [PubMed] [Google Scholar]
- Born W., Hall L., Dallas A., Boymel J., Shinnick T., Young D., Brennan P., O'Brien R. Recognition of a peptide antigen by heat shock--reactive gamma delta T lymphocytes. Science. 1990 Jul 6;249(4964):67–69. doi: 10.1126/science.1695022. [DOI] [PubMed] [Google Scholar]
- Daniel T. M., Ferguson L. E. Purification and Characterization of Two Proteins from Culture Filtrates of Mycobacterium tuberculosis H(37)Ra Strain. Infect Immun. 1970 Feb;1(2):164–168. doi: 10.1128/iai.1.2.164-168.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Libero G., Casorati G., Giachino C., Carbonara C., Migone N., Matzinger P., Lanzavecchia A. Selection by two powerful antigens may account for the presence of the major population of human peripheral gamma/delta T cells. J Exp Med. 1991 Jun 1;173(6):1311–1322. doi: 10.1084/jem.173.6.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisch P., Malkovsky M., Kovats S., Sturm E., Braakman E., Klein B. S., Voss S. D., Morrissey L. W., DeMars R., Welch W. J. Recognition by human V gamma 9/V delta 2 T cells of a GroEL homolog on Daudi Burkitt's lymphoma cells. Science. 1990 Nov 30;250(4985):1269–1273. doi: 10.1126/science.1978758. [DOI] [PubMed] [Google Scholar]
- Griffin J. P., Harshan K. V., Born W. K., Orme I. M. Kinetics of accumulation of gamma delta receptor-bearing T lymphocytes in mice infected with live mycobacteria. Infect Immun. 1991 Nov;59(11):4263–4265. doi: 10.1128/iai.59.11.4263-4265.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn H., Kaufmann S. H. The role of cell-mediated immunity in bacterial infections. Rev Infect Dis. 1981 Nov-Dec;3(6):1221–1250. doi: 10.1093/clinids/3.6.1221. [DOI] [PubMed] [Google Scholar]
- Haregewoin A., Soman G., Hom R. C., Finberg R. W. Human gamma delta+ T cells respond to mycobacterial heat-shock protein. Nature. 1989 Jul 27;340(6231):309–312. doi: 10.1038/340309a0. [DOI] [PubMed] [Google Scholar]
- Havlir D. V., Ellner J. J., Chervenak K. A., Boom W. H. Selective expansion of human gamma delta T cells by monocytes infected with live Mycobacterium tuberculosis. J Clin Invest. 1991 Feb;87(2):729–733. doi: 10.1172/JCI115053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holoshitz J., Koning F., Coligan J. E., De Bruyn J., Strober S. Isolation of CD4- CD8- mycobacteria-reactive T lymphocyte clones from rheumatoid arthritis synovial fluid. Nature. 1989 May 18;339(6221):226–229. doi: 10.1038/339226a0. [DOI] [PubMed] [Google Scholar]
- Inoue T., Yoshikai Y., Matsuzaki G., Nomoto K. Early appearing gamma/delta-bearing T cells during infection with Calmétte Guérin bacillus. J Immunol. 1991 Apr 15;146(8):2754–2762. [PubMed] [Google Scholar]
- Janis E. M., Kaufmann S. H., Schwartz R. H., Pardoll D. M. Activation of gamma delta T cells in the primary immune response to Mycobacterium tuberculosis. Science. 1989 May 12;244(4905):713–716. doi: 10.1126/science.2524098. [DOI] [PubMed] [Google Scholar]
