Abstract
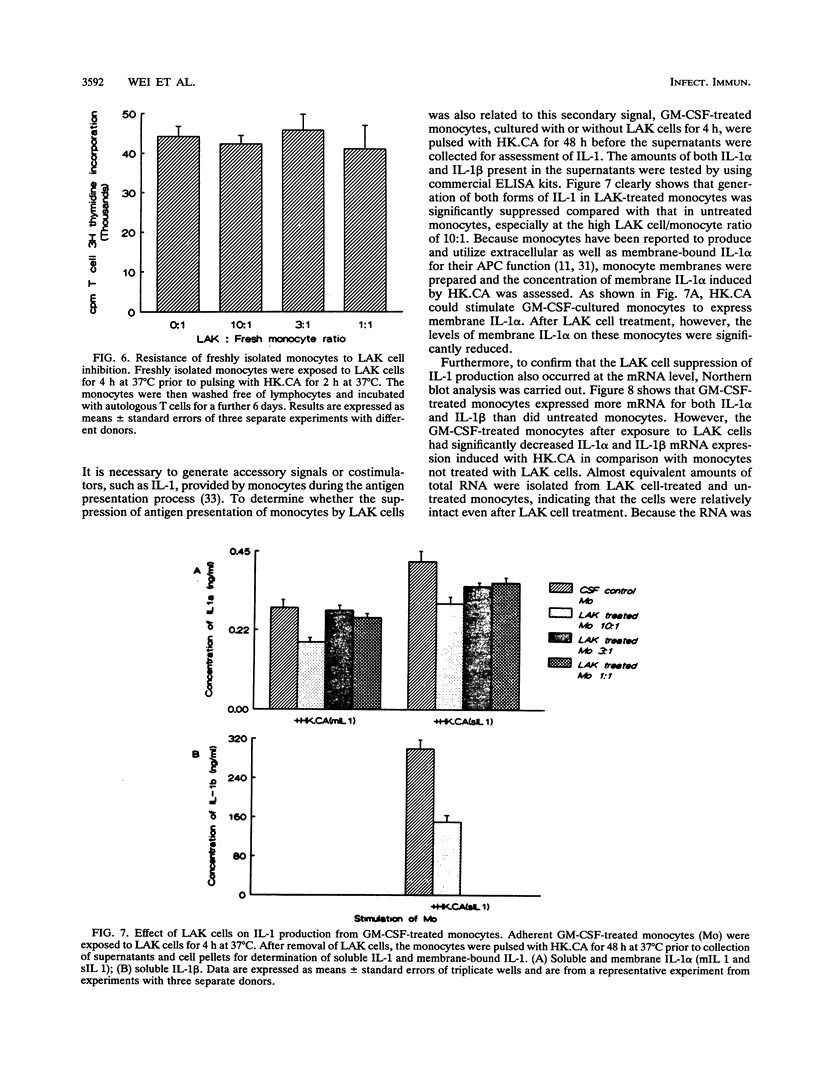
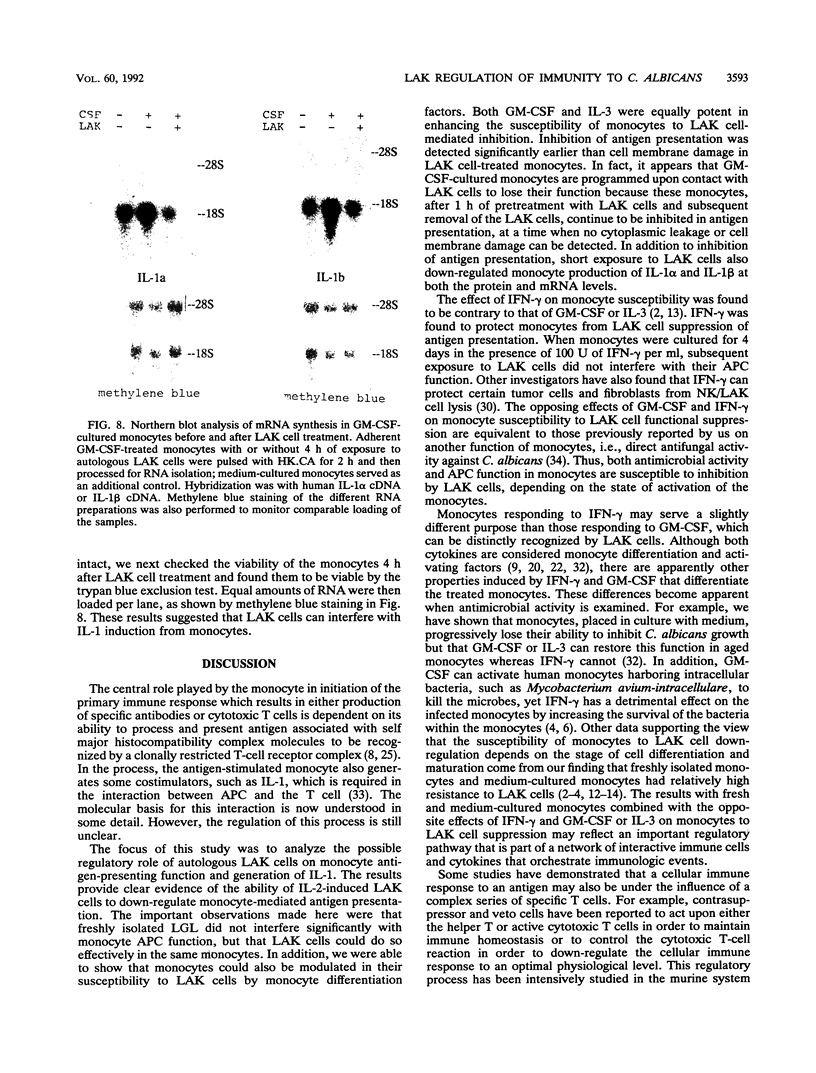
Monocytes are important accessory cells in the activation of T cells for specific antigen recognition yet little is known of their regulation. We demonstrated here that interleukin-2 (IL-2)-induced human lymphokine-activated killer (LAK) cells can inhibit monocyte antigen presentation, depending on the state of differentiation of the monocytes. Adherent monocytes cultured for 4 days in medium or granulocyte-macrophage colony-stimulating factor (GM-CSF) were found to equally process and present intact Candida albicans to autologous Percoll gradient-isolated T cells, as measured by [3H]thymidine uptake. However, only the GM-CSF-cultured monocytes were functionally inhibited by autologous 4-day IL-2-induced LAK cells. Even soluble candidal cell wall mannoprotein antigens could not be presented by these monocytes after exposure to LAK cells. Pretreatment of these monocytes with LAK cells for 1 h, followed by subsequent removal of the nonadherent LAK cells, was sufficient to cause significant inhibition, with maximal inhibition observed after 4 h. Northern (RNA) blot analysis indicated that mRNA expression for IL-1 alpha and IL-1 beta in response to C. albicans stimulation was also down-regulated in GM-CSF-cultured monocytes exposed to LAK cells. Interestingly, freshly isolated, Percoll gradient-purified large granular lymphocytes did not suppress antigen presentation in GM-CSF-treated monocytes. Another important finding was the inability of LAK cells to suppress the ability of freshly isolated or gamma interferon-cultured monocytes, which are resistant to LAK cell-mediated lysis, to present antigen to T cells. In contrast, IL-3 was similar to GM-CSF in inducing LAK cell susceptibility in monocytes. Taken together, these results indicated that IL-2 can induce LAK cells to down-regulate antigen presentation function in a select set of monocytes that have been activated by colony-stimulating factor (GM-CSF and IL-3) but not by gamma interferon. LAK cells may therefore play an important role in regulation of monocytes and their function, depending on their differentiation state.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ausiello C. M., Palma C., Spagnoli G. C., Piazza A., Casciani C. U., Cassone A. Cytotoxic effectors in human peripheral blood mononuclear cells induced by a mannoprotein complex of Candida albicans: a comparison with interleukin 2-activated killer cells. Cell Immunol. 1989 Jul;121(2):349–359. doi: 10.1016/0008-8749(89)90033-6. [DOI] [PubMed] [Google Scholar]
