Abstract
Objective
To determine whether serum Zic4 antibodies associate with paraneoplastic neurologic disorders (PND) and small-cell lung cancer (SCLC), and the association of these antibodies with other onconeuronal immunities associated with SCLC.
Design/Methods
The authors studied 498 patients (215 with PND and 283 without PND or without cancer). The presence of antibodies was tested with immunoblots of Zic4, HuD, and CRMP5 proteins. The tumor expression of these proteins was determined by immunohistochemistry.
Results
Zic4 antibodies were identified in 61 patients. Ninety-two percent of patients with Zic4 antibodies had SCLC; detection of these antibodies segregated with the presence of PND (p = 0.031). Intrathecal synthesis of Zic4 antibodies was demonstrated in 5/7 patients with PND. None of 175 control patients without PND or cancer had Zic4 antibodies. Because of the robust association between Zic autoimmunity and SCLC, all patients were tested for other SCLC-related antibodies; concurrent Zic4, Hu, or CRMP5 antibodies occurred in the serum or CSF of 27% of SCLC patients with PND. Patients with isolated Zic4 antibodies were more likely to develop predominant cerebellar dysfunction than patients with several immunities (p < 0.001). Tumors of patients with and without onconeuronal antibodies coexpressed Zic, Hu, and CRMP5 proteins, indicating that the tumor expression of these antigens is necessary, but not sufficient, for immunologic activation.
Conclusions
In patients with neurologic symptoms of unknown cause detection of Zic4 antibodies predicts a neoplasm, usually a SCLC, and suggests that the neurologic disorder is paraneoplastic. Detection of Zic4 antibodies often associates with anti-Hu or CRMP5 antibodies. Patients with isolated Zic4 antibodies are more likely to develop cerebellar dysfunction than those with concurrent immunities.
The Zic genes encode zinc-finger proteins that are expressed in the developing and mature CNS and have critical roles in the development of the cerebellum.1–3 Antibodies to Zic proteins have been identified in a patient with subacute cerebellar degeneration and in a few patients with small-cell lung cancer (SCLC).4,5 We postulated that in patients with neurologic disease of unknown etiology, detection of Zic4 antibodies represents paraneoplastic immunity associated with CNS dysfunction or SCLC. The data presented here support this hypothesis and emphasize the multiplicity and heterogeneity of paraneoplastic immunity associated to SCLC.
Materials and methods
Sera, tissues, and plasmids
A total of 498 sera were studied. These included 167 patients with paraneoplastic neurologic disorders (PND) and SCLC or neuroendocrine tumors, 48 with PND and other tumors, 108 cancer patients without PND (74 SCLC, 11 brain tumors, 8 Hodgkin’s lymphoma, 8 colon cancer, and 7 testicular tumors), 155 patients with non-cancer related neurologic disorders (40 idiopathic late-onset cerebellar degeneration, 32 dementia, 20 multiple sclerosis (MS), 18 retinitis/optic neuropathy of unknown cause, 17 opsoclonus, 12 sensorimotor neuropathy, 5 inherited cerebellar degeneration, 4 subacute development of movement disorders, 4 seizures refractory to treatment, 3 angiitis of the CNS), and 20 normal blood donors. Paraffin-embedded tumors were provided by the tumor procurement service at the University of Arkansas for Medical Sciences. The human Zic4 gene was cloned as reported.5
Criteria for PND of the CNS
Patients were considered to have PND when they developed a characteristic neurologic syndrome in association with cancer and no other etiology was identified, or a paraneoplastic antibody was detected in serum or CSF. Characteristic neurologic syndromes included one or more of the following: limbic encephalitis, brainstem encephalitis, cerebellar degeneration, myelitis, autonomic dysfunction, and sensorimotor or sensory neuropathy.