- Kabelitz D., Bender A., Prospero T., Wesselborg S., Janssen O., Pechhold K. The primary response of human gamma/delta + T cells to Mycobacterium tuberculosis is restricted to V gamma 9-bearing cells. J Exp Med. 1991 Jun 1;173(6):1331–1338. doi: 10.1084/jem.173.6.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabelitz D., Bender A., Schondelmaier S., Schoel B., Kaufmann S. H. A large fraction of human peripheral blood gamma/delta + T cells is activated by Mycobacterium tuberculosis but not by its 65-kD heat shock protein. J Exp Med. 1990 Mar 1;171(3):667–679. doi: 10.1084/jem.171.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modlin R. L., Pirmez C., Hofman F. M., Torigian V., Uyemura K., Rea T. H., Bloom B. R., Brenner M. B. Lymphocytes bearing antigen-specific gamma delta T-cell receptors accumulate in human infectious disease lesions. Nature. 1989 Jun 15;339(6225):544–548. doi: 10.1038/339544a0. [DOI] [PubMed] [Google Scholar]
- Munk M. E., Gatrill A. J., Kaufmann S. H. Target cell lysis and IL-2 secretion by gamma/delta T lymphocytes after activation with bacteria. J Immunol. 1990 Oct 15;145(8):2434–2439. [PubMed] [Google Scholar]
- Murray C. J., Styblo K., Rouillon A. Tuberculosis in developing countries: burden, intervention and cost. Bull Int Union Tuberc Lung Dis. 1990 Mar;65(1):6–24. [PubMed] [Google Scholar]
- O'Brien R. L., Happ M. P., Dallas A., Palmer E., Kubo R., Born W. K. Stimulation of a major subset of lymphocytes expressing T cell receptor gamma delta by an antigen derived from Mycobacterium tuberculosis. Cell. 1989 May 19;57(4):667–674. doi: 10.1016/0092-8674(89)90135-9. [DOI] [PubMed] [Google Scholar]
- Patel S. S., Wacholtz M. C., Duby A. D., Thiele D. L., Lipsky P. E. Analysis of the functional capabilities of CD3+CD4-CD8- and CD3+CD4+CD8+ human T cell clones. J Immunol. 1989 Aug 15;143(4):1108–1117. [PubMed] [Google Scholar]
- Pfeffer K., Schoel B., Gulle H., Kaufmann S. H., Wagner H. Primary responses of human T cells to mycobacteria: a frequent set of gamma/delta T cells are stimulated by protease-resistant ligands. Eur J Immunol. 1990 May;20(5):1175–1179. doi: 10.1002/eji.1830200534. [DOI] [PubMed] [Google Scholar]
- Rich E. A., Ellner J. J. Preliminary characterization of accessory cells in the nonadherent fraction of human blood mononuclear cells. Cell Immunol. 1986 Dec;103(2):339–351. doi: 10.1016/0008-8749(86)90094-8. [DOI] [PubMed] [Google Scholar]
- Rivas A., Koide J., Cleary M. L., Engleman E. G. Evidence for involvement of the gamma, delta T cell antigen receptor in cytotoxicity mediated by human alloantigen-specific T cell clones. J Immunol. 1989 Mar 15;142(6):1840–1846. [PubMed] [Google Scholar]
- Shinnick T. M., Krat C., Schadow S. Isolation and restriction site maps of the genes encoding five Mycobacterium tuberculosis proteins. Infect Immun. 1987 Jul;55(7):1718–1721. doi: 10.1128/iai.55.7.1718-1721.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triebel F., Faure F., Graziani M., Jitsukawa S., Lefranc M. P., Hercend T. A unique V-J-C-rearranged gene encodes a gamma protein expressed on the majority of CD3+ T cell receptor-alpha/beta- circulating lymphocytes. J Exp Med. 1988 Feb 1;167(2):694–699. doi: 10.1084/jem.167.2.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuyuguchi I., Kawasumi H., Ueta C., Yano I., Kishimoto S. Increase of T-cell receptor gamma/delta-bearing T cells in cord blood of newborn babies obtained by in vitro stimulation with mycobacterial cord factor. Infect Immun. 1991 Sep;59(9):3053–3059. doi: 10.1128/iai.59.9.3053-3059.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y. J., Tian W. T., Snider R. M., Rittershaus C., Rogers P., LaManna L., Ip S. H. Signal transduction of gamma/delta T cell antigen receptor with a novel mitogenic anti-delta antibody. J Immunol. 1988 Sep 1;141(5):1476–1479. [PubMed] [Google Scholar]


