Abstract
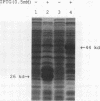
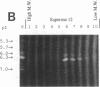
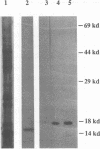
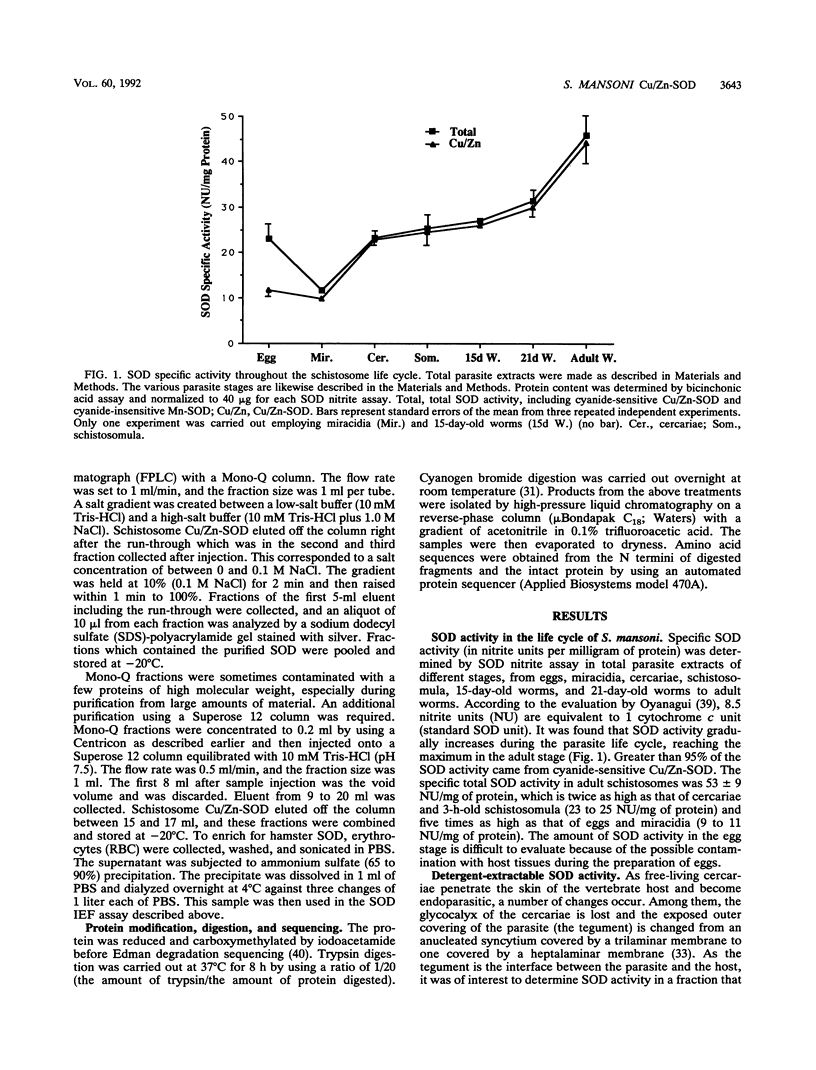
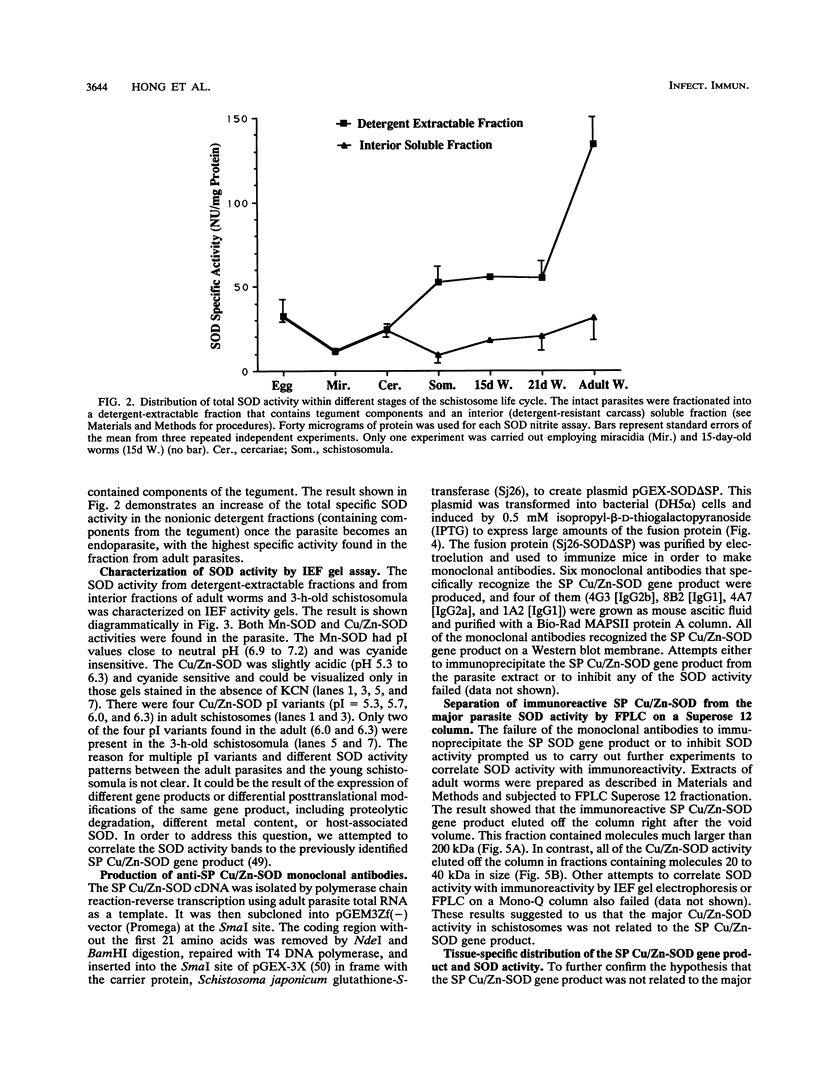
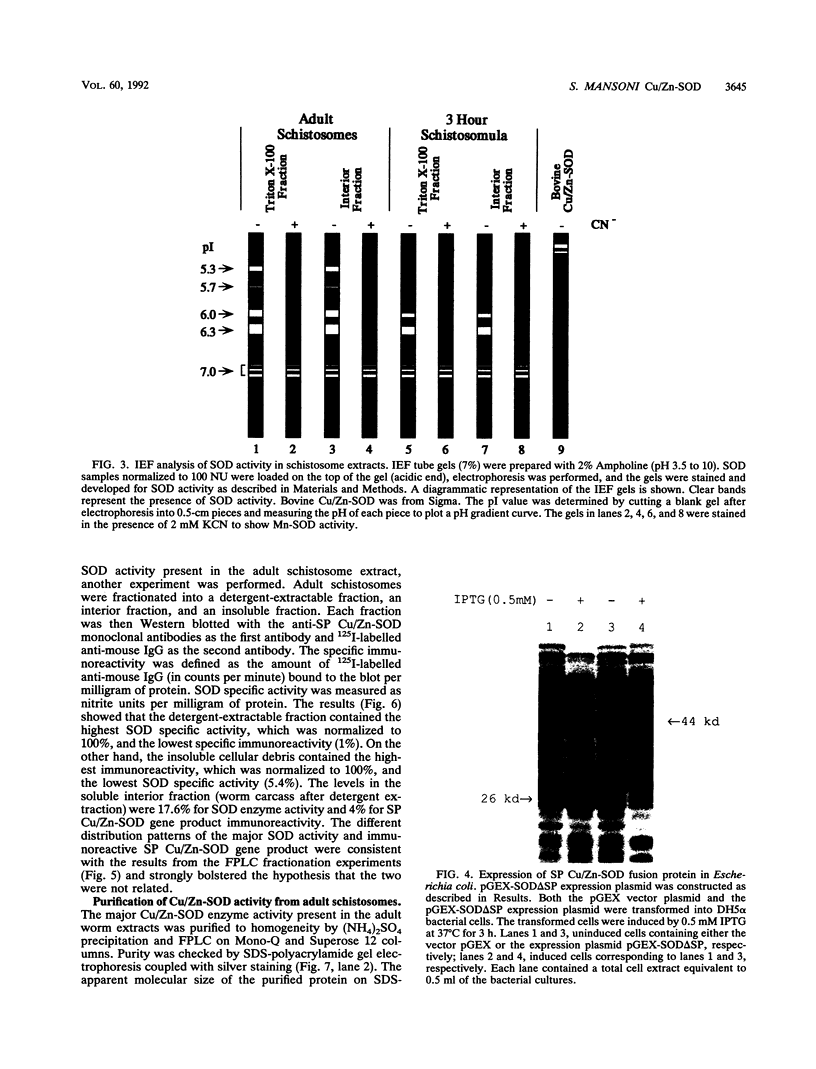
Our laboratories previously isolated a putative extracellular or membrane-associated Cu/Zn superoxide dismutase (Cu/Zn-SOD) gene, designated a signal peptide-containing (SP) Cu/Zn-SOD, from Schistosoma mansoni. SOD activity was thus investigated throughout the life cycle of S. mansoni and found in all stages: eggs, miracidia, cercariae, schistosomula, lung-stage worms, and adult worms. The adult worms had the highest SOD activity (53 +/- 9 nitrite units), which was five times higher than that of eggs or miracidia and twice as high as that of 3-h-old mechanically transformed schistosomula. Cu/Zn-SOD constituted over 95% of the total SOD activity found in S. mansoni, compared with that of Mn-SOD. Most of Cu/Zn-SOD specific activity was associated with a detergent-extractable fraction of the parasite. Isoelectric focusing gel electrophoresis analysis revealed that there were four major pI variants of Cu/Zn-SOD present in the adult worms. Only two of these Cu/Zn-SOD pI variants were present in the 3-h-old mechanically transformed schistosomula. Fast protein liquid chromatography gel filtration fractionation of adult parasite extract was carried out to correlate the SP Cu/Zn-SOD with the SOD activity by using anti-SP Cu/Zn-SOD monoclonal antibodies, which separated the immunoreactive gene product and the SOD activity into different fractions. Quantitative tissue fractionation also revealed a discordant distribution of the