Recombinant proteins and immunoblot analysis
Recombinant Zic4 (100 μg/mL), HuD (50 μg/mL), and CRMP5 (100 μg/mL) were obtained as previously reported.6 Immunoblots of fusion proteins were tested with patients’ sera (diluted 1:750) or CSF (1:10) using a secondary biotinylated goat anti-human immunoglobulin G (IgG) antibody (1:2000) and a standard avidin-biotin-peroxidase method (Vector, Burlingame, CA). The titers of Zic4 and anti-Hu antibodies were obtained by serial serum dilutions with immunoblots of Zic4 and HuD proteins until the reactive band was no longer visible. Titers were not obtained for anti-CRMP5 antibodies. Analysis of intrathecal synthesis of Zic4 antibodies was performed as reported.7
Immunohistochemistry
To avoid reactivity with the endogenous IgG contained in human tumors, all immunohistochemical studies with human tissues utilized IgG isolated from patients’ sera and labeled with biotin, as reported.8 Paraffin-embedded tissues were deparaffinized and the antigens retrieved, as reported.9 Serial tissue sections were subsequently incubated with biotin-labeled IgG containing anti-Zic4, anti-Hu, or anti-CRMP5 antibodies, diluted 1:50, and the reactivity developed with the avidin-biotin-peroxidase method.6 Biotin-labeled IgG from a normal individual served as control. Immunocompetition assays between each biotin-labeled antibody (anti-Zic4, anti-Hu, or anti-CRMP5) and sera harboring only one of these antibodies were used to confirm the reactivity of each onconeuronal antibody with tumor tissue, as reported.6
Statistics
The χ2 test was used to evaluate the significance of the association of Zic4 antibodies with other onconeuronal antibodies, as well as the significance of the detection of onconeuronal antibodies in cancer patients with and without PND. If the expected frequencies were less than 5, the χ2 test with Yates’ correction was employed.
Results
Clinical and immunologic associations of antibodies to Zic4
Zic4 antibodies were identified in 61 patients with PND or cancer (figure 1), but not in the 175 patients with non-cancer related neurologic disorders or normal individuals. Forty-nine of the 61 patients with Zic4 antibodies had PND. The main clinical features of these patients are shown in table 1. Nine of 49 patients had Zic4 antibodies without other onconeuronal antibodies, and these patients were more likely to develop pure or predominant cerebellar dysfunction (p < 0.001). Eight of these patients had predominant cerebellar dysfunction with no other symptoms of CNS involvement during the course of the disease, and the other patient developed cognitive problems and symptoms of limbic encephalitis. The eight patients with predominant cerebellar dysfunction had previously been tested for P/Q type VGCC antibodies and three were positive: one had Lambert-Eaton myasthenic syndrome (LEMS) whereas the other two did not have electrophysiologic evidence of LEMS.
Figure 1.
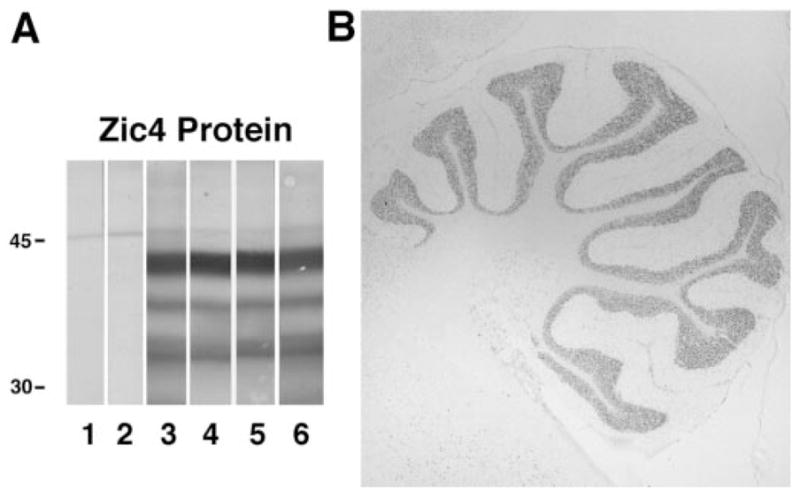
Immunoblot and immunohistochemistry of Zic4 antibodies. (A) Immunoblot of recombinant Zic4 protein with sera of four small-cell lung cancer (SCLC) patients with paraneoplastic neurologic disorders (PND) and Zic4 antibodies (lanes 3 to 6), serum of a normal individual (lane 1), and serum of a patient with SCLC without Zic4 antibodies (lane 2). (B) Immunolabeling of rat cerebellum with Zic4 antibodies. The predominant reactivity corresponds to the granule cell layer of the cerebellum; minor reactivity is barely perceived in other areas (brainstem at bottom left, and brain at top left). Section not counter-stained (×10).
Table 1.
