Abstract
We demonstrate that interactions between multimeric receptors and multivalent ligands are dramatically enhanced by recruiting a complementary templating receptor such as an endogenous multimeric protein but only when individual ligands are attached to a polymer as preorganized, covalent, heterobifunctional pairs. This effect cannot be replicated by a multivalent ligand if the same recognition elements are independently arrayed on the scaffold. Application of this principle offers an approach to create high-avidity inhibitors for multimeric receptors. Judicious selection of the ligand that engages the templating protein allows appropriate effector function to be incorporated in the polymeric construct, thereby providing an opportunity for therapeutic applications. The power of this approach is exemplified by the design of exceptionally potent Escherichia coli Shiga toxin antagonists that protect transgenic mice that constitutively express a human pentraxin, serum amyloid P component.
Keywords: heterobifunctional ligand, multivalency, Shiga toxin
Efficient inhibition of multivalent proteins that bind their ligands with weak intrinsic affinity may be achieved by using multivalency (1–3). Presentation of ligands and drugs on polymeric scaffolds offers numerous advantages from a pharmaceutical perspective: superior pharmacokinetic profile, higher activity and stability, and lower toxicity can often be achieved by conjugating a drug to a polymer (4). Additionally, a polymer is a convenient vehicle that permits the physical incorporation of several structural elements into one molecular entity to create a combination of properties (5). Herein, we describe our discovery of an important breakthrough in the design of multifunctional polymeric inhibitors that exhibit significantly enhanced avidity for their receptors by mediating formation of specific complexes of an endogenous multivalent protein with a multimeric target (Fig. 1). As a case study, we demonstrate that polymeric inhibitors–adaptors containing preordered heterobifunctional ligands that recognize Shiga toxin Type 1 (Stx1) produced by enterohemorrhagic Escherichia coli and human serum amyloid P component (HuSAP) can be tuned to achieve unprecedented in vivo activity.
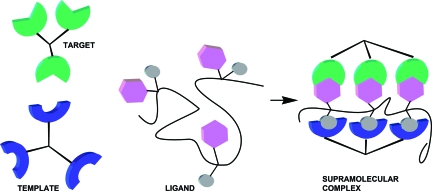
Fig. 1.
Schematic representation of the proposed concept of polymeric preordered heterobifunctional ligands.
Shiga toxins (Stx) belong to the same family of AB5 toxins as cholera and heat-labile toxins and can cause hemolytic-uremic syndrome. The radially symmetric pentameric Stx1 B-subunit binds to cell-surface glycolipids via its functional ligand, the Pk-trisaccharide, α-d-Galp (1–4)-β-d-Galp (1–4)-β-d-Glcp. Several Stx antagonists containing synthetic derivatives of the Pk-trisaccharide in various multivalent formats (6–12) have been proposed. Because the intrinsic interaction between Stx1 and individual Pk-trisaccharide sequences is extremely weak (KD = 2 × 10−3 M), a multivalent display of Pk ligands is necessary to achieve sufficient binding avidity. Because of their cross-linking capabilities, some multivalent ligands, e.g., radially symmetric dendrimeric STARFISH inhibitors, are able to induce the formation of face-to-face complexes between two copies of Stx1, thereby increasing the millimolar activity of Pk-trisaccharide inhibitors 1,000,000-fold (6, 13). However in vivo protective activity was far less impressive (14).
Heterobifunctional ligand–adaptors designed to bind both a target protein and an endogenous multivalent protein template with matching spatial arrangement of binding sites are able to mediate highly stable supramolecular assemblies (15–21). Recently, it was demonstrated that a heterobifunctional ligand can be designed to mediate the face-to-face interaction between bacterial AB5 toxins and HuSAP (15, 16, 21), which leads to the occlusion of all of the carbohydrate-binding sites in the Stx1 or cholera toxin B pentamers, thereby preventing the interaction between the toxin and its glycolipid receptor on host cells. HuSAP is a circulating plasma protein, a member of the highly conserved pentraxin family, and a component of the innate immune system. HuSAP is constitutively produced by the liver (22) and may be involved in reticuloendothelial system (RES)-mediated clearance of the by-products of inflammation and apoptosis. Structurally, the doughnut-shaped HuSAP pentamer resembles the B5 subunit of Stx1, with radially arranged binding sites presented on one face of the ring. With homobifunctional ligands such as those based on d-proline (23) or pyruvate acetals of glycerol (24) it forms decameric face-to-face complexes reminiscent of the STARFISH-mediated Stx1 dimer (6). When HuSAP is used as a template protein, the relatively high physiological concentration of the HuSAP mitigates low intrinsic affinity for its ligand (25), cyclic pyruvate ketal (CP), and facilitates formation of a strong ternary complex. We term the entropy-driven self-assembly of the sandwich-shaped heteromultimeric protein complex the supramolecular inhibition effect. The recently reported templated clustering of a membrane-bound protein, siglec CD22 (26), suggests that this effect may not be confined to proteins in solution but could also operate between membrane receptors, a soluble effector template and a heterobifunctional ligand pair, provided the membrane receptors are able to cluster in microdomains, thereby achieving a spatial distribution that is complementary to the templating protein.