gene product compared with that of Cu/Zn-SOD activity. These results indicated the existence of another Cu/Zn-SOD(s) in the parasite. Purification of the Cu/Zn-SOD activity from the adult worms showed that it represented the two lower-pI variants found in both adult worms and 3-h-old schistosomula. Peptide sequence analysis of the purified Cu/Zn-SOD confirmed that there is a second form of Cu/Zn-SOD in the parasite.
Full text
PDF

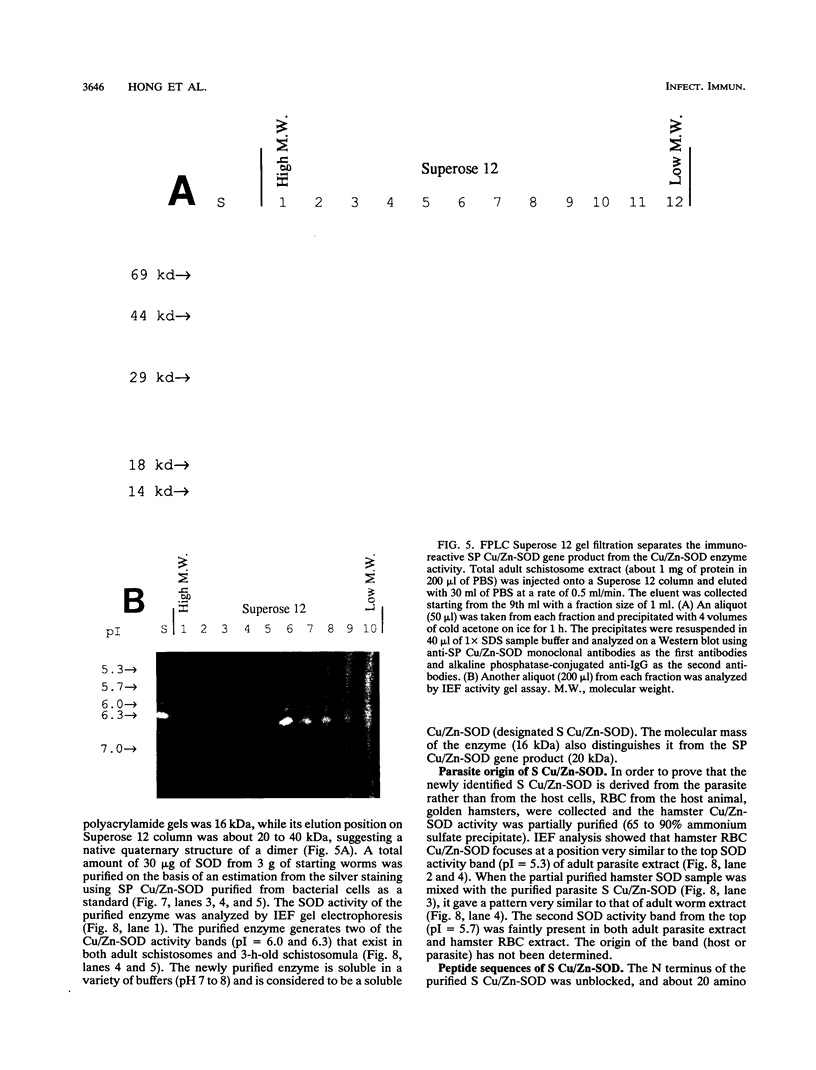
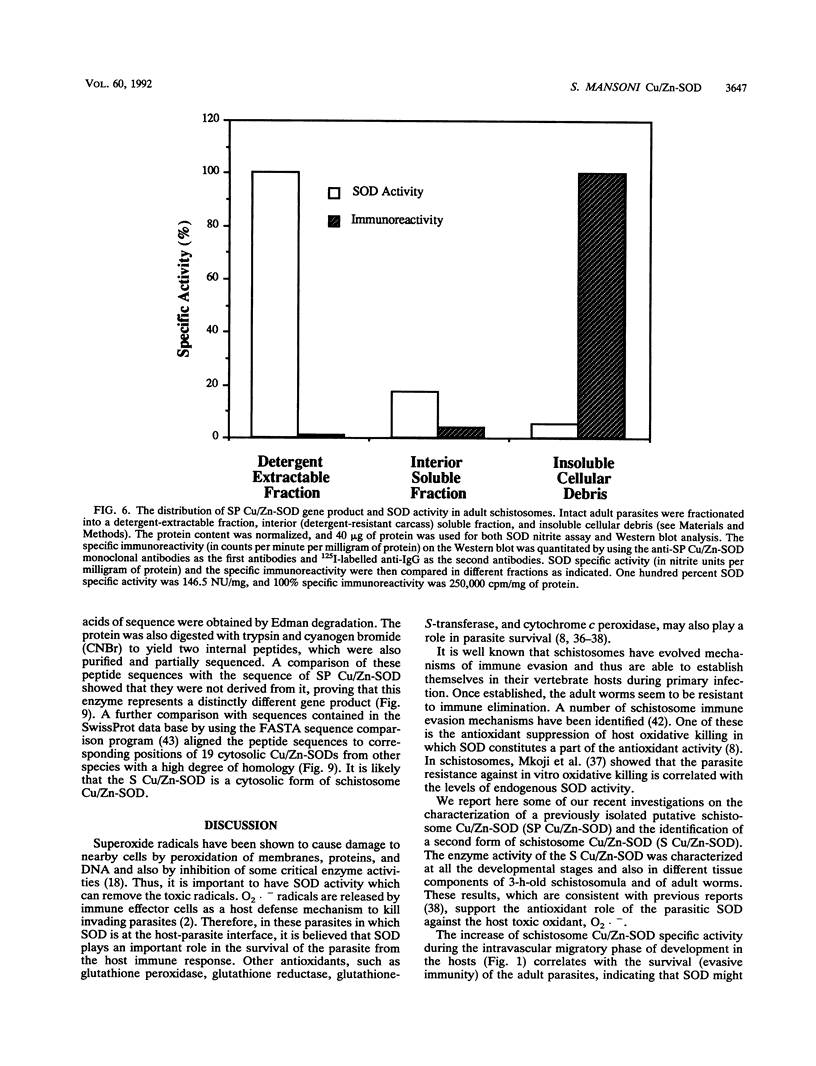
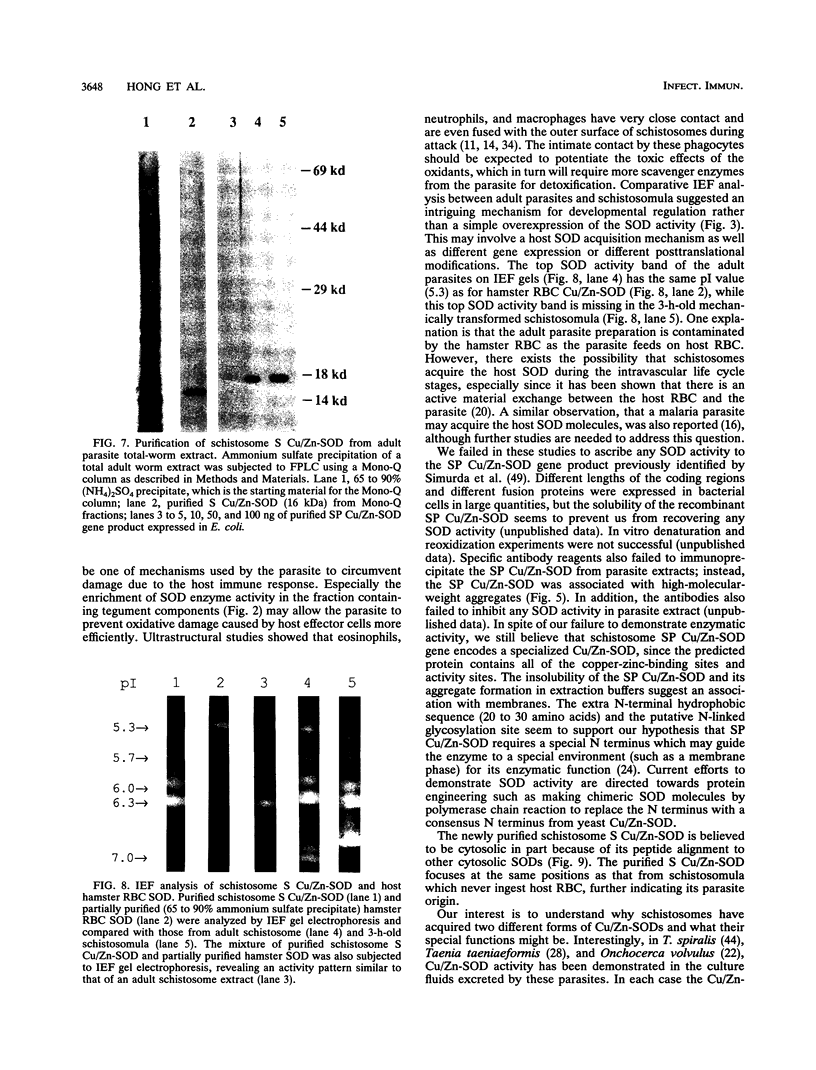
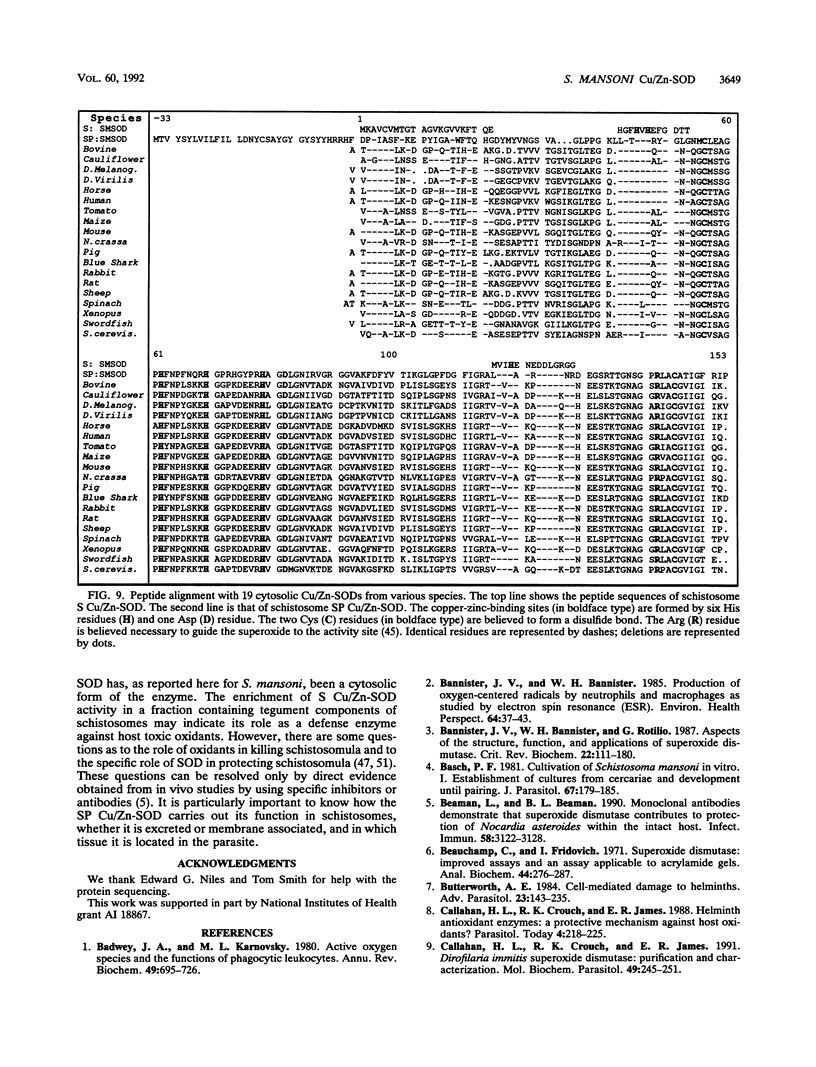


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badwey J. A., Karnovsky M. L. Active oxygen species and the functions of phagocytic leukocytes. Annu Rev Biochem. 1980;49:695–726. doi: 10.1146/annurev.bi.49.070180.003403. [DOI] [PubMed] [Google Scholar]
- Bannister J. V., Bannister W. H. Production of oxygen-centered radicals by neutrophils and macrophages as studied by electron spin resonance (ESR). Environ Health Perspect. 1985 Dec;64:37–43. doi: 10.1289/ehp.856437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannister J. V., Bannister W. H., Rotilio G. Aspects of the structure, function, and applications of superoxide dismutase. CRC Crit Rev Biochem. 1987;22(2):111–180. doi: 10.3109/10409238709083738. [DOI] [PubMed] [Google Scholar]
- Basch P. F. Cultivation of Schistosoma mansoni in vitro. I. Establishment of cultures from cercariae and development until pairing. J Parasitol. 1981 Apr;67(2):179–185. [PubMed] [Google Scholar]