Clinical characteristics of 49 patients with PND and Zic4 antibodies
| Immunity | Anti-Zic4, 9 patients | Anti-Zic4 and anti-Hu and/or anti-CRMP5 antibodies, 40 patients |
|---|---|---|
| Median age (range), y | 67 (59–85) | 66 (4.5–85) |
| Male, n | 8 | 19 |
| Predominant syndrome at diagnosis, n | ||
| Cerebellar (p < 0.001)* | 8 (1 with LEMS) | 10 (1 with LEMS) |
| Sensory neuropathy | — | 9 |
| Limbic encephalitis | — | 3 |
| Brainstem | — | 1 |
| Sensorimotor neuropathy | — | 2 |
| Orthostatic hypotension | — | 1 |
| Multifocal | 1 (with LEMS) | 14† (3 with LEMS) |
| Development of PND, n (mo) | ||
| Before tumor diagnosis | 5 (1–10, median 3) | 26 (2–26, median 3) |
| At tumor diagnosis | 1 | 6 |
| After tumor diagnosis | 2 (3 and 3) | 8 (2–48, median 4) |
| Tumor type, n | ||
| Small-cell lung cancer | 8 | 36 |
| Other | — | 4‡ |
| No tumor | 1§ | — |
| Limited tumor stage at diagnosis, n | 6/7 | 30/35 |
Chi-square with Yates’ correction.
Eleven of these patients had symptoms of cerebellar dysfunction along with other prominent symptoms associated with the following: brainstem encephalopathy (6), sensory neuronopathy (5), short-term memory deficits (3), seizures (4), myelopathy (2), orthostatic hypotension (1).
One patient had adenocarcinoma of the lung with anti-Hu antibodies, 1 neuroblastoma, 1 a Merkel cell carcinoma, and 1 radiologic evidence of a lung tumor.
Mediastinal adenopathy but biopsy negative for tumor.
PND = paraneoplastic neurologic disorders; LEMS = Lambert-Eaton myasthenic syndrome.
Forty of the 49 (82%) patients with PND and Zic4 antibodies harbored additional onconeuronal antibodies (29 anti-Hu, 2 anti-CRMP5, 9 anti-Hu and anti-CRMP5). These patients developed the clinical features shown in table 1. Overall, in 21 of these patients symptoms of cerebellar dysfunction were documented: 10 as a predominant cerebellar syndrome and 11 in the context of multifocal deficits (encephalomyelitis). Four patients had LEMS associated with PND of the CNS, and all were seropositive for P/Q type VGCC antibodies; the other 36 patients were not tested for P/Q type VGCC antibodies. No other clinical differences were observed between these 40 patients and the 9 Zic4 antibody positive patients without anti-Hu or anti-CRMP5 antibodies.
Thirty-one of the 49 patients developed PND before (median 3 months) the tumor diagnosis and 10 patients after (median 4 months); in 7 patients the PND and tumor were diagnosed during the same month. The associated tumors included 44 SCLC, 1 adenocarcinoma of the lung in a patient with anti-Hu antibodies (likely representing a mixed tumor), 1 neuroblastoma, 1 Merkel cell tumor, 1 patient with radiographic evidence of a lung tumor, and 1 patient with mediastinal adenopathies but no evidence of cancer.
The CSF was examined in nine Zic4 seropositive patients and all contained Zic4 antibodies. Intrathecal synthesis of Zic4 was demonstrated in five of seven patients. Seven patients with SCLC and PND who did not harbor Zic4 antibodies in serum were also negative in the CSF.
Analysis of antibodies to Zic4, HuD, and CRMP5 in patients with SCLC or neuroendocrine tumors
Because of the strong association between the presence of Zic4 antibodies and SCLC or neuroendocrine tumors, we analyzed the sera of all 498 patients for antibodies to other SCLC-related onconeuronal antibodies (anti-Hu and anti-CRMP5). These antibodies were only identified in patients with PND or SCLC. Of 167 patients with PND and SCLC or neuroendocrine tumors 141 had one (n = 96; 57%) or more (n = 45; 27%) of the three indicated antibodies and 26 (16%) were negative. A summary of all the immunities as well as the frequency of overlapping antibodies is shown in table 2. Note that although all three antibodies can be detected in patients with and without PND, in this study, the co-presence of anti-Hu and Zic4 antibodies was significantly associated with PND. Patients who harbored several antibodies in their sera also had the same antibodies in the CSF (data not shown).
Table 2.
