Abstract
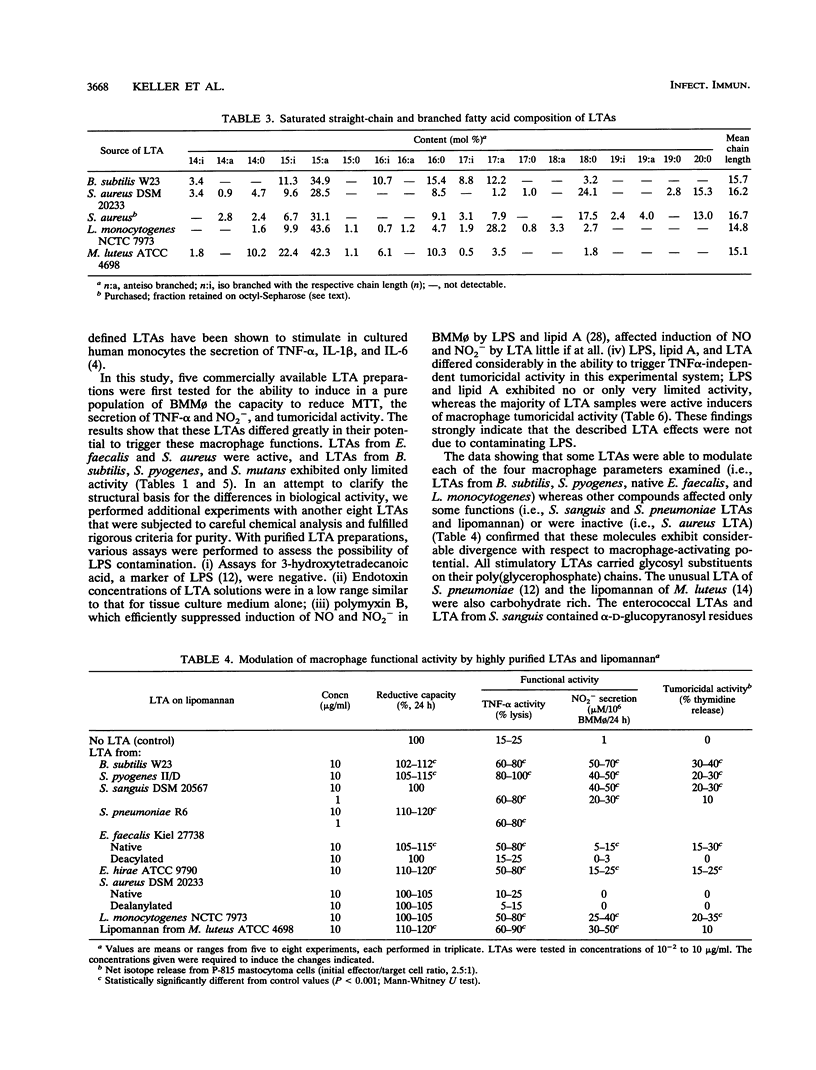
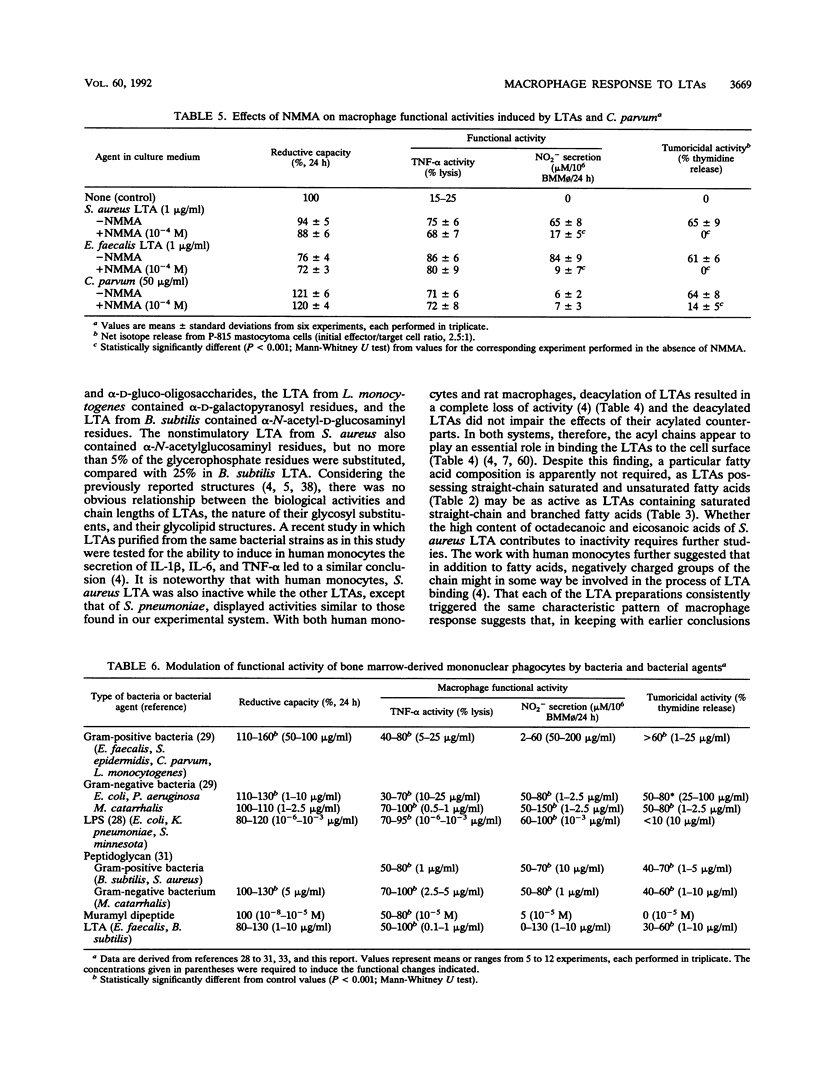
Lipoteichoic acids (LTAs) from various bacterial species, including Staphylococcus aureus, Streptococcus pyogenes, Streptococcus pneumoniae, Enterococcus faecalis, and Listeria monocytogenes, were examined for the ability to induce secretory and cellular responses in a pure population of bone marrow-derived mononuclear phagocytes. Some of the highly purified LTAs, in particular LTAs from Bacillus subtilis, S. pyogenes, E. faecalis, and Enterococcus hirae, were able to affect each of the macrophage parameters measured, i.e., reductive capacity, secretion of tumor necrosis factor and nitrite, and tumoricidal activity. As after stimulation with whole organisms or other bacterial products, secretion of tumor necrosis factor induced by these LTAs reached its maximum within the first few hours of the interaction, while secretion of nitrite and tumoricidal activity required 24 to 36 h for full expression. Other purified LTAs, i.e., LTAs from Streptococcus sanguis, S. pneumoniae, and L. monocytogenes, as well as lipomannan from Micrococcus luteus affected only some of these parameters, while native LTA from S. aureus was inactive. There was no obvious correlation between biological activity and chain length, kind of glycosyl substituents, glycolipid structures, or fatty acid composition of LTAs. Deacylation of LTAs resulted in a complete loss of activity, and deacylated LTAs did not impair the activity of their acylated counterparts, suggesting that acyl chains may be essential for binding of LTA to the cell surface. The results demonstrate that some LTA species are potent inducers of macrophage secretory and cellular activities.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam A., Ciorbaru R., Ellouz F., Petit J. F., Lederer E. Adjuvant activity of monomeric bacterial cell wall peptidoglycans. Biochem Biophys Res Commun. 1974 Feb 4;56(3):561–567. doi: 10.1016/0006-291x(74)90640-8. [DOI] [PubMed] [Google Scholar]
- Auguet M., Lonchampt M. O., Delaflotte S., Goulin-Schulz J., Chabrier P. E., Braquet P. Induction of nitric oxide synthase by lipoteichoic acid from Staphylococcus aureus in vascular smooth muscle cells. FEBS Lett. 1992 Feb 3;297(1-2):183–185. doi: 10.1016/0014-5793(92)80356-l. [DOI] [PubMed] [Google Scholar]
- Beachey E. H., Courtney H. S. Bacterial adherence: the attachment of group A streptococci to mucosal surfaces. Rev Infect Dis. 1987 Sep-Oct;9 (Suppl 5):S475–S481. doi: 10.1093/clinids/9.supplement_5.s475. [DOI] [PubMed] [Google Scholar]
- Bhakdi S., Klonisch T., Nuber P., Fischer W. Stimulation of monokine production by lipoteichoic acids. Infect Immun. 1991 Dec;59(12):4614–4620. doi: 10.1128/iai.59.12.4614-4620.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brade L., Brade H., Fischer W. A 28 kDa protein of normal mouse serum binds lipopolysaccharides of gram-negative and lipoteichoic acids of gram-positive bacteria. Microb Pathog. 1990 Nov;9(5):355–362. doi: 10.1016/0882-4010(90)90069-3. [DOI] [PubMed] [Google Scholar]
