Abstract
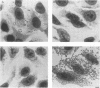
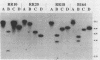
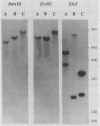
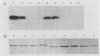
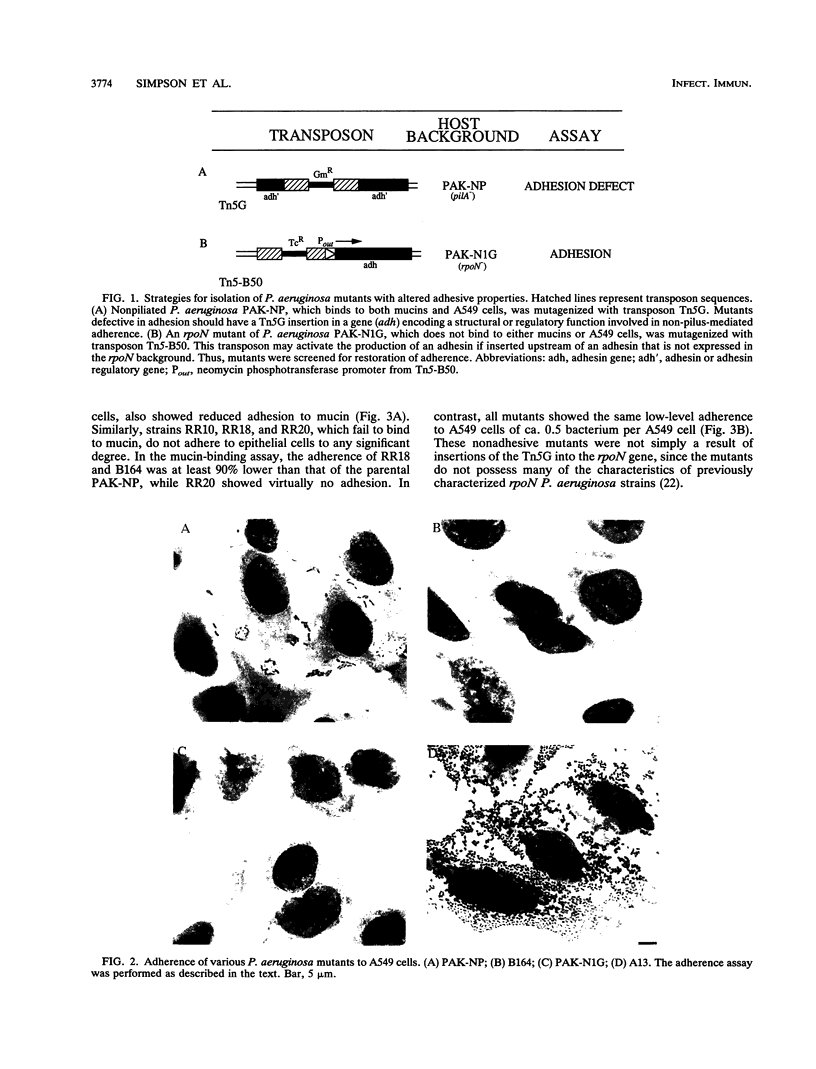
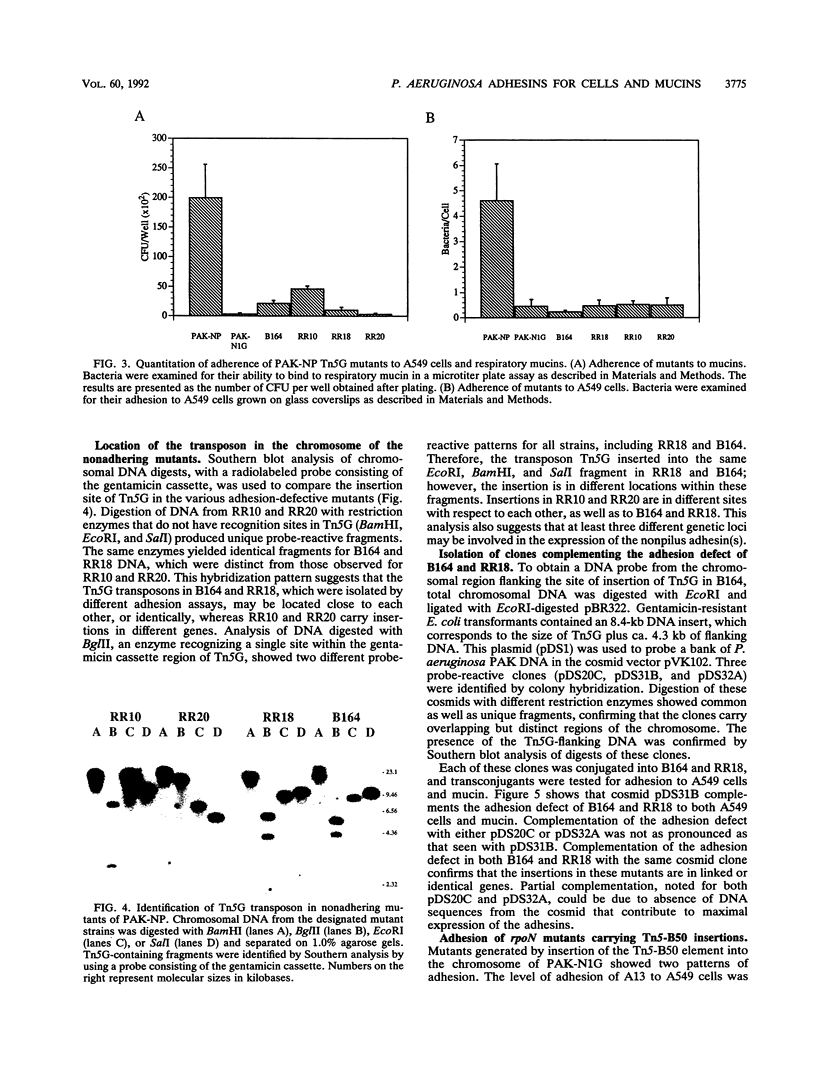
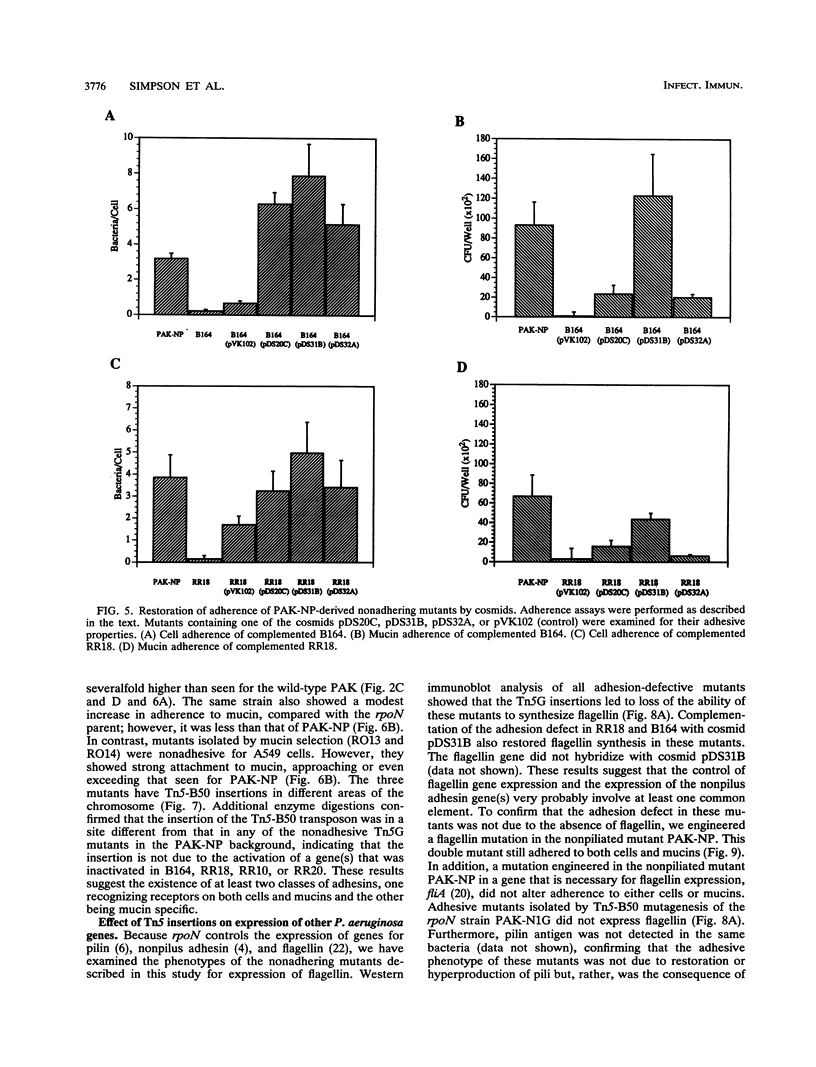
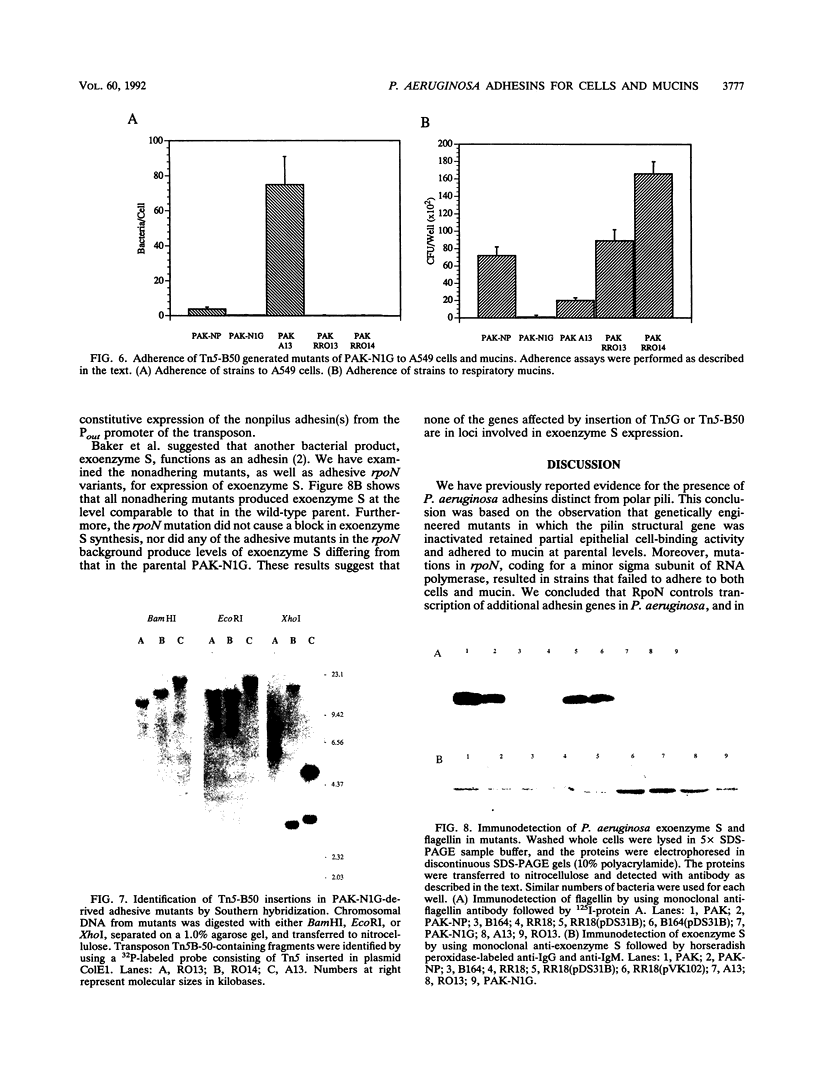
Infection of mucosal tissues by the opportunistic pathogen Pseudomonas aeruginosa is initiated by attachment of the bacterium to host tissues. To gain a better understanding of this interaction, we used two methods to isolate mutants of P. aeruginosa with altered adherence to cultured A549 cells and to mucins. First, from a population of nonpiliated mutants of P. aeruginosa mutagenized with transposon Tn5G, we have isolated variants that are defective in binding to both A549 cells and respiratory mucins. Using a cloned transposon plus flanking DNA from one such mutant as a DNA probe, we have isolated plasmids from a cosmid bank, which, upon reintroduction to the original mutants, restored adhesion to both A549 cells and mucin. The second strategy to identify genes involved in adhesion used mutagenesis of P. aeruginosa N1G, an rpoN mutant which is unable to bind to either A549 cells or mucin, with transposon Tn5 containing an outward-directed promoter. From this bank of mutagenized P. aeruginosa N1G, two classes of adhesion variants were isolated; one class attached to A549 cells and to mucin, and