- Beaman L., Beaman B. L. Monoclonal antibodies demonstrate that superoxide dismutase contributes to protection of Nocardia asteroides within the intact host. Infect Immun. 1990 Sep;58(9):3122–3128. doi: 10.1128/iai.58.9.3122-3128.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971 Nov;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Butterworth A. E. Cell-mediated damage to helminths. Adv Parasitol. 1984;23:143–235. doi: 10.1016/s0065-308x(08)60287-0. [DOI] [PubMed] [Google Scholar]
- Callahan H. L., Crouch R. K., James E. R. Dirofilaria immitis superoxide dismutase: purification and characterization. Mol Biochem Parasitol. 1991 Dec;49(2):245–251. doi: 10.1016/0166-6851(91)90068-h. [DOI] [PubMed] [Google Scholar]
- Callahan H. L., Crouch R. K., James E. R. Helminth anti-oxidant enzymes: a protective mechanism against host oxidants? Parasitol Today. 1988 Aug;4(8):218–225. doi: 10.1016/0169-4758(88)90162-7. [DOI] [PubMed] [Google Scholar]
- Capron A., Dessaint J. P., Capron M., Ouma J. H., Butterworth A. E. Immunity to schistosomes: progress toward vaccine. Science. 1987 Nov 20;238(4830):1065–1072. doi: 10.1126/science.3317823. [DOI] [PubMed] [Google Scholar]
- Caulfield J. P., Korman G., Butterworth A. E., Hogan M., David J. R. Partial and complete detachment of neutrophils and eosinophils from schistosomula: evidence for the establishment of continuity between a fused and normal parasite membrane. J Cell Biol. 1980 Jul;86(1):64–76. doi: 10.1083/jcb.86.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claflin L., Williams K. Mouse myeloma--spleen cell hybrids: Enhanced hybridization frequencies and rapid screening procedures. Curr Top Microbiol Immunol. 1978;81:107–109. doi: 10.1007/978-3-642-67448-8_16. [DOI] [PubMed] [Google Scholar]