- Carswell E. A., Old L. J., Kassel R. L., Green S., Fiore N., Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney H., Ofek I., Simpson W. A., Beachey E. H. Characterization of lipoteichoic acid binding to polymorphonuclear leukocytes of human blood. Infect Immun. 1981 May;32(2):625–631. doi: 10.1128/iai.32.2.625-631.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVuono J., Panos C. Effect of L-form Streptococcus pyogenes and of lipoteichoic acid on human cells in tissue culture. Infect Immun. 1978 Oct;22(1):255–265. doi: 10.1128/iai.22.1.255-265.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doe W. F., Henson P. M. Macrophage stimulation by bacterial lipopolysaccharides. I. Cytolytic effect on tumor target cells. J Exp Med. 1978 Aug 1;148(2):544–556. doi: 10.1084/jem.148.2.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedel B. A., Jackson R. W. Activation of the alternative complement pathway by a streptococcal lipoteichoic acid. Infect Immun. 1978 Oct;22(1):286–287. doi: 10.1128/iai.22.1.286-287.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer W., Koch H. U., Haas R. Improved preparation of lipoteichoic acids. Eur J Biochem. 1983 Jul 1;133(3):523–530. doi: 10.1111/j.1432-1033.1983.tb07495.x. [DOI] [PubMed] [Google Scholar]
- Fischer W., Koch H. U., Rösel P., Fiedler F. Alanine ester-containing native lipoteichoic acids do not act as lipoteichoic acid carrier. Isolation, structural and functional characterization. J Biol Chem. 1980 May 25;255(10):4557–4562. [PubMed] [Google Scholar]
- Fischer W. One-step purification of bacterial lipid macroamphiphiles by hydrophobic interaction chromatography. Anal Biochem. 1991 May 1;194(2):353–358. doi: 10.1016/0003-2697(91)90240-t. [DOI] [PubMed] [Google Scholar]
- Fischer W. Physiology of lipoteichoic acids in bacteria. Adv Microb Physiol. 1988;29:233–302. doi: 10.1016/s0065-2911(08)60349-5. [DOI] [PubMed] [Google Scholar]
- Fischer W. Purification and fractionation of lipopolysaccharide from gram-negative bacteria by hydrophobic interaction chromatography. Eur J Biochem. 1990 Dec 12;194(2):655–661. doi: 10.1111/j.1432-1033.1990.tb15665.x. [DOI] [PubMed] [Google Scholar]
- Gold M. R., Miller C. L., Mishell R. I. Soluble non-cross-linked peptidoglycan polymers stimulate monocyte-macrophage inflammatory functions. Infect Immun. 1985 Sep;49(3):731–741. doi: 10.1128/iai.49.3.731-741.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn H., Kaufmann S. H. The role of cell-mediated immunity in bacterial infections. Rev Infect Dis. 1981 Nov-Dec;3(6):1221–1250. doi: 10.1093/clinids/3.6.1221. [DOI] [PubMed] [Google Scholar]
- Hamada S., Yamamoto T., Koga T., McGhee J. R., Michalek S. M., Yamamoto S. Chemical properties and immunobiological activities of streptococcal lipoteichoic acids. Zentralbl Bakteriol Mikrobiol Hyg A. 1985 Apr;259(2):228–243. doi: 10.1016/s0176-6724(85)80054-7. [DOI] [PubMed] [Google Scholar]