Results and Discussion
Supramolecular Scaffolding.
Our previous attempts to address the issue of possible cooperativity between multivalency and supramolecular inhibition effects using a STARFISH-type dendrimer-based scaffold resulted in a very moderate increase of activity and, thus, were inconclusive (21). One possible reason was the unfavorable orientation of the Pk-trisaccharide bound to the Stx1 surface. In addition, the proximity of opposing proteins in the face-to-face or ternary complex greatly reduces the intervening space available to accommodate the scaffolding components of the multivalent ligand. The configuration of a putative supramolecular complex calls for a peripheral rather than a radial topology of the scaffolding.
To this end, we synthesized and evaluated a set of polymer-based ligands containing either independently distributed Pk (shown in Fig. 2 as its methyl glycoside compound 1) and CP (Fig. 2, compound 2) head groups or prearranged heterobifunctional CP-Pk ligands [polymers A and B, Fig. 3; for information regarding synthesis, see supporting information (SI) Text and Fig. S1]. Whereas the former polymer contains two types of independently bound head groups with specificities for the two multivalent proteins, the latter presents the same two functionalities as a single structural entity. Solid-phase binding-inhibition studies (Fig. 3 and Table 1) demonstrate the crucial importance of prearranging the two different functionalities on the polymer scaffold. Whereas the preorganized polymer of type B shows a substantial 6,000-fold increase in inhibitory activity for Stx1 in the presence of HuSAP, the polymer of type A with “random” presentation of univalent head groups was completely devoid of HuSAP-dependent activity. The remarkable nanomolar activity of polymer B is achieved at a low ligand payload of only 2.6 molar percent (Table 1) in sharp contrast to the previously reported polyacrylamide-based Shiga toxin inhibitors that required a much higher density of the pendant Pk-ligand to achieve submicromolar activities (9, 27). The corresponding unimeric heterobifunctional analog of polymer B, ligand 3, was originally designed to be able to simultaneously bridge five Ca2+-dependent binding sites of HuSAP with the five most avid binding sites of Stx1 (designated as sites 2), which resulted in enhancement of its inhibitory activity by a factor of 300 in the presence of HuSAP (21). Thus, induction of a ternary complex is a prerequisite for activity enhancement, whereas, simple recruitment of HuSAP into a Stx1-inhibitor complex is not sufficient to influence the extent of inhibition.
Fig. 2.
Structures of univalent ligands 1 and 2, unimeric heterobifunctional ligands 3, (S)-BAIT and (R)-BAIT and polymeric ligands (S)-PolyBAIT and (R)-PolyBAIT.
Fig. 3.
Inhibition of Stx1 binding to Pk-BSA glycoconjugate-coated ELISA microtiter plates by polymers A and B. Heterobivalent ligands presented on polymeric scaffolds. Polymer A presents two binding functionalities that are randomly distributed throughout a linear scaffold. Polymer B affords preorganized presentation of the two functionalities.
Table 1.