- Damian R. T. Molecular mimicry: parasite evasion and host defense. Curr Top Microbiol Immunol. 1989;145:101–115. doi: 10.1007/978-3-642-74594-2_9. [DOI] [PubMed] [Google Scholar]
- Dessaint J. P., Capron A., Joseph M., Bazin H. Cytophilic binding of IgE to the macrophage. II. Immunologic release of lysosomal enzyme from macrophages by IgE and anti-IgE in the rat: a new mechanism of macrophage activation. Cell Immunol. 1979 Aug;46(1):24–34. doi: 10.1016/0008-8749(79)90242-9. [DOI] [PubMed] [Google Scholar]
- Duvall R. H., DeWitt W. B. An improved perfusion technique for recovering adult schistosomes from laboratory animals. Am J Trop Med Hyg. 1967 Jul;16(4):483–486. doi: 10.4269/ajtmh.1967.16.483. [DOI] [PubMed] [Google Scholar]
- Fairfield A. S., Meshnick S. R., Eaton J. W. Malaria parasites adopt host cell superoxide dismutase. Science. 1983 Aug 19;221(4612):764–766. doi: 10.1126/science.6348944. [DOI] [PubMed] [Google Scholar]
- Fletcher M., LoVerde P. T., Woodruff D. S. Genetic variation in Schistosoma mansoni: enzyme polymorphisms in populations from Africa, Southwest Asia, South America, and the West Indies. Am J Trop Med Hyg. 1981 Mar;30(2):406–421. doi: 10.4269/ajtmh.1981.30.406. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases. An adaptation to a paramagnetic gas. J Biol Chem. 1989 May 15;264(14):7761–7764. [PubMed] [Google Scholar]
- Fridovich I. Superoxide radical: an endogenous toxicant. Annu Rev Pharmacol Toxicol. 1983;23:239–257. doi: 10.1146/annurev.pa.23.040183.001323. [DOI] [PubMed] [Google Scholar]
- Goldring O. L., Sher A., Smithers S. R., McLaren D. J. Host antigens and parasite antigens of murine Schistosoma mansoni. Trans R Soc Trop Med Hyg. 1977;71(2):144–148. doi: 10.1016/0035-9203(77)90083-9. [DOI] [PubMed] [Google Scholar]