the other class restored binding of the rpoN mutant to mucin but not to A549 cells. These findings suggest that P. aeruginosa can express at least two adhesins distinct from pili, one recognizing receptors shared by epithelial cells and mucins and the other recognizing mucins alone.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker N. R., Minor V., Deal C., Shahrabadi M. S., Simpson D. A., Woods D. E. Pseudomonas aeruginosa exoenzyme S is an adhesion. Infect Immun. 1991 Sep;59(9):2859–2863. doi: 10.1128/iai.59.9.2859-2863.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker N., Hansson G. C., Leffler H., Riise G., Svanborg-Edén C. Glycosphingolipid receptors for Pseudomonas aeruginosa. Infect Immun. 1990 Jul;58(7):2361–2366. doi: 10.1128/iai.58.7.2361-2366.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi E., Mehl T., Nunn D., Lory S. Interaction of Pseudomonas aeruginosa with A549 pneumocyte cells. Infect Immun. 1991 Mar;59(3):822–828. doi: 10.1128/iai.59.3.822-828.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimoto K. S., Lory S. Formation of pilin in Pseudomonas aeruginosa requires the alternative sigma factor (RpoN) of RNA polymerase. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1954–1957. doi: 10.1073/pnas.86.6.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimoto K. S., Lory S. Identification of pilR, which encodes a transcriptional activator of the Pseudomonas aeruginosa pilin gene. J Bacteriol. 