Immunologic associations: Antibodies to Zic4, Hu, and CRMP5 proteins
| Associations | Anti-Zic4+ | Anti-Hu+ | Anti-CRMP5+ |
|---|---|---|---|
| PND with SCLC or neuroendocrine tumors* (n = 167) | 49† | 125‡ | 18§ |
| PND with other tumors (n = 48) | 0 | 0 | 0 |
| Cancer without PND | |||
| SCLC (n = 74) | 12† | 14‡ | 7§ |
| Other (n = 34) | 0 | 0 | 0 |
| Miscellaneous neurologic disorders without cancer (n = 155) | 0 | 0 | 0 |
| Normal individuals (n = 20) | 0 | 0 | 0 |
Overlap of antibodies: 9 patients had Zic4 + Hu + CRMP5 antibodies; 29 patients had Zic4 and Hu antibodies; 2 patients had Zic4 and CRMP5 antibodies; 5 patients had Hu and CRMP5 antibodies.
Association of anti-Zic4 antibodies and PND, p = 0.031.
Association of anti-Hu antibodies and PND, p < 0.001.
Association of anti-CRMP5 antibodies and PND, p = 0.75.
PND = paraneoplastic neurologic disorders; SCLC = small-cell lung cancer.
There was overlap of Zic4 titers between the 49 patients with PND and the 12 patients without PND: titers ranged from 1:750 to 1:192,000 (median 1:24,000) in the PND group and from 1:750 to 1:96,000 (median 1:12,000) in the non-PND group. The anti-Hu antibody titers ranged from 1:6000 to 1:1,536,000 (median 1:48,000) for PND patients and from 1:750 to 1:24,000 (median 1:3000) for the non-PND patients.
Expression of Zic and other paraneoplastic antigens in tumors
The frequent detection of concurrent anti-Zic4, anti-Hu, or anti-CRMP5 antibodies in SCLC patients with PND suggested that the target antigens are coexpressed by tumors. To test this hypothesis we examined five paraffin-embedded tumors for expression of Zic4, Hu, and CRMP5 proteins. Tumors included three SCLC from patients without PND and without onconeuronal antibodies, one SCLC from a patient with PND and Zic4 antibodies, and one Merkel cell tumor from a patient with PND and anti-Zic4 and anti-Hu antibodies. Biotin-labeled IgG containing only anti-Zic4, anti-Hu, or anti-CRMP5 antibodies were used to immunohistochemically probe tumor sections. These studies showed coexpression of immunoreactive Zic, Hu, and CRMP5 proteins in all five tumors. Comparison of consecutive tumor sections indicated that some tumor areas had homogeneous expression of the three antigens whereas other areas showed unequal expression (figure 2).
Figure 2.
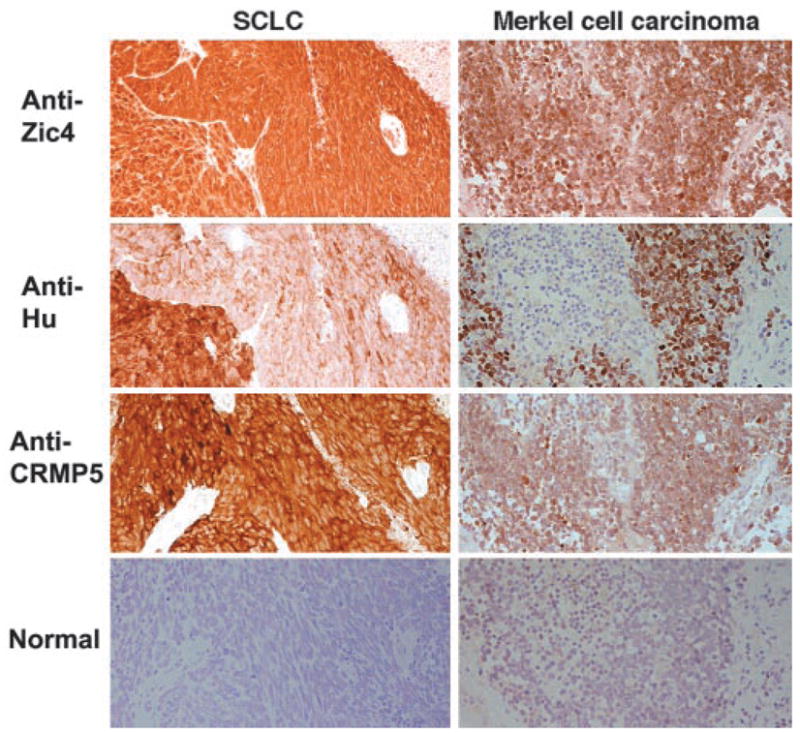
Coexpression of immunoreactive Zic4, Hu, and CRMP5 proteins by tumors. Consecutive sections of small-cell lung cancer (SCLC) from a patient without paraneoplastic neurologic disorders (PND) and without antineuronal antibodies and a Merkel cell carcinoma of a patient with PND and anti-Zic4 and anti-Hu antibodies, incubated with biotinylated immunoglobulin G (IgG) from sera of patients harboring only anti-Zic4, anti-Hu, or anti-CRMP antibodies. In both tumors there are areas with intense coexpression of the three antigens (Zic, Hu, CRMP proteins) and other areas with unequal antigen expression (i.e., in the SCLC there is an extensive area with minimal expression of Hu proteins but intense expression of Zic and CRMP proteins). Row “normal” corresponds to biotinylated IgG from a normal individual and serves as a negative control. The specific reactivity of each biotinylated IgG (anti-Zic4, anti-Hu, and anti-CRMP) was confirmed with immunocompetition assays (sections counterstained with hematoxylin, ×200).