- Gregoire R. J., Shi M. H., Rekosh D. M., Loverde P. T. Protective monoclonal antibodies from mice vaccinated or chronically infected with Schistosoma mansoni that recognize the same antigens. J Immunol. 1987 Dec 1;139(11):3792–3801. [PubMed] [Google Scholar]
- Henkle K. J., Liebau E., Müller S., Bergmann B., Walter R. D. Characterization and molecular cloning of a Cu/Zn superoxide dismutase from the human parasite Onchocerca volvulus. Infect Immun. 1991 Jun;59(6):2063–2069. doi: 10.1128/iai.59.6.2063-2069.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjalmarsson K., Marklund S. L., Engström A., Edlund T. Isolation and sequence of complementary DNA encoding human extracellular superoxide dismutase. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6340–6344. doi: 10.1073/pnas.84.18.6340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazura J. W., Meshnick S. R. Scavenger enzymes and resistance to oxygen mediated damage in Trichinella spiralis. Mol Biochem Parasitol. 1984 Jan;10(1):1–10. doi: 10.1016/0166-6851(84)90013-6. [DOI] [PubMed] [Google Scholar]
- Lazdins J. K., Stein M. J., David J. R., Sher A. Schistosoma mansoni: rapid isolation and purification of schistosomula of different developmental stages by centrifugation on discontinuous density gradients of Percoll. Exp Parasitol. 1982 Feb;53(1):39–44. doi: 10.1016/0014-4894(82)90090-x. [DOI] [PubMed] [Google Scholar]
- Leid R. W., Suquet C. M. A superoxide dismutase of metacestodes of Taenia taeniaeformis. Mol Biochem Parasitol. 1986 Mar;18(3):301–311. doi: 10.1016/0166-6851(86)90087-3. [DOI] [PubMed] [Google Scholar]