1992 Jun;174(11):3514–3521. doi: 10.1128/jb.174.11.3514-3521.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauf V. C., Nester E. W. Wide host range cloning vectors: a cosmid clone bank of an Agrobacterium Ti plasmid. Plasmid. 1982 Jul;8(1):45–54. doi: 10.1016/0147-619x(82)90040-3. [DOI] [PubMed] [Google Scholar]
- Kustu S., Santero E., Keener J., Popham D., Weiss D. Expression of sigma 54 (ntrA)-dependent genes is probably united by a common mechanism. Microbiol Rev. 1989 Sep;53(3):367–376. doi: 10.1128/mr.53.3.367-376.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leong S. A., Ditta G. S., Helinski D. R. Heme biosynthesis in Rhizobium. Identification of a cloned gene coding for delta-aminolevulinic acid synthetase from Rhizobium meliloti. J Biol Chem. 1982 Aug 10;257(15):8724–8730. [PubMed] [Google Scholar]
- Nunn D. N., Lory S. Components of the protein-excretion apparatus of Pseudomonas aeruginosa are processed by the type IV prepilin peptidase. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):47–51. doi: 10.1073/pnas.89.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramphal R., Carnoy C., Fievre S., Michalski J. C., Houdret N., Lamblin G., Strecker G., Roussel P. Pseudomonas aeruginosa recognizes carbohydrate chains containing type 1 (Gal beta 1-3GlcNAc) or type 2 (Gal beta 1-4GlcNAc) disaccharide units. Infect Immun. 1991 Feb;59(2):700–704. doi: 10.1128/iai.59.2.700-704.