- Harrop P. J., O'Grady R. L., Knox K. W., Wicken A. J. Stimulation of lysosomal enzyme release from macrophages by lipoteichoic acid. J Periodontal Res. 1980 Sep;15(5):492–501. doi: 10.1111/j.1600-0765.1980.tb00307.x. [DOI] [PubMed] [Google Scholar]
- Hazuda D. J., Lee J. C., Young P. R. The kinetics of interleukin 1 secretion from activated monocytes. Differences between interleukin 1 alpha and interleukin 1 beta. J Biol Chem. 1988 Jun 15;263(17):8473–8479. [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Vavrin Z. Macrophage cytotoxicity: role for L-arginine deiminase and imino nitrogen oxidation to nitrite. Science. 1987 Jan 23;235(4787):473–476. doi: 10.1126/science.2432665. [DOI] [PubMed] [Google Scholar]
- Hummell D. S., Swift A. J., Tomasz A., Winkelstein J. A. Activation of the alternative complement pathway by pneumococcal lipoteichoic acid. Infect Immun. 1985 Feb;47(2):384–387. doi: 10.1128/iai.47.2.384-387.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson D. E., Jackson G. D., Wicken A. J. Induction of IgM immunological memory to lipoteichoic acids in rabbits. Part I. Int Arch Allergy Appl Immunol. 1981;65(2):198–202. doi: 10.1159/000232756. [DOI] [PubMed] [Google Scholar]
- Keller R., Bassetti S., Keist R., Mülsch A., Klauser S. Induction of nitric oxide synthase is a necessary precondition for expression of tumor necrosis factor-independent tumoricidal activity by activated macrophages. Biochem Biophys Res Commun. 1992 May 15;184(3):1364–1371. doi: 10.1016/s0006-291x(05)80033-6. [DOI] [PubMed] [Google Scholar]
- Keller R., Gehri R., Keist R., Huf E., Kayser F. H. The interaction of macrophages and bacteria: a comparative study of the induction of tumoricidal activity and of reactive nitrogen intermediates. Cell Immunol. 1991 Apr 15;134(1):249–256. doi: 10.1016/0008-8749(91)90348-f. [DOI] [PubMed] [Google Scholar]
- Keller R., Gehri R., Keist R. The interaction of macrophages and bacteria: Escherichia coli species, bacterial lipopolysaccharide, and lipid A differ in their ability to induce tumoricidal activity and the secretion of reactive nitrogen intermediates in macrophages. Cell Immunol. 1992 Apr 15;141(1):47–58. doi: 10.1016/0008-8749(92)90126-a. [DOI] [PubMed] [Google Scholar]
- Keller R., Geiges M., Keist R. L-arginine-dependent reactive nitrogen intermediates as mediators of tumor cell killing by activated macrophages. Cancer Res. 1990 Mar 1;50(5):1421–1425. [PubMed] [Google Scholar]
- Keller R., Keist R., Frei K. Lymphokines and bacteria, that induce tumoricidal activity, trigger a different secretory response in macrophages. Eur J Immunol. 1990 Mar;20(3):695–698. doi: 10.1002/eji.1830200334. [DOI] [PubMed] [Google Scholar]
- Keller R., Keist R., Klauser S., Schweiger A. The macrophage response to bacteria: flow of L-arginine through the nitric oxide and urea pathways and induction of tumoricidal activity. Biochem Biophys Res Commun. 1991 Jun 14;177(2):821–827. doi: 10.1016/0006-291x(91)91863-8. [DOI] [PubMed] [Google Scholar]
