Abstract
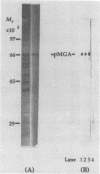
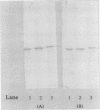
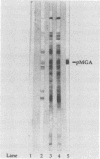
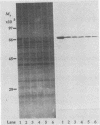
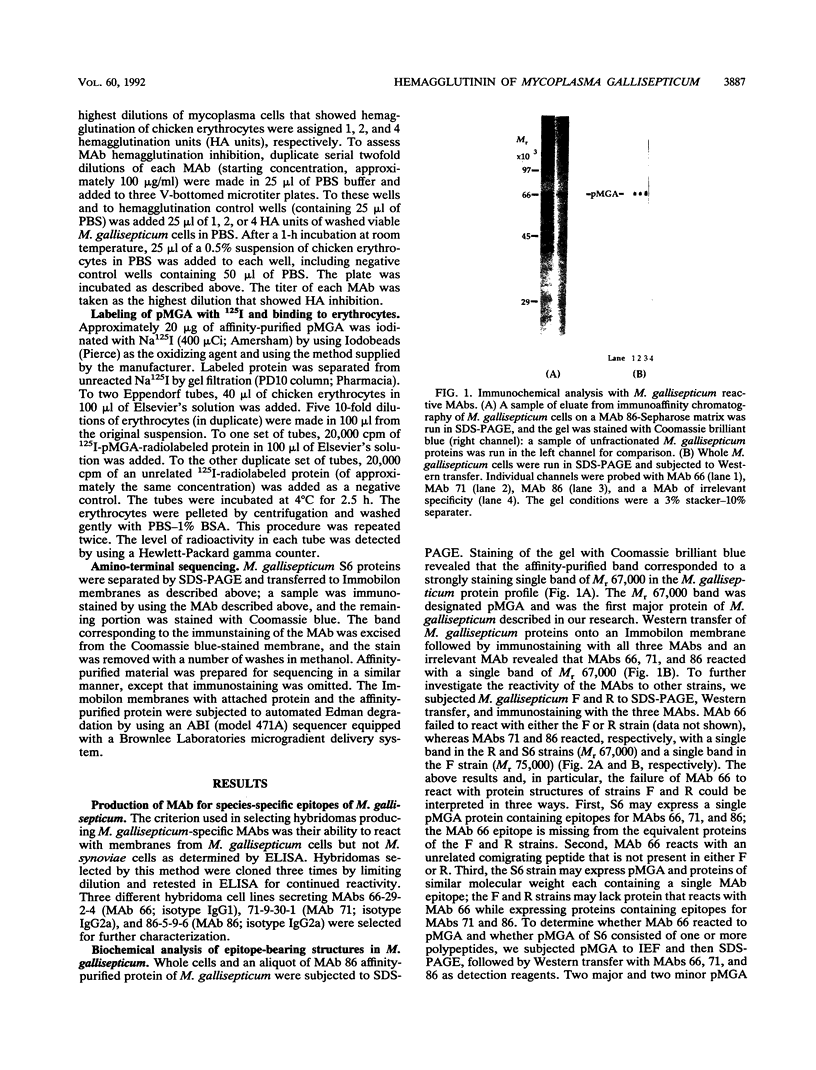
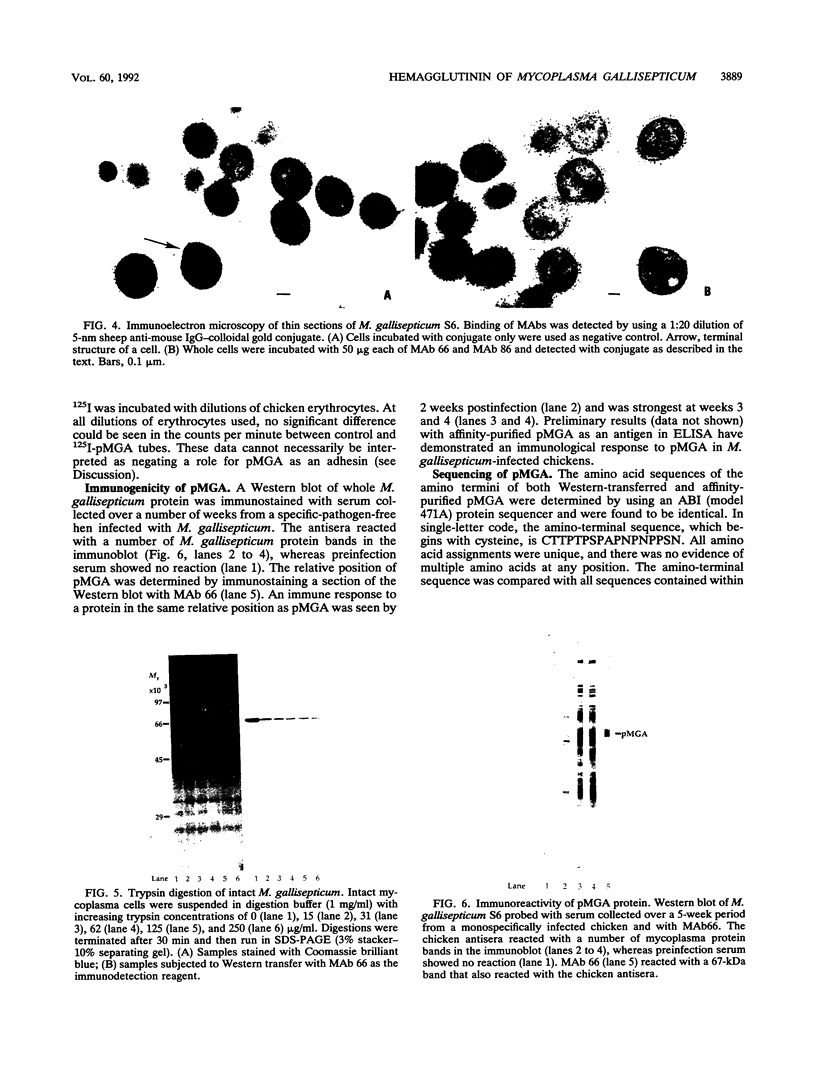
Mycoplasma gallisepticum cell membranes were used to immunize mice to produce monoclonal antibodies to cell surface proteins. Three monoclonal antibodies were chosen for further characterization. All three reacted in immunoblots with an M. gallisepticum protein band of M(r) approximately 67,000 (designated pMGA). By using immunoelectron microscopy, pMGA was shown to be located on the cell surface. When M. gallisepticum whole cells were treated with up to 250 micrograms of trypsin per ml for 30 min, the only major protein lost from the cell surface as judged by sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by Western immunoblot transfer was pMGA. Two of the pMGA-specific monoclonal antibodies inhibited hemagglutination of chicken erythrocytes by M. gallisepticum S6, suggesting a role for pMGA in the attachment of M. gallisepticum to chicken erythrocytes. Sequencing the amino terminus of pMGA yielded 17 amino acids with no significant homology with the Mycoplasma pneumoniae attachment protein P1 or any other protein in the GenBank, Swiss-Prot, and EMBL data bases.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avakian A. P., Kleven S. H., Ley D. H. Comparison of Mycoplasma gallisepticum strains and identification of immunogenic integral membrane proteins with Triton X-114 by immunoblotting. Vet Microbiol. 1991 Nov;29(3-4):319–328. doi: 10.1016/0378-1135(91)90139-7. [DOI] [PubMed] [Google Scholar]
- Banai M., Kahane I., Razin S., Bredt W. Adherence of Mycoplasma gallisepticum to human erythrocytes. Infect Immun. 1978 Aug;21(2):365–372. doi: 10.1128/iai.21.2.365-372.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley L. D., Snyder D. B., Van Deusen R. A. Identification of species-specific and interspecies-specific polypeptides of Mycoplasma gallisepticum and Mycoplasma synoviae. Am J Vet Res. 1988 Apr;49(4):511–515. [PubMed] [Google Scholar]