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramphal R., Koo L., Ishimoto K. S., Totten P. A., Lara J. C., Lory S. Adhesion of Pseudomonas aeruginosa pilin-deficient mutants to mucin. Infect Immun. 1991 Apr;59(4):1307–1311. doi: 10.1128/iai.59.4.1307-1311.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramphal R., Pier G. B. Role of Pseudomonas aeruginosa mucoid exopolysaccharide in adherence to tracheal cells. Infect Immun. 1985 Jan;47(1):1–4. doi: 10.1128/iai.47.1.1-4.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiman L., Ishimoto K., Lory S., Prince A. The effect of piliation and exoproduct expression on the adherence of Pseudomonas aeruginosa to respiratory epithelial monolayers. J Infect Dis. 1990 Mar;161(3):541–548. doi: 10.1093/infdis/161.3.541. [DOI] [PubMed] [Google Scholar]
- Simon R., Quandt J., Klipp W. New derivatives of transposon Tn5 suitable for mobilization of replicons, generation of operon fusions and induction of genes in gram-negative bacteria. Gene. 1989 Aug 1;80(1):161–169. doi: 10.1016/0378-1119(89)90262-x. [DOI] [PubMed] [Google Scholar]
- Starnbach M. N., Lory S. The fliA (rpoF) gene of Pseudomonas aeruginosa encodes an alternative sigma factor required for flagellin synthesis. Mol Microbiol. 1992 Feb;6(4):459–469. doi: 10.1111/j.1365-2958.1992.tb01490.x. [DOI] [PubMed] [Google Scholar]