- Marklund S. L. Extracellular superoxide dismutase and other superoxide dismutase isoenzymes in tissues from nine mammalian species. Biochem J. 1984 Sep 15;222(3):649–655. doi: 10.1042/bj2220649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund S. L. Properties of extracellular superoxide dismutase from human lung. Biochem J. 1984 May 15;220(1):269–272. doi: 10.1042/bj2200269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsudaira P. Limited N-terminal sequence analysis. Methods Enzymol. 1990;182:602–613. doi: 10.1016/0076-6879(90)82047-6. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- McLaren D. J., Ramalho-Pinto F. J., Smithers S. R. Ultrastructural evidence for complement and antibody-dependent damage to schistosomula of Schistosoma mansoni by rat eosinophils in vitro. Parasitology. 1978 Dec;77(3):313–324. doi: 10.1017/s0031182000050277. [DOI] [PubMed] [Google Scholar]
- Mclaren D. J., Hockley D. J. Blood flukes have a double outer membrane. Nature. 1977 Sep 8;269(5624):147–149. doi: 10.1038/269147a0. [DOI] [PubMed] [Google Scholar]
- Michalski W. P., Prowse S. J. Superoxide dismutases in Eimeria tenella. Mol Biochem Parasitol. 1991 Aug;47(2):189–195. doi: 10.1016/0166-6851(91)90178-9. [DOI] [PubMed] [Google Scholar]
- Mkoji G. M., Smith J. M., Prichard R. K. Antioxidant systems in Schistosoma mansoni: correlation between susceptibility to oxidant killing and the levels of scavengers of hydrogen peroxide and oxygen free radicals. Int J Parasitol. 1988 Jul;18(5):661–666. doi: 10.1016/0020-7519(88)90101-4. [DOI] [PubMed] [Google Scholar]
- Mkoji G. M., Smith J. M., Prichard R. K. Antioxidant systems in Schistosoma mansoni: evidence for their role in protection of the adult worms against oxidant killing. Int J Parasitol. 1988 Jul;18(5):667–673. doi: 10.1016/0020-7519(88)90102-6. [DOI] [PubMed] [Google Scholar]