Properties and inhibitory activities of univalent, unimeric heterobifunctional, and polymer-bound ligands
| Inhibitor | Ligand/acrylamide ratio | Inhibition of Stx1*† |
Inhibition of Stx1 in the presence of SAP* |
Inhibition of SAP*‡ |
|---|---|---|---|---|
| IC50, mol × L−1 | ||||
| Polymer A | 1:19 | 5.8 × 10−7§ | 5.4 × 10−7§ | 1.2 × 10−6 |
| Polymer B | 1:36 | 1.4 × 10−5§ | 2.3 × 10−9§ | 6.4 × 10−7 |
| (S)-PolyBAIT | 1:19 | 1.1 × 10−4 | 2.7 × 10−9¶ | 1.2 × 10−7 |
| (R)-PolyBAIT | 1:21 | 1.7 × 10−4 | N/A‖ | 8.0 × 10−8 |
| 1 | – | 2.1 × 10−3 (13) | – | – |
| 2 | – | – | – | 5.3 × 10−4 (24) |
| 3 | – | 8.5 × 10−3 (21) | 3 × 10−5 (21) | – |
| (S)-BAIT | – | 3.2 × 10−3** (15) | 5.6 × 10−7 (15) | 1.9 × 10−3 (15) |
| (R)-BAIT | – | 6.0 × 10−3** (15) | N/A‖ (15) | 1.2 × 10−3 (15) |
*In case of polymers, activity is calculated per active binding fragment.
‡Inhibition of SAP binding to ELISA plate coated with d-proline analog (24).
§Binding data from Fig. 3.
¶Binding data from Fig. 4.
‖N/A, not active, i.e., IC50 is higher than 1 mg/ml, unable to attain 100% inhibition at 10 mg/ml.
**Binding data obtained by FT-ICR mass spectrometry (15).
Having established the synergistic relationship between multivalency and supramolecular inhibition effects, we designed a more efficient Stx1 antagonist, (S)-PolyBAIT. This polymeric ligand (molecular mass ≈80 kDa, Fig. 2; for information regarding synthesis, see SI Text and Fig. S2) contains head groups that are derived from a small heterobifunctional ligand, (S)-BAIT, with a molecular mass of 574 Da, whose activity rivals that of STARFISH (molecular mass ≈9 kDa) in solid-phase binding inhibition and Vero cell cytotoxicity-neutralization assays (15). The design of (S)-BAIT (15) and its less rigid analogues (21) was based on the crystal structure of the complex between HuSAP and a cyclic pyruvate ketal of glycerol 2 (24), which binds in a Ca2+-dependent manner with a KD of ≈1 mM (Table 1). The crystal structures for HuSAP-CP5 (24) and Stx1-Pk5 (6) complexes suggested the possibility that a heterobivalent ligand containing both Pk and CP would induce face-to-face aggregation between HuSAP and Stx1. As recently reported by us, this prediction was realized when the pyruvate ketal of glycerol 2 was tethered to the Pk trisaccharide 1 via its anomeric center to create 3 (21), and when the pyruvate was integrated with the glucopyranose residue of the Pk trisaccharide to create the (S)-BAIT molecule (15). Both 3 and (S)-BAIT displayed Stx1 binding activity in a HuSAP- and Ca2+-dependent manner (15, 21), and the stoichiometry of the ligand-mediated complex was established by a number of techniques (15). The observed free-energy gain originates from minimizing entropy losses in two ways. First, the heterobifunctional ligand (S)-BAIT (15) holds the pyruvate ketal in a rigid conformation, whereas 3 possess several additional degrees of rotational freedom for each of the single bonds of the tether. Second, the increased degree of preorganization of the ligand due to its interaction with the protein template (HuSAP in this case) correctly positions the Pk head groups for binding Stx1. The relative inhibitory potency of the univalent ligands, unimeric heterobifunctional ligands, and polymeric ligands for their cognate proteins illustrates the additional benefit of choosing (S)-and (R)-PolyBAITs for in vivo testing. The differential activity in the presence and absence of HuSAP of the unimeric and polymeric ligands underscores the role of HuSAP in enhancing the protective properties of the preordered heterobifunctional polymer (Table 1).
In Vitro Efficacy.