- Clyde W. A., Jr, Hu P. C. Antigenic determinants of the attachment protein of Mycoplasma pneumoniae shared by other pathogenic Mycoplasma species. Infect Immun. 1986 Feb;51(2):690–692. doi: 10.1128/iai.51.2.690-692.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallo S. F., Baseman J. B. Cross-hybridization between the cytadhesin genes of Mycoplasma pneumoniae and Mycoplasma genitalium and genomic DNA of Mycoplasma gallisepticum. Microb Pathog. 1990 May;8(5):371–375. doi: 10.1016/0882-4010(90)90096-9. [DOI] [PubMed] [Google Scholar]
- Feldner J., Göbel U., Bredt W. Mycoplasma pneumoniae adhesin localized to tip structure by monoclonal antibody. Nature. 1982 Aug 19;298(5876):765–767. doi: 10.1038/298765a0. [DOI] [PubMed] [Google Scholar]
- Frey M. L., Hanson R. P., Andrson D. P. A medium for the isolation of avian mycoplasmas. Am J Vet Res. 1968 Nov;29(11):2163–2171. [PubMed] [Google Scholar]
- Hansen E. J., Wilson R. M., Baseman J. B. Two-dimensional gel electrophoretic comparison of proteins from virulent and avirulent strains of Mycoplasma pneumoniae. Infect Immun. 1979 May;24(2):468–475. doi: 10.1128/iai.24.2.468-475.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Higgins P. A., Whithear K. G. Detection and differentiation of Mycoplasma gallisepticum and M. synoviae antibodies in chicken serum using enzyme-linked immunosorbent assay. Avian Dis. 1986 Jan-Mar;30(1):160–168. [PubMed] [Google Scholar]
- Hu P. C., Cole R. M., Huang Y. S., Graham J. A., Gardner D. E., Collier A. M., Clyde W. A., Jr Mycoplasma pneumoniae infection: role of a surface protein in the attachment organelle. Science. 1982 Apr 16;216(4543):313–315. doi: 10.1126/science.6801766. [DOI] [PubMed] [Google Scholar]
- Hu P. C., Collier A. M., Baseman J. B. Surface parasitism by Mycoplasma pneumoniae of respiratory epithelium. J Exp Med. 1977 May 1;145(5):1328–1343. doi: 10.1084/jem.145.5.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inamine J. M., Denny T. P., Loechel S., Schaper U., Huang C. H., Bott K. F., Hu P. C. Nucleotide sequence of the P1 attachment-protein gene of Mycoplasma pneumoniae. Gene. 1988 Apr 29;64(2):217–229. doi: 10.1016/0378-1119(88)90337-x. [DOI] [PubMed] [Google Scholar]