- Nare B., Smith J. M., Prichard R. K. Schistosoma mansoni: levels of antioxidants and resistance to oxidants increase during development. Exp Parasitol. 1990 May;70(4):389–397. doi: 10.1016/0014-4894(90)90122-s. [DOI] [PubMed] [Google Scholar]
- Oyanagui Y. Reevaluation of assay methods and establishment of kit for superoxide dismutase activity. Anal Biochem. 1984 Nov 1;142(2):290–296. doi: 10.1016/0003-2697(84)90467-6. [DOI] [PubMed] [Google Scholar]
- Ozols J. Amino acid analysis. Methods Enzymol. 1990;182:587–601. doi: 10.1016/0076-6879(90)82046-5. [DOI] [PubMed] [Google Scholar]
- Pearce E. J., Sher A. Mechanisms of immune evasion in schistosomiasis. Contrib Microbiol Immunol. 1987;8:219–232. [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoads M. L. Trichinella spiralis: identification and purification of superoxide dismutase. Exp Parasitol. 1983 Aug;56(1):41–54. doi: 10.1016/0014-4894(83)90095-4. [DOI] [PubMed] [Google Scholar]
- Rossi F. The O2- -forming NADPH oxidase of the phagocytes: nature, mechanisms of activation and function. Biochim Biophys Acta. 1986 Nov 4;853(1):65–89. doi: 10.1016/0304-4173(86)90005-4. [DOI] [PubMed] [Google Scholar]
- Sanchez-Moreno M., Monteoliva M., Fatou A., García-Ruiz M. A. Superoxide dismutase from Ascaris suum. Parasitology. 1988 Oct;97(Pt 2):345–353. doi: 10.1017/s0031182000058546. [DOI] [PubMed] [Google Scholar]
- Scott P., James S., Sher A. The respiratory burst is not required for killing of intracellular and extracellular parasites by a lymphokine-activated macrophage cell line. Eur J Immunol. 1985 Jun;15(6):553–558. doi: 10.1002/eji.1830150605. [DOI] [PubMed] [Google Scholar]