A polyacrylamide copolymer (S)-PolyBAIT was evaluated in solid-phase binding-inhibition and cytotoxicity-neutralization assays. In the presence of HuSAP, (S)-PolyBAIT demonstrated high activity (IC50 6 ng/ml or 2.7 nM per sugar unit) in a solid-phase Stx1-binding inhibition assay (Fig. 4). At the same time, a polymer, (R)-PolyBAIT (Fig. 2; for information regarding synthesis, see SI Text and Fig. S3) constructed from an inactive heterobifunctional ligand (R)-BAIT (15) failed to significantly inhibit Stx1 binding even at 1 mg/ml in the presence of HuSAP. It should be noted that the activities of both (R)-PolyBAIT and (S)-PolyBAIT with respect to each protein in separate inhibition assays are comparable (Table 1), but only the (S)-isomer shows synergy, thus confirming the importance of optimal orientation of binding moieties in the composite heterobifunctional ligands. The inhibitory activity of (S)-PolyBAIT in the Vero cell cytotoxicity-neutralization assay (IC50 ≈8 ng/ml) was similar to that observed for the solid-phase binding-inhibition assay (IC50 ≈13 ng/ml). This was in sharp contrast to our previous experience with STARFISH-type Pk-dendrimers, which exhibited a significant drop in activity in this cytotoxicity-neutralization assay (6, 14). The HuSAP-dependent activity enhancement should be attributed to the ability of (S)-PolyBAIT to mediate formation of a stable ternary complex with Stx1 and HuSAP (see SI Text and Figs. S6 and S7).
Fig. 4.
HuSAP-dependent inhibitory activities of the multimeric heterobivalent inhibitor (S)-PolyBAIT and its unimeric analog (S)-BAIT. (A) Inhibition of Stx1 binding to Pk-BSA glycoconjugate-coated ELISA microtiter plates. (B) Vero cell cytotoxicity neutralization assay. (STARFISH is a decavalent Pk-trisaccharide-containing compound; Fig. S4).
In Vivo Efficacy.
We next tested the efficacy of (S)-PolyBAIT in preventing Stx1-mediated Shigatoxemia in mice. Two different protocols were developed by using transgenic (tg) mice that constitutively express HuSAP at a stable physiological concentration (28). In the first format, groups of HuSAP-tg mice were treated intravenously with a lethal dose (LD100) of Stx1 premixed with (S)-PolyBAIT, (S)-BAIT, or (R)-PolyBAIT. Whereas, 100% of the HuSAP-tg mice in the Stx1/(S)-PolyBAIT-treated group developed no signs of Shigatoxemia during the 7-day course of the experiment, 100% of the mice in the Stx1/saline, Stx1/(S)-BAIT- and Stx1/(R)-PolyBAIT-treatment groups developed Shigatoxemia within 72–110 h and were euthanized (Fig. 5). In a similar in vivo Stx1 mouse challenge experiment, the STARFISH-type ligand (6), at its maximum achievable dose of 25 μg/g of body weight, was completely ineffective. The failure of the hydrophilic, low-molecular-mass (S)-BAIT in these in vivo experiments is attributed to its extremely short, <30-min, half-life in circulation.
Fig. 5.
Protection of C57BL6 mice, transgenic for HuSAP, from Stx1-mediated Shigatoxemia. (Upper Left) Mice were injected via tail vein with a mixture of LD100 of Stx1 and inhibitor. (Lower Left) Mice were challenged with an LD100 of Stx1 via dorsal s.c. injection, followed immediately by 100-μl tail vein injections of (S)-PolyBAIT, (R)-PolyBAIT, or saline. (Right) Biodistribution of Stx1-125I after injection of 20 ng/g in HuSAP tg mice via tail vein. The mice were then euthanized at 4 h.
The second protocol more closely mimicked the clinical situation where toxin slowly enters the blood from the intestine and is then delivered via the circulation to its target organs. In this protocol, an LD100 of Stx1 was first administered s.c. into the backs of HuSAP-tg mice. This was immediately followed by a single i.v. injection of (S)-PolyBAIT. In this experiment, 100% of the HuSAP-tg mice were again protected by (S)-PolyBAIT at a dose as low as 1 μg/g of mouse body weight, whereas all mice in the control groups (no inhibitor, (R)-PolyBAIT, or HuSAP-negative mice) developed signs of Shigatoxemia and were euthanized within 72–110 h of receiving Stx1 (Fig. 5). Previously, only humanized Stx1-specific monoclonal antibodies demonstrated this level of protection in mice (29).