Discussion
We report the detection of Zic4 antibodies in 61 patients with PND or cancer, but not in 175 patients with non-cancer related neurologic disorders or normal individuals. The presence of Zic4 antibodies had a robust association with SCLC, and studies to determine the presence of other SCLC-related immunities demonstrated co-occurrence of Zic4, Hu, or CRMP5 antibodies in the serum or CSF of 27% of SCLC patients with PND.
Patients who had Zic4 antibodies (without anti-Hu or anti-CRMP5) were more likely to develop a pure or predominant cerebellar syndrome than patients with several antineuronal antibodies. Fifty-two percent of patients with several antibodies developed cerebellar dysfunction, but in approximately half of these patients the cerebellar symptoms occurred in the context of multifocal neurologic deficits characteristic of encephalomyelitis. Overall, 65% of patients with Zic4 antibodies developed neurologic symptoms before the diagnosis of the tumor.
The detection of Zic4 antibodies significantly associated with PND, but similar serum titers were present in 16% of SCLC patients without PND. Two possibilities that are not mutually exclusive may account for this finding. First, in some patients, the Zic4 antibodies are markers of PND; these patients likely include those with intrathecal synthesis anti-bodies. Second, in other patients, the Zic4 antibodies associate with cancer rather than PND as occurred in 9% of patients with anti-CRMP5 and 19% of patients with anti-Hu antibodies. Although the anti-Hu serum titers of patients without PND are usually lower than those with PND,10 overlapping titers occasionally occur (two non-PND patients had anti-Hu titers overlapping those with PND; data not shown). Titers of CRMP5 antibodies were not determined and studies done by others have not compared serum titers with the development of PND.11,12 Further studies are needed to determine whether intrathecal synthesis of Zic4 antibodies correlates better than serum titers with the development of PND. At this stage, a practical implication of the current study is that in patients with neurologic symptoms of unknown cause, detection of Zic4 antibodies predicts a neoplasm, usually a SCLC, and suggests that the neurologic disorder is paraneoplastic.
Excluding patients with other onconeuronal immunities, we estimate that the detection of Zic4 antibodies would have contributed to the diagnosis of PND in 9 of 35 (26%) patients with SCLC. Although in retrospect one can argue that four of these nine patients had P/Q-type VGCC antibodies (two with LEMS) that could have led to the diagnosis of PND,13 four of the other five patients (one was not tested) did not harbor P/Q-type VGCC antibodies. In the current series testing only for anti-Hu antibodies would have missed 14 of 42 (33%) patients with SCLC and PND who were positive for Zic4, CRMP5, or both antibodies.