- Scott P., Pearce E., Cheever A. W., Coffman R. L., Sher A. Role of cytokines and CD4+ T-cell subsets in the regulation of parasite immunity and disease. Immunol Rev. 1989 Dec;112:161–182. doi: 10.1111/j.1600-065x.1989.tb00557.x. [DOI] [PubMed] [Google Scholar]
- Simurda M. C., van Keulen H., Rekosh D. M., LoVerde P. T. Schistosoma mansoni: identification and analysis of an mRNA and a gene encoding superoxide dismutase (Cu/Zn). Exp Parasitol. 1988 Oct;67(1):73–84. doi: 10.1016/0014-4894(88)90010-0. [DOI] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Smith J. M., Mkoji G. M., Prichard R. K. Depression of hydrogen peroxide dependent killing of schistosomula in vitro by peritoneal exudate cells from Schistosoma mansoni infected mice. Am J Trop Med Hyg. 1989 Feb;40(2):186–194. doi: 10.4269/ajtmh.1989.40.186. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Smithers S. R., Terry R. J. Immunity in schistosomiasis. Ann N Y Acad Sci. 1969 Oct 6;160(2):826–840. doi: 10.1111/j.1749-6632.1969.tb15904.x. [DOI] [PubMed] [Google Scholar]
- Tibell L., Hjalmarsson K., Edlund T., Skogman G., Engström A., Marklund S. L. Expression of human extracellular superoxide dismutase in Chinese hamster ovary cells and characterization of the product. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6634–6638. doi: 10.1073/pnas.84.19.6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignali D. A., Bickle Q. D., Taylor M. G. Immunity to Schistosoma mansoni in vivo: contradiction or clarification? Immunol Today. 1989 Dec;10(12):410–416. doi: 10.1016/0167-5699(89)90038-8. [DOI] [PubMed] [Google Scholar]