One of HuSAP's normal physiological functions may be to bind to the cellular debris produced during apoptosis and inflammation (25), thereby redirecting potentially harmful inflammatory by-products to the RES where they may be catabolically disposed. A biodistribution experiment revealed the in vivo fate of (S)-PolyBAIT-Stx1-HuSAP complexes. In addition to its ability to inhibit Stx1 binding to Pk receptors, (S)-PolyBAIT redirected 125I-labeled Stx1 from the kidney to the liver (Fig. 5). Thus, the protective effect of (S)-PolyBAIT in vivo can be attributed to three factors: prolonged bioavailability in the circulation, promotion of stable Stx1-HuSAP complexes, and redirection of these complexes to the RES for disposal. We estimate the physiological concentration of HuSAP to be appropriately equal to the effective dose of polymeric ligand, both higher than the IC50 of polymeric ligands containing CP head groups (Table 1). This suggests that the polymeric inhibitors will circulate in the HuSAP-bound form, which may affect their biodistributions.
Stx2, often cited as the most clinically significant toxin produced by E. coli O157, cannot be evaluated in this animal model because it was shown that HuSAP complexes Stx2 in the absence of ligand (28, 30, 31). Nevertheless, we have demonstrated that our heterobifunctional ligand does promote the formation of Stx2-HuSAP complexes (15), thus providing strong evidence that the mechanism described here would also operate in vivo against Stx2. It could also be envisioned that recently described ligands that have a high affinity for Stx2 could be integrated into the polymeric ligand construct in place of the Pk trisaccharide (32).
Conclusions
We have demonstrated that covalently preorganized heterobifunctional ligands displayed on a polymeric scaffold create a class of multivalent inhibitors whose activity for a target protein is enhanced by several orders of magnitude in the presence of a templating protein that organizes the ligand for optimal binding to the target. In the specific example of Shiga-like toxins released during infections by E. coli O157, it could be envisioned that the highly active antagonists reported here could be administered with appropriate antibiotics. Although antibiotic therapy alone is not used in practice because of the increased toxin load that results from toxin released by killed bacteria, such dual therapy may be an attractive option for the most severe E. coli infections.
The concept of multifunctional multivalency delineated here should find broader application in therapies and diagnostics. In separate work (26), a unimeric heterobifunctional sialic acid containing a trisaccharide tethered to a nitrophenol ligand engaged the siglec CD22 with high avidity when nitrophenol-specific IgM antibody was used to template the ligands. This demonstrated that both soluble and membrane-associated receptors can be targeted by this approach, because membrane-associated receptors generally possess lateral mobility, thereby permitting the adoption of optimal spacing to complement that of the templating protein. Recognition of host cell-surface antigens is an essential stage of any infection, colonization, and metastasis. Heterobifunctional inhibitors/mediators designed to recognize both cell-surface receptors and antibodies may target malignant B cells (26) or provide instant adaptation of the host immune system to a novel pathogen or toxin and buy necessary time for development of an efficient adaptive immune response in an immunologically naïve host (19, 20). Multivalent supramolecular binding systems of the type described herein that can attract humoral or cellular components of the host immune system to the site of interaction have the potential to provide a first line of defense against a multitude of pathogenic organisms and malignancies. Because the same physical principles apply to both biological systems and artificial molecular mechanisms (33), the prearranged multivalent ligands–adaptors may also find use in engineering of nanoscale molecular machinery.
Materials and Methods
Synthetic procedures for new compounds, gel-permeation chromatography, gel electrophoresis, and dynamic light scattering are presented in SI Text.
Biological Evaluation.
The solid-phase binding-inhibition and Vero cell cytotoxicity-neutralization assays are fully described in previous reports (6, 15, 24).
Mouse Challenge Trials.
Transgenic mice C57BL/6-Tg(APSC)1Imeg (34), which exhibit liver-specific expression of HuSAP at a stable circulating serum concentration of 30–40 μg/ml, were used in the Stx1-mediated Shigatoxemia experiments. All experiments were conducted in a double-blind, placebo-controlled manner. All animal protocols were reviewed and approved (Protocol Number MO4002) by the Faculty of Medicine, University of Calgary Animal Welfare Committee and were performed according to the Guidelines published by the Canadian Council on Animal Care, (Vol 1, 2nd Ed).
Protocol A.