- Blanchard D. K., Djeu J. Y. Protection of cultured human monocytes from lymphokine-activated killer-mediated lysis by IFN-gamma. J Immunol. 1988 Dec 1;141(11):4067–4073. [PubMed] [Google Scholar]
- Blanchard D. K., Michelini-Norris M. B., Djeu J. Y. Interferon decreases the growth inhibition of Mycobacterium avium-intracellulare complex by fresh human monocytes but not by culture-derived macrophages. J Infect Dis. 1991 Jul;164(1):152–157. doi: 10.1093/infdis/164.1.152. [DOI] [PubMed] [Google Scholar]
- Blanchard D. K., Michelini-Norris M. B., Friedman H., Djeu J. Y. Lysis of mycobacteria-infected monocytes by IL-2-activated killer cells: role of LFA-1. Cell Immunol. 1989 Apr 1;119(2):402–411. doi: 10.1016/0008-8749(89)90254-2. [DOI] [PubMed] [Google Scholar]
- Blanchard D. K., Michelini-Norris M. B., Pearson C. A., McMillen S., Djeu J. Y. Production of granulocyte-macrophage colony-stimulating factor (GM-CSF) by monocytes and large granular lymphocytes stimulated with Mycobacterium avium-M. intracellulare: activation of bactericidal activity by GM-CSF. Infect Immun. 1991 Jul;59(7):2396–2402. doi: 10.1128/iai.59.7.2396-2402.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard D. K., Serbousek D., Djeu J. Y. Induction of human monocyte susceptibility to lymphokine-activated killer cell lysis by granulocyte-macrophage colony-stimulating factor. Cancer Res. 1989 Sep 15;49(18):5037–5043. [PubMed] [Google Scholar]
- Blanchard D. K., Stewart W. E., 2nd, Klein T. W., Friedman H., Djeu J. Y. Cytolytic activity of human peripheral blood leukocytes against Legionella pneumophila-infected monocytes: characterization of the effector cell and augmentation by interleukin 2. J Immunol. 1987 Jul 15;139(2):551–556. [PubMed] [Google Scholar]
- Braciale T. J., Morrison L. A., Sweetser M. T., Sambrook J., Gething M. J., Braciale V. L. Antigen presentation pathways to class I and class II MHC-restricted T lymphocytes. Immunol Rev. 1987 Aug;98:95–114. doi: 10.1111/j.1600-065x.1987.tb00521.x. [DOI] [PubMed] [Google Scholar]
- Buckle A. M., Jayaram Y., Hogg N. Colony-stimulating factors and interferon-gamma differentially affect cell surface molecules shared by monocytes and neutrophils. Clin Exp Immunol. 1990 Aug;81(2):339–345. doi: 10.1111/j.1365-2249.1990.tb03342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A. Biology of interleukin 1. FASEB J. 1988 Feb;2(2):108–115. [PubMed] [Google Scholar]
- Djeu J. Y., Blanchard D. K. Interferon-gamma-induced alterations of monocyte susceptibility to lysis by autologous lymphokine-activated killer (LAK) cells. Int J Cancer. 1988 Sep 15;42(3):449–454. doi: 10.1002/ijc.2910420323. [DOI] [PubMed] [Google Scholar]
