Abstract
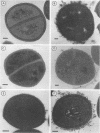
Many strains of Staphylococcus saprophyticus cause direct hemagglutination of sheep erythrocytes. For a high proportion of clinical isolates, a surface protein (Ssp) that is apparently not involved in this property has been described. In this study, S. saprophyticus CCM883, a hemagglutinating but Ssp-negative strain, was used for the identification, purification, and characterization of a 160-kDa surface polypeptide that appears to be the major component of the hemagglutinin. Expression of the protein required the addition to the growth medium of EDTA in micromolar quantities, suggesting an inhibitory role for some unidentified metal ion. The protein was purified by means of Sephacryl S-300 chromatography, and antisera were raised in rabbits. Antibody against this protein inhibited the hemagglutination of two other, unrelated strains and was used to demonstrate, by electron microscopy, the presence of the protein on the surface of the cells. In a confirmatory experiment, the purified antigen was incubated with erythrocytes and binding was detected by the Western immunoblot technique with the antibody to the 160-kDa polypeptide. These experiments indicate that this surface protein is the hemagglutinin of S. saprophyticus.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almeida R. J., Jorgensen J. H. Comparison of adherence and urine growth rate properties of Staphylococcus saprophyticus and Staphylococcus epidermidis. Eur J Clin Microbiol. 1984 Dec;3(6):542–545. doi: 10.1007/BF02013615. [DOI] [PubMed] [Google Scholar]
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Gatermann S., John J., Marre R. Staphylococcus saprophyticus urease: characterization and contribution to uropathogenicity in unobstructed urinary tract infection of rats. Infect Immun. 1989 Jan;57(1):110–116. doi: 10.1128/iai.57.1.110-116.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatermann S., Kreft B., Marre R., Wanner G. Identification and characterization of a surface-associated protein (Ssp) of Staphylococcus saprophyticus. Infect Immun. 1992 Mar;60(3):1055–1060. doi: 10.1128/iai.60.3.1055-1060.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatermann S., Marre R. Cloning and expression of Staphylococcus saprophyticus urease gene sequences in Staphylococcus carnosus and contribution of the enzyme to virulence. Infect Immun. 1989 Oct;57(10):2998–3002. doi: 10.1128/iai.57.10.2998-3002.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatermann S., Marre R., Heesemann J., Henkel W. Hemagglutinating and adherence properties of Staphylococcus saprophyticus: epidemiology and virulence in experimental urinary tract infection of rats. FEMS Microbiol Immunol. 1988 Dec;1(3):179–185. doi: 10.1111/j.1574-6968.1988.tb02372.x. [DOI] [PubMed] [Google Scholar]
- Gunnarsson A., Mårdh P. A., Lundblad A., Svensson S. Oligosaccharide structures mediating agglutination of sheep erythrocytes by Staphylococcus saprophyticus. Infect Immun. 1984 Jul;45(1):41–46. doi: 10.1128/iai.45.1.41-46.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovelius B., Mårdh P. A. Haemagglutination by Staphylococcus saprophyticus and other staphylococcal species. Acta Pathol Microbiol Scand B. 1979 Feb;87B(1):45–50. doi: 10.1111/j.1699-0463.1979.tb02401.x. [DOI] [PubMed] [Google Scholar]
- Hovelius B., Mårdh P. A. Staphylococcus saprophyticus as a common cause of urinary tract infections. Rev Infect Dis. 1984 May-Jun;6(3):328–337. doi: 10.1093/clinids/6.3.328. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lindahl M., Holmberg O., Jonsson P. Adhesive proteins of haemagglutinating Staphylococcus aureus isolated from bovine mastitis. J Gen Microbiol. 1990 May;136(5):935–939. doi: 10.1099/00221287-136-5-935. [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Stinson M. W., Jones C. A. Binding of Todd-Hewitt broth antigens by Streptococcus mutans. Infect Immun. 1983 Jun;40(3):1140–1145. doi: 10.1128/iai.40.3.1140-1145.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teti G., Chiofalo M. S., Tomasello F., Fava C., Mastroeni P. Mediation of Staphylococcus saprophyticus adherence to uroepithelial cells by lipoteichoic acid. Infect Immun. 1987 Mar;55(3):839–842. doi: 10.1128/iai.55.3.839-842.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallmark G., Arremark I., Telander B. Staphylococcus saprophyticus: a frequent cause of acute urinary tract infection among female outpatients. J Infect Dis. 1978 Dec;138(6):791–797. doi: 10.1093/infdis/138.6.791. [DOI] [PubMed] [Google Scholar]