HuSAP-tg mice (n = 4–6 animals per group) were intravenously injected with a lethal dose (LD100 20 ng/g of body weight) of Stx1 that was premixed in a total volume of 100 μl with either (S)-PolyBAIT (2.5 μg/g of body weight) or (R)-PolyBAIT (25 μg/g of body weight) in physiological saline solution. Forty-eight hours after challenge, the mice were monitored for signs of lethargy and immediately euthanized by CO2 asphyxia. On the 10th day, all surviving mice were euthanized.
Protocol B.
HuSAP-tg mice (n = 4–6 animals per group) were given a dorsal s.c. injection of Stx1 (LD100 20 ng/g of body weight) in saline with 0.375 μg/g of body weight of saponin, immediately followed by a single i.v. injection of (S)-PolyBAIT (3.15, 1.0, or 0.315 μg/g of body weight) in physiological saline solution or (R)-PolyBAIT (100 μg/g of body weight in physiological saline solution). HuSAP-negative mice served as a third control group. Forty-eight hours after challenge, the mice were monitored for signs of lethargy and immediately euthanized by CO2 asphyxia. On the 10th day, all surviving mice were euthanized.
Gel-Permeation Chromatography (GPC).
GPC was carried out by using a Waters HPLC liquid-chromatography system. Two running buffers were used in GPC binding experiments: Buffer 1, [10 mM Tris·HCl (pH 7.4), 0.14 M NaCl] and Buffer 2, (10 mM Tris·HCl (pH 7.4), 0.14 M NaCl, 2.5 mM CaCl2, 50 μg/ml]. The elution curves are shown in Fig. S5.
Estimation of (S)-PolyBAIT Molecular Mass.
GPC of polyacrylamide-based polymers was conducted on an Ultrahydrogel 1000 column in series with an Ultrahydrogel 500 column in PBS (pH 7.2).
Purification of the Ternary Complex Among HuSAP, (S)-PolyBAIT, and Stx1 B5-Subunit.
Protein experiments were conducted by using a Shodex KW-803 column run at a flow rate of 0.5 ml/min and temperature of 20°C with UV detection at 220 nm. The sample volume applied was 100 μl and contained HuSAP (13 μl at 1 mg/ml), Stx1 B subunit with HIS tag (11 μl at 0.45 mg/ml), and Buffer 2 (76 μl).
The complex Stx1B-(S)-PolyBAIT-HuSAP was collected as a fraction with retention time between 5.5 and 8 min, washed four times with Buffer 2 on an Amicon Ultra centrifugal device with a 5,000 molecular mass cut-off membrane. The solution containing the collected complex was used in SDS/PAGE experiments. The elution curves are shown in Fig. S6.
Acrylamide-Gel Electrophoresis.
Tricine–SDS/PAGE was performed on a 16.5% gel (3% cross-linker) with sample buffer containing 6% of 2-mercaptoethanol. Gels were stained with Coomassie Brilliant blue G-250. The composition of complexes formed among HuSAP, PolyBAIT, and Stx1B5 are shown in Fig. S7.
PolyBAIT Molecular Mass Determination by Dynamic Light Scattering (DLS).
The hydrodynamic radius (Rh) analysis was carried out at 25°C by using a Protein Solutions DynaPro Molecular Sizing Instrument (Proterion). DLS measurements were accepted only if the light intensity count rate was steady to within ±20% and the polydispersity index reading was <0.2. The hydrodynamic radius was calculated by using the cumulant monomodal model. The molecular mass was estimated by using a standard curve (molecular mass = (RhFactor × Rh)∧Power). Parameters for polyacrylamides were derived from measurements of standard polyacrylamide samples (RhFactor = 1.78277, Power = 1.4244). The determined average molecular mass of (S)-PolyBAIT was ≈80 kDa. For additional information, see SI Appendix, which contains supporting spectra.
Supplementary Material
Acknowledgments.
This work was supported by a grant from Alberta Ingenuity Foundation. T.G. is the recipient of a Natural Sciences and Engineering Research Council (NSERC) Canada Graduate Scholarship.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0804919105/DCSupplemental.