- Strom M. S., Lory S. Cloning and expression of the pilin gene of Pseudomonas aeruginosa PAK in Escherichia coli. J Bacteriol. 1986 Feb;165(2):367–372. doi: 10.1128/jb.165.2.367-372.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totten P. A., Lara J. C., Lory S. The rpoN gene product of Pseudomonas aeruginosa is required for expression of diverse genes, including the flagellin gene. J Bacteriol. 1990 Jan;172(1):389–396. doi: 10.1128/jb.172.1.389-396.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishwanath S., Ramphal R. Tracheobronchial mucin receptor for Pseudomonas aeruginosa: predominance of amino sugars in binding sites. Infect Immun. 1985 May;48(2):331–335. doi: 10.1128/iai.48.2.331-335.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods D. E., Straus D. C., Johanson W. G., Jr, Bass J. A. Factors influencing the adherence of Pseudomonas aeruginosa to mammalian buccal epithelial cells. Rev Infect Dis. 1983 Nov-Dec;5 (Suppl 5):S846–S851. doi: 10.1093/clinids/5.supplement_5.s846. [DOI] [PubMed] [Google Scholar]
- Woods D. E., Straus D. C., Johanson W. G., Jr, Berry V. K., Bass J. A. Role of pili in adherence of Pseudomonas aeruginosa to mammalian buccal epithelial cells. Infect Immun. 1980 Sep;29(3):1146–1151. doi: 10.1128/iai.29.3.1146-1151.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward H., Horsey B., Bhavanandan V. P., Davidson E. A. Isolation, purification, and properties of respiratory mucus glycoproteins. Biochemistry. 1982 Feb 16;21(4):694–701. doi: 10.1021/bi00533a017. [DOI] [PubMed] [Google Scholar]