The frequent presence of several antibodies (Zic4, Hu, or CRMP5) in the serum of patients with SCLC results from the co-expression of the three antigens by these types of tumors. We found expression of the three antigens in the five tumors examined, three from patients without PND and without onconeuronal antibodies, and two from patients with antibody-associated PND. The fact that tumors that express all three antigens may induce some but not all three antibodies implies that other factors (i.e., presentation of peptides to the immunologic system, or the patient’s haplotype, among others) contribute to determining the repertoire of paraneoplastic immunity.14,15
The Zic proteins have important roles in the development of the nervous system, and comprise a family of five zinc-finger proteins with extensive sequence homology (range 52% to 62% identity).1,2 Consistent with this homology, 29 of 30 sera of patients with Zic4 antibodies also reacted with human Zic1 protein, and some sera reacted with Zic2, suggesting epitope sharing between the Zic proteins (Bataller, unpublished data). We have not determined the reactivity against Zic3 and Zic5.
In animal studies, mutations of different Zic genes result in an extensive array of neurologic abnormalities, including cerebellar malformation, holoprosen-cephaly, spina bifida, and sensorimotor gait abnormalities.16–20 Mutant Zic1 mice are regarded as models of the Joubert’s syndrome, a human autosomal recessive disorder characterized by hindbrain and cerebellar malformation.16,21 The predominant cerebellar phenotype that results from genetic disruption of the Zic genes and that is associated with paraneoplastic immunity to Zic proteins, along with the detection of intrathecal synthesis of Zic4 antibodies in PND patients, suggests that immunity against Zic proteins (antibody-, T-cell mediated, or both) may contribute to the cerebellar degeneration. It is tempting to speculate that patients with several antibodies had more widespread neurologic dysfunction as a result of concurrent immune mechanisms targeting several more widely expressed antigens (i.e., Hu and CRMP proteins).
Analysis for Zic4 antibodies should be considered in patients suspected to have PND, particularly if they have risk factors for SCLC (i.e., smokers). Detection of these antibodies frequently accompanies other paraneoplastic immunities that could assist in the diagnosis, but in some patients the Zic4 antibodies are the only detectable marker of PND. Prospective studies are needed to determine whether Zic4-positive patients without PND eventually develop cerebellar dysfunction.
Acknowledgments
One paraffin-embedded tumor was provided by Dr. John Greenlee (Salt Lake City, UT). The plasmid with the human CRMP5 gene was a gift of Dr. Jerome Honnorat (Lyon, France).
Supported in part by NCI RO1CA/89054 and the Charles A. Dana Neuroscience Research Program Award (J.D.) and the Fulbright Fellowship Program and the Spanish Society of Neurology (L.B.).
Footnotes
Presented in part at the 127th annual meeting of the American Neurological Association; New York, NY; October 2002.
References
- 1.Yokota N, Aruga J, Takai S, et al. Predominant expression of human zic in cerebellar granule cell lineage and medulloblastoma. Cancer Res. 1996;56:377–383. [PubMed] [Google Scholar]
- 2.Aruga J, Yokota N, Hashimoto M, Furuichi T, Fukuda M, Mikoshiba K. A novel zinc finger protein, zic, is involved in neurogenesis, especially in the cell lineage of cerebellar granule cells. J Neurochem. 1994;63:1880–1890. doi: 10.1046/j.1471-4159.1994.63051880.x. [DOI] [PubMed] [Google Scholar]
- 3.Aruga J, Tohmonda T, Homma S, Mikoshiba K. Zic1 promotes the expansion of dorsal neural progenitors in spinal cord by inhibiting neuronal differentiation. Dev Biol. 2002;244:329–341. doi: 10.1006/dbio.2002.0598. [DOI] [PubMed] [Google Scholar]