References
- 1.Gestwicki JE, Cairo CW, Strong LE, Oetjen KA, Kiessling LL. Influencing receptor–ligand binding mechanisms with multivalent ligand architecture. J Am Chem Soc. 2002;124:14922–14933. doi: 10.1021/ja027184x. [DOI] [PubMed] [Google Scholar]
- 2.Lundquist JJ, Toone EJ. The cluster glycoside effect. Chem Rev. 2002;102:555–578. doi: 10.1021/cr000418f. [DOI] [PubMed] [Google Scholar]
- 3.Mammen M, Choi SK, Whitesides GM. Polyvalent interactions in biological systems: Implications for design and use of multivalent ligands and inhibitors. Angew Chem. 1998;37:2755–2794. doi: 10.1002/(SICI)1521-3773(19981102)37:20<2754::AID-ANIE2754>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 4.Duncan R. The dawning era of polymer therapeutics. Nat Rev Drug Discov. 2003;2:347–360. doi: 10.1038/nrd1088. [DOI] [PubMed] [Google Scholar]
- 5.Krishnamurthy VM, et al. Promotion of opsonization by antibodies and phagocytosis of Gram-positive bacteria by a bifunctional polyacrylamide. Biomaterials. 2006;27:3663–3674. doi: 10.1016/j.biomaterials.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Kitov PI, et al. Shiga-like toxins are neutralized by tailored multivalent carbohydrate ligands. Nature. 2000;403:669–672. doi: 10.1038/35001095. [DOI] [PubMed] [Google Scholar]
- 7.Nishikawa K, et al. A therapeutic agent with oriented carbohydrates for treatment of infections by Shiga toxin-producing Escherichia coli O157: H7. Proc Natl Acad Sci USA. 2002;99:7669–7674. doi: 10.1073/pnas.112058999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Isobe H, et al. Synthesis of fullerene glycoconjugates via a copper-catalyzed Huisgen cycloaddition reaction. Org Lett. 2007;9:4611–4614. doi: 10.1021/ol702128z. [DOI] [PubMed] [Google Scholar]
- 9.Watanabe M, et al. Oral therapeutic agents with highly clustered globotriose for treatment of Shiga toxigenic Escherichia coli infections. J Infect Dis. 2004;189:360–368. doi: 10.1086/381124. [DOI] [PubMed] [Google Scholar]
- 10.Neri P, et al. Monovalent Gb(3)-/Gb(2)-derivatives conjugated with a phosphatidyl residue: A novel class of Shiga toxin-neutralizing agent. Biol Pharmaceut Bull. 2007;30:1697–1701. doi: 10.1248/bpb.30.1697. [DOI] [PubMed] [Google Scholar]
- 11.Paton AW, Morona R, Paton JC. A new biological agent for treatment of Shiga toxigenic Escherichia coli infections and dysentery in humans. Nat Med. 2000;6:265–270. doi: 10.1038/73111. [DOI] [PubMed] [Google Scholar]
- 12.Armstrong GD, et al. A phase-I study of chemically synthesized verotoxin (Shiga-like toxin) Pk-trisaccharide receptors attached to chromosorb for preventing hemolytic-uremic syndrome. J Infect Dis. 1995;171:1042–1045. doi: 10.1093/infdis/171.4.1042. [DOI] [PubMed] [Google Scholar]
- 13.Kitov PI, Bundle DR. On the nature of the multivalency effect: A thermodynamic model. J Am Chem Soc. 2003;125:16271–16284. doi: 10.1021/ja038223n. [DOI] [PubMed] [Google Scholar]
- 14.Mulvey GL, et al. Assessment in mice of the therapeutic potential of tailored, multivalent Shiga toxin carbohydrate ligands. J Infect Dis. 2003;187:640–649. doi: 10.1086/373996. [DOI] [PubMed] [Google Scholar]
- 15.Kitov PI, et al. An entropically efficient supramolecular inhibition strategy for Shiga toxins. Angew Chem. 2008;47:672–676. doi: 10.1002/anie.200704064. [DOI] [PubMed] [Google Scholar]
- 16.Liu JY, et al. Protein heterodimerization through ligand-bridged multivalent pre-organization: Enhancing ligand binding toward both protein targets. J Am Chem Soc. 2005;127:2044–2045. doi: 10.1021/ja043817r. [DOI] [PubMed] [Google Scholar]