- Djeu J. Y., Blanchard D. K. Lysis of human monocytes by lymphokine-activated killer cells. Cell Immunol. 1988 Jan;111(1):55–65. doi: 10.1016/0008-8749(88)90050-0. [DOI] [PubMed] [Google Scholar]
- Djeu J. Y., Widen R., Blanchard D. K. Susceptibility of monocytes to lymphokine-activated killer cell lysis: effect of granulocyte-macrophage colony-stimulating factor and interleukin-3. Blood. 1989 Apr;73(5):1264–1271. [PubMed] [Google Scholar]
- Fink P. J., Shimonkevitz R. P., Bevan M. J. Veto cells. Annu Rev Immunol. 1988;6:115–137. doi: 10.1146/annurev.iy.06.040188.000555. [DOI] [PubMed] [Google Scholar]
- Grimm E. A., Mazumder A., Zhang H. Z., Rosenberg S. A. Lymphokine-activated killer cell phenomenon. Lysis of natural killer-resistant fresh solid tumor cells by interleukin 2-activated autologous human peripheral blood lymphocytes. J Exp Med. 1982 Jun 1;155(6):1823–1841. doi: 10.1084/jem.155.6.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanier L. L., Le A. M., Civin C. I., Loken M. R., Phillips J. H. The relationship of CD16 (Leu-11) and Leu-19 (NKH-1) antigen expression on human peripheral blood NK cells and cytotoxic T lymphocytes. J Immunol. 1986 Jun 15;136(12):4480–4486. [PubMed] [Google Scholar]
- Lotze M. T., Matory Y. L., Rayner A. A., Ettinghausen S. E., Vetto J. T., Seipp C. A., Rosenberg S. A. Clinical effects and toxicity of interleukin-2 in patients with cancer. Cancer. 1986 Dec 15;58(12):2764–2772. doi: 10.1002/1097-0142(19861215)58:12<2764::aid-cncr2820581235>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Morstyn G., Burgess A. W. Hemopoietic growth factors: a review. Cancer Res. 1988 Oct 15;48(20):5624–5637. [PubMed] [Google Scholar]
- Ortaldo J. R., Herberman R. B. Heterogeneity of natural killer cells. Annu Rev Immunol. 1984;2:359–394. doi: 10.1146/annurev.iy.02.040184.002043. [DOI] [PubMed] [Google Scholar]
- Perussia B., Kobayashi M., Rossi M. E., Anegon I., Trinchieri G. Immune interferon enhances functional properties of human granulocytes: role of Fc receptors and effect of lymphotoxin, tumor necrosis factor, and granulocyte-macrophage colony-stimulating factor. J Immunol. 1987 Feb 1;138(3):765–774. [PubMed] [Google Scholar]
- Perussia B., Trinchieri G., Jackson A., Warner N. L., Faust J., Rumpold H., Kraft D., Lanier L. L. The Fc receptor for IgG on human natural killer cells: phenotypic, functional, and comparative studies with monoclonal antibodies. J Immunol. 1984 Jul;133(1):180–189. [PubMed] [Google Scholar]