- Keller R., Keist R., Van der Meide P. H., Groscurth P., Aguet M., Leist T. P. Induction, maintenance, and reinduction of tumoricidal activity in bone marrow-derived mononuclear phagocytes by Corynebacterium parvum. Evidence for the involvement of a T cell- and interferon-gamma-independent pathway of macrophage activation. J Immunol. 1987 Apr 1;138(7):2366–2371. [PubMed] [Google Scholar]
- Keller R., Keist R., Wechsler A., Leist T. P., van der Meide P. H. Mechanisms of macrophage-mediated tumor cell killing: a comparative analysis of the roles of reactive nitrogen intermediates and tumor necrosis factor. Int J Cancer. 1990 Oct 15;46(4):682–686. doi: 10.1002/ijc.2910460422. [DOI] [PubMed] [Google Scholar]
- Koch H. U., Haas R., Fischer W. The role of lipoteichoic acid biosynthesis in membrane lipid metabolism of growing Staphylococcus aureus. Eur J Biochem. 1984 Jan 16;138(2):357–363. doi: 10.1111/j.1432-1033.1984.tb07923.x. [DOI] [PubMed] [Google Scholar]
- Leopold K., Fischer W. Separation of the poly(glycerophosphate) lipoteichoic acids of Enterococcus faecalis Kiel 27738, Enterococcus hirae ATCC 9790 and Leuconostoc mesenteroides DSM 20343 into molecular species by affinity chromatography on concanavalin A. Eur J Biochem. 1991 Mar 14;196(2):475–482. doi: 10.1111/j.1432-1033.1991.tb15839.x. [DOI] [PubMed] [Google Scholar]
- Levy R., Kotb M., Nagauker O., Majumdar G., Alkan M., Ofek I., Beachey E. H. Stimulation of oxidative burst in human monocytes by lipoteichoic acids. Infect Immun. 1990 Feb;58(2):566–568. doi: 10.1128/iai.58.2.566-568.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisi P. J., Chu C. W., Koch G. A., Endres S., Lonnemann G., Dinarello C. A. Development and use of a radioimmunoassay for human interleukin-1 beta. Lymphokine Res. 1987 Summer;6(3):229–244. [PubMed] [Google Scholar]
- Loos M., Clas F., Fischer W. Interaction of purified lipoteichoic acid with the classical complement pathway. Infect Immun. 1986 Sep;53(3):595–599. doi: 10.1128/iai.53.3.595-599.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G. A., Urban J., Jackson R. W. Effects of a streptococcal lipoteichoic acid on host responses in mice. Infect Immun. 1976 May;13(5):1408–1417. doi: 10.1128/iai.13.5.1408-1417.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Myers P. R., Minor R. L., Jr, Guerra R., Jr, Bates J. N., Harrison D. G. Vasorelaxant properties of the endothelium-derived relaxing factor more closely resemble S-nitrosocysteine than nitric oxide. Nature. 1990 May 10;345(6271):161–163. doi: 10.1038/345161a0. [DOI] [PubMed] [Google Scholar]
- Mülsch A., Mordvintcev P., Vanin A. F., Busse R. The potent vasodilating and guanylyl cyclase activating dinitrosyl-iron(II) complex is stored in a protein-bound form in vascular tissue and is released by thiols. FEBS Lett. 1991 Dec 9;294(3):252–256. doi: 10.1016/0014-5793(91)81441-a. [DOI] [PubMed] [Google Scholar]