- 17.Lu YJ, Low PS. Folate targeting of haptens to cancer cell surfaces mediates immunotherapy of syngeneic murine tumors. Cancer Immunol Immunother. 2002;51:153–162. doi: 10.1007/s00262-002-0266-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu YJ, Sega E, Leamon CP, Low PS. Folate receptor-targeted immunotherapy of cancer: Mechanism and therapeutic potential. Adv Drug Deliv Rev. 2004;56:1161–1176. doi: 10.1016/j.addr.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 19.Owen RM, et al. Bifunctional ligands that target cells displaying the avb3 integrin. ChemBioChem. 2007;8:68–82. doi: 10.1002/cbic.200600339. [DOI] [PubMed] [Google Scholar]
- 20.Shokat KM, Schultz PG. Redirecting the immune-response—Ligand-mediated immunogenicity. J Am Chem Soc. 1991;113:1861–1862. [Google Scholar]
- 21.Solomon D, et al. Heterobifunctional multivalent inhibitor-adaptor mediates specific aggregation between Shiga toxin and a pentraxin. Org Lett. 2005;7:4369–4372. doi: 10.1021/ol051529+. [DOI] [PubMed] [Google Scholar]
- 22.Pepys MB, et al. Biology of serum amyloid P-component. Ann NY Acad Sci. 1982;389:286–298. doi: 10.1111/j.1749-6632.1982.tb22144.x. [DOI] [PubMed] [Google Scholar]
- 23.Pepys MB, et al. Targeted pharmacological depletion of serum amyloid P component for treatment of human amyloidosis. Nature. 2002;417:254–259. doi: 10.1038/417254a. [DOI] [PubMed] [Google Scholar]
- 24.Ho JGS, et al. Ligand-assisted aggregation of proteins—Dimerization of serum amyloid P component by bivalent ligands. J Biol Chem. 2005;280:31999–32008. doi: 10.1074/jbc.M504403200. [DOI] [PubMed] [Google Scholar]
- 25.Pepys MB, et al. Amyloid P component. A critical review. Amyloid. 1997;4:274–295. [Google Scholar]
- 26.O'Reilly MK, et al. Bi-functional CD22 ligands use multimeric immunoglobulins as protein scaffolds in assembly of immune complexes on B cells. J Am Chem Soc. 2008;130:7736–7745. doi: 10.1021/ja802008q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neri P, et al. Neutralizing activity of polyvalent Gb(3), Gb(2) and galacto-trehalose models against Shiga toxins. Microbiol Immunol. 2007;51:581–592. doi: 10.1111/j.1348-0421.2007.tb03944.x. [DOI] [PubMed] [Google Scholar]
- 28.Armstrong GD, et al. Human serum amyloid P component protects against Escherichia coli O157: H7 Shiga toxin 2 in vivo: Therapeutic implications for hemolytic-uremic syndrome. J Infect Dis. 2006;193:1120–1124. doi: 10.1086/501472. [DOI] [PubMed] [Google Scholar]
- 29.Mukherjee J, et al. Production and characterization of protective human antibodies against Shiga toxin 1. Infect Immun. 2002;70:5896–5899. doi: 10.1128/IAI.70.10.5896-5899.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kimura T, Tani S, Matsumoto Y, Takeda T . Serum amyloid P component is the Shiga toxin 2-neutralizing factor in human blood. J Biol Chem. 2001;276:41576–41579. doi: 10.1074/jbc.M107819200. [DOI] [PubMed] [Google Scholar]
- 31.Marcato P, Vander HK, Mulvey GL, Armstrong GD. Infect Immun. 2003;71:6075–6078. doi: 10.1128/IAI.71.10.6075-6078.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kale RR, et al. Differentiation between structurally homologous Shiga 1 and Shiga 2 toxins by using synthetic glycoconjugates. Angew Chem. 2008;47:1265–1268. doi: 10.1002/anie.200703680. [DOI] [PubMed] [Google Scholar]
- 33.Badjic JD, Nelson A, Cantrill SJ, Turnbull WB, Stoddart JF. Multivalency and cooperativity in supramolecular chemistry. Acc Chem Res. 2005;38:723–732. doi: 10.1021/ar040223k. [DOI] [PubMed] [Google Scholar]
- 34.Zhao X, Araki K, Miyazaki J, Yamamura K. Developmental and liver-specific expression directed by the serum amyloid-P component promoter in transgenic mice. J Biochem. 1992;111:736–738. doi: 10.1093/oxfordjournals.jbchem.a123828. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.