- Rosenberg S. A., Lotze M. T., Muul L. M., Leitman S., Chang A. E., Ettinghausen S. E., Matory Y. L., Skibber J. M., Shiloni E., Vetto J. T. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl J Med. 1985 Dec 5;313(23):1485–1492. doi: 10.1056/NEJM198512053132327. [DOI] [PubMed] [Google Scholar]
- Schwartz R. H. T-lymphocyte recognition of antigen in association with gene products of the major histocompatibility complex. Annu Rev Immunol. 1985;3:237–261. doi: 10.1146/annurev.iy.03.040185.001321. [DOI] [PubMed] [Google Scholar]
- Sone S., Inamura N., Singh S. M., Okubo A., Yanagawa H., Nakanishi M., Ogura T. Killing of alveolar macrophages and of monocytes that have responded to granulocyte-macrophage colony-stimulating factor by human lymphokine-activated killer cells. Jpn J Cancer Res. 1989 Jul;80(7):662–669. doi: 10.1111/j.1349-7006.1989.tb01694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timonen T., Ortaldo J. R., Herberman R. B. Characteristics of human large granular lymphocytes and relationship to natural killer and K cells. J Exp Med. 1981 Mar 1;153(3):569–582. doi: 10.1084/jem.153.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torosantucci A., Palma C., Boccanera M., Ausiello C. M., Spagnoli G. C., Cassone A. Lymphoproliferative and cytotoxic responses of human peripheral blood mononuclear cells to mannoprotein constituents of Candida albicans. J Gen Microbiol. 1990 Nov;136(11):2155–2163. doi: 10.1099/00221287-136-11-2155. [DOI] [PubMed] [Google Scholar]
- Trinchieri G. Biology of natural killer cells. Adv Immunol. 1989;47:187–376. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchieri G., Granato D., Perussia B. Interferon-induced resistance of fibroblasts to cytolysis mediated by natural killer cells: specificity and mechanism. J Immunol. 1981 Jan;126(1):335–340. [PubMed] [Google Scholar]
- Unanue E. R., Allen P. M. The basis for the immunoregulatory role of macrophages and other accessory cells. Science. 1987 May 1;236(4801):551–557. doi: 10.1126/science.2437650. [DOI] [PubMed] [Google Scholar]
- Wang M., Friedman H., Djeu J. Y. Enhancement of human monocyte function against Candida albicans by the colony-stimulating factors (CSF): IL-3, granulocyte-macrophage-CSF, and macrophage-CSF. J Immunol. 1989 Jul 15;143(2):671–677. [PubMed] [Google Scholar]
- Weaver C. T., Unanue E. R. The costimulatory function of antigen-presenting cells. Immunol Today. 1990 Feb;11(2):49–55. doi: 10.1016/0167-5699(90)90018-5. [DOI] [PubMed] [Google Scholar]
- Wei S., Serbousek D., McMillen S., Blanchard D. K., Djeu J. Y. Suppression of human monocyte function against Candida albicans by autologous IL-2-induced lymphokine-activated killer cells. J Immunol. 1991 Jan 1;146(1):337–342. [PubMed] [Google Scholar]