- 4.Gure AO, Stockert E, Scanlan MJ, et al. Serological identification of embryonic neural proteins as highly immunogenic tumor antigens in small cell lung cancer. Proc Natl Acad Sci USA. 2000;97:4198–4203. doi: 10.1073/pnas.97.8.4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bataller L, Wade DF, Fuller GN, Rosenfeld MR, Dalmau J. Cerebellar degeneration and autoimmunity to zinc-finger proteins of the cerebellum. Neurology. 2002;59:1985–1987. doi: 10.1212/01.wnl.0000038352.01415.ce. [DOI] [PubMed] [Google Scholar]
- 6.Dalmau J, Gultekin SH, Voltz R, et al. Ma1, a novel neuron- and testis-specific protein, is recognized by the serum of patients with paraneoplastic neurological disorders. Brain. 1999;122:27–39. doi: 10.1093/brain/122.1.27. [DOI] [PubMed] [Google Scholar]
- 7.Voltz R, Gultekin SH, Rosenfeld MR, et al. A serologic marker of paraneoplastic limbic and brain-stem encephalitis in patients with testicular cancer. N Engl J Med. 1999;340:1788–1795. doi: 10.1056/NEJM199906103402303. [DOI] [PubMed] [Google Scholar]
- 8.Furneaux HM, Rosenblum MK, Dalmau J, et al. Selective expression of Purkinje-cell antigens in tumor tissue from patients with paraneoplastic cerebellar degeneration. N Engl J Med. 1990;322:1844–1851. doi: 10.1056/NEJM199006283222604. [DOI] [PubMed] [Google Scholar]
- 9.Cattoretti G, Pileri S, Parravicini C, et al. Antigen unmasking on formalin-fixed, paraffin-embedded tissue sections. J Pathol. 1993;171:83–98. doi: 10.1002/path.1711710205. [DOI] [PubMed] [Google Scholar]
- 10.Dalmau J, Furneaux HM, Gralla RJ, Kris MG, Posner JB. Detection of the anti-Hu antibody in the serum of patients with small cell lung cancer—a quantitative western blot analysis. Ann Neurol. 1990;27:544–552. doi: 10.1002/ana.410270515. [DOI] [PubMed] [Google Scholar]
- 11.Honnorat J, Antoine JC, Derrington E, Aguera M, Belin MF. Antibodies to a subpopulation of glial cells and a 66 kDa developmental protein in patients with paraneoplastic neurological syndromes. J Neurol Neuro-surg Psychiatry. 1996;61:270–278. doi: 10.1136/jnnp.61.3.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu Z, Kryzer TJ, Griesmann GE, Kim K-K, Benarroch EE, Lennon VA. CRMP-5 neuronal autoantibody: marker of lung cancer and thymoma-related autoimmunity. Ann Neurol. 2001;49:146–154. [PubMed] [Google Scholar]
- 13.Graus F, Lang B, Pozo-Rosich P, Saiz A, Casamitjana R, Vincent A. P/Q type calcium-channel antibodies in paraneoplastic cerebellar degeneration with lung cancer. Neurology. 2002;59:764–766. doi: 10.1212/wnl.59.5.764. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka M, Tanaka K, Tsuji S, et al. Cytotoxic T cell activity against the peptide, AYRARALEL, from Yo protein of patients with the HLA A24 or B27 supertype and paraneoplastic cerebellar degeneration. J Neurol Sci. 2001;188:61–65. doi: 10.1016/s0022-510x(01)00548-2. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka M, Tanaka K. HLA A24 in paraneoplastic cerebellar degeneration with anti-Yo antibody. Neurology. 1996;47:606–607. doi: 10.1212/wnl.47.2.606. [DOI] [PubMed] [Google Scholar]
- 16.Ogura H, Aruga J, Mikoshiba K. Behavioral abnormalities of Zic1 and Zic2 mutant mice: implications as models for human neurological disorders. Behav Genet. 2001;31:317–324. doi: 10.1023/a:1012235510600. [DOI] [PubMed] [Google Scholar]
- 17.Aruga J, Mizugishi K, Koseki H, et al. Zic1 regulates the patterning of vertebral arches in cooperation with Gli3. Mech Dev. 1999;89:141–150. doi: 10.1016/s0925-4773(99)00220-8. [DOI] [PubMed] [Google Scholar]
- 18.Aruga J, Minowa O, Yaginuma H, et al. Mouse Zic1 is involved in cerebellar development. J Neurosci. 1998;18:284–293. doi: 10.1523/JNEUROSCI.18-01-00284.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aruga J, Yozu A, Hayashizaki Y, Okazaki Y, Chapman VM, Mikoshiba K. Identification and characterization of Zic4, a new member of the mouse Zic gene family. Gene. 1996;172:291–294. doi: 10.1016/0378-1119(96)00111-4. [DOI] [PubMed] [Google Scholar]
- 20.Aruga J, Inoue T, Hoshino J, Mikoshiba K. Zic2 controls cerebellar development in cooperation with zic1. J Neurosci. 2002;22:218–225. doi: 10.1523/JNEUROSCI.22-01-00218.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barreirinho MS, Teixeira J, Moreira NC, Calcada BS, Goncalvez S, Barbot MC. Joubert’s syndrome: report of 12 cases. Rev Neurol. 2001;32:812–817. [PubMed] [Google Scholar]