- Kahane I., Granek J., Reisch-Saada A. The adhesins of Mycoplasma gallisepticum and M. pneumoniae. Ann Microbiol (Paris) 1984 Jan-Feb;135A(1):25–32. doi: 10.1016/s0769-2609(84)80055-1. [DOI] [PubMed] [Google Scholar]
- Krause D. C., Baseman J. B. Inhibition of mycoplasma pneumoniae hemadsorption and adherence to respiratory epithelium by antibodies to a membrane protein. Infect Immun. 1983 Mar;39(3):1180–1186. doi: 10.1128/iai.39.3.1180-1186.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause D. C., Leith D. K., Baseman J. B. Reacquisition of specific proteins confers virulence in Mycoplasma pneumoniae. Infect Immun. 1983 Feb;39(2):830–836. doi: 10.1128/iai.39.2.830-836.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts D. D., Olson L. D., Barile M. F., Ginsburg V., Krivan H. C. Sialic acid-dependent adhesion of Mycoplasma pneumoniae to purified glycoproteins. J Biol Chem. 1989 Jun 5;264(16):9289–9293. [PubMed] [Google Scholar]
- Rosengarten R., Wise K. S. The Vlp system of Mycoplasma hyorhinis: combinatorial expression of distinct size variant lipoproteins generating high-frequency surface antigenic variation. J Bacteriol. 1991 Aug;173(15):4782–4793. doi: 10.1128/jb.173.15.4782-4793.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su C. J., Tryon V. V., Baseman J. B. Cloning and sequence analysis of cytadhesin P1 gene from Mycoplasma pneumoniae. Infect Immun. 1987 Dec;55(12):3023–3029. doi: 10.1128/iai.55.12.3023-3029.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong W. K., Gerhard B., Guo Z. M., Kilburn D. G., Warren A. J., Miller R. C., Jr Characterization and structure of an endoglucanase gene cenA of Cellulomonas fimi. Gene. 1986;44(2-3):315–324. doi: 10.1016/0378-1119(86)90196-4. [DOI] [PubMed] [Google Scholar]
- Yogev D., Rosengarten R., Watson-McKown R., Wise K. S. Molecular basis of Mycoplasma surface antigenic variation: a novel set of divergent genes undergo spontaneous mutation of periodic coding regions and 5' regulatory sequences. EMBO J. 1991 Dec;10(13):4069–4079. doi: 10.1002/j.1460-2075.1991.tb04983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]