- Nauciel C., Fleck J., Martin J. P., Mock M., Nguyen-Huy H. Adjuvant activity of bacterial peptidoglycans on the production of delayed hypersensitivity and on antibody response. Eur J Immunol. 1974 May;4(5):352–356. doi: 10.1002/eji.1830040509. [DOI] [PubMed] [Google Scholar]
- Ohshima Y., Ko H. L., Beuth J., Pulverer G. Immunostimulating staphylococcal lipoteichoic acid prevents pulmonary tumor colonization in BALB/c-mice. Zentralbl Bakteriol Mikrobiol Hyg A. 1988 Nov;270(1-2):213–218. doi: 10.1016/s0176-6724(88)80156-1. [DOI] [PubMed] [Google Scholar]
- Ohshima Y., Ko H. L., Beuth J., Roszkowski K., Roszkowski W. Biological properties of staphylococcal lipoteichoic acid and related macromolecules. Zentralbl Bakteriol. 1990 Dec;274(3):359–365. doi: 10.1016/s0934-8840(11)80693-6. [DOI] [PubMed] [Google Scholar]
- Smialowicz R. J., Schwab J. H. Cytotoxicity of rat macrophages activated by persistent or biodegradable bacterial cell walls. Infect Immun. 1977 Sep;17(3):599–606. doi: 10.1128/iai.17.3.599-606.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart-Tull D. E. The immunological activities of bacterial peptidoglycans. Annu Rev Microbiol. 1980;34:311–340. doi: 10.1146/annurev.mi.34.100180.001523. [DOI] [PubMed] [Google Scholar]
- Stuehr D. J., Marletta M. A. Synthesis of nitrite and nitrate in murine macrophage cell lines. Cancer Res. 1987 Nov 1;47(21):5590–5594. [PubMed] [Google Scholar]
- Tosato G., Seamon K. B., Goldman N. D., Sehgal P. B., May L. T., Washington G. C., Jones K. D., Pike S. E. Monocyte-derived human B-cell growth factor identified as interferon-beta 2 (BSF-2, IL-6). Science. 1988 Jan 29;239(4839):502–504. doi: 10.1126/science.2829354. [DOI] [PubMed] [Google Scholar]
- Usami H., Yamamoto A., Yamashita W., Sugawara Y., Hamada S., Yamamoto T., Kato K., Kokeguchi S., Ohokuni H., Kotani S. Antitumour effects of streptococcal lipoteichoic acids on Meth A fibrosarcoma. Br J Cancer. 1988 Jan;57(1):70–73. doi: 10.1038/bjc.1988.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanin A. F. Endothelium-derived relaxing factor is a nitrosyl iron complex with thiol ligands. FEBS Lett. 1991 Sep 2;289(1):1–3. doi: 10.1016/0014-5793(91)80894-9. [DOI] [PubMed] [Google Scholar]
- Weinreb B. D., Shockman G. D., Beachey E. H., Swift A. J., Winkelstein J. A. The ability to sensitize host cells for destruction by autologous complement is a general property of lipoteichoic acid. Infect Immun. 1986 Nov;54(2):494–499. doi: 10.1128/iai.54.2.494-499.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whigham E. A., Kleinman R. Bacteria-immune system interactions. IX. Modulation of human and murine cytotoxic reactions by crude lipoteichoic acid extracted from Staphylococcus aureus. J Immunopharmacol. 1983;5(1-2):77–91. doi: 10.3109/08923978309026444. [DOI] [PubMed] [Google Scholar]
- Wicken A. J., Knox K. W. Bacterial cell surface amphiphiles. Biochim Biophys Acta. 1980 May 27;604(1):1–26. doi: 10.1016/0005-2736(80)90583-0. [DOI] [PubMed] [Google Scholar]


