Abstract
Summary
The inability to properly balance energy intake and expenditure with nutrient supply forms the basis for some of today's most pressing health issues including diabetes and obesity. Mechanisms of nutrient homeostasis may also lie at the root of dietary restriction, a manipulation whereby reduced nutrient availability extends lifespan and ameliorates age-related deteriorations in many species. The traditional belief that the most important aspect of the diet is its energetic (i.e., caloric) content is currently under scrutiny, and hypotheses that focus on more subtle characteristics revolving around composition are beginning to emerge. Using Drosophila melanogaster, we asked whether diet composition alone, independent of its caloric content, was sufficient to impact behavior, physiology, and lifespan. We found that providing flies with a yeast-rich diet produced lean, reproductively-competent animals with reduced feeding rates. Excess dietary sugar, on the other hand, promoted obesity, which was magnified during aging. Addition of dietary yeast often limited or reversed the phenotypic changes associated with increased dietary sugar and vice versa, and dietary imbalance was associated with reduced lifespan. Our data reveal that diet composition, alone and in combination with overall caloric intake, modulates lifespan, consumption, and fat deposition in flies, and they provide a useful foundation for dissecting the underlying genetic mechanisms that link specific nutrients with important aspects of general health and longevity.
Keywords: Aging, Metabolism, Dietary Restriction, Longevity, Drosophila, Calories
Introduction
Continuing gains in human health and longevity, which developed societies have enjoyed for well over a century, are currently under threat from a rising tide of obesity (Olshansky et al. 2005). Nearly 3/4 of the U.S. population is overweight, and roughly 1/3 is clinically obese. These individuals have an elevated risk of diabetes and cardiovascular disease, as well as increased incidence and severity of many age-related afflictions, including inflammation and cancer. In stark contrast to the effects of over-nutrition, however, is the practice of dietary restriction whereby nutrient intake is reduced to 60% of what might be considered normal or ad libitum. Dietary restriction extends lifespan in a range of species, and delays or eliminates a wide range of diseases and age-dependent deteriorations in mammals (Longo & Finch 2003). The contrasting effects of diet on health and longevity highlight the need for a more comprehensive understanding of the costs and benefits associated with particular diets and the biological changes that underlie them.
The traditional belief that molecular mechanisms responsible for dietary restriction and obesity are predominantly regulated by the energetic (i.e., caloric) content of available nutrients is currently being challenged by hypotheses that focus on more subtle characteristics of the diet. For example, sensory perception of specific dietary components alone, without increased consumption, is sufficient to increase adiposity and decrease lifespan in Drosophila (Libert et al. 2007). In addition, dietary components may act independently of their role in nutrition to modulate intracellular signaling pathways directly. In Drosophila, the impact of dietary yeast on longevity is dependent on the TOR signaling pathway (Kapahi et al. 2004). Lastly, the relative concentrations of key nutrients in the diet may lead to ingestion of severe deficits of some nutrients and excesses in others as the animals seek to secure a minimal amount of each (Simpson & Raubenheimer 2007). When dietary protein is scarce, caterpillars will consume larger amounts of energy-rich food and will store the excess energy as fat, which leads to obesity (Warbrick-Smith et al. 2006). Indeed, when given a choice, insects and spiders may independently regulate their intake of protein versus non-protein energy in a way that seems to balance the evolutionary fitness costs associated with individual nutrient overload or shortage (Mayntz et al. 2005).
Much of the work from invertebrate systems that characterizes the myriad effects of diet is proving relevant to mammalian aging and physiology. A combination of taste, smell, texture, and appearance influence human food assessment, and resulting preferences and aversions may be linked with the nutritional value of the perceived food (Goff & Klee 2006). In rats, artificial sweeteners in the diet promoted increased caloric intake, increased body weight, and increased adiposity, suggesting a mechanistic link between sweetness perception and metabolic processes (Swithers & Davidson 2008). Studies involving manipulation of single dietary components (e.g., sucrose and amino acids) in rodents have revealed significant effects on lifespan (Miller et al. 2005) Zimmerman, 2003 #1056; Preuss, 1997 #1269}, while others that have claimed support for calories as the driving force in modulation of longevity are coming under increasing scrutiny (Simpson & Raubenheimer 2007). Mice fed complementary diets containing different levels of protein and carbohydrate regulated intake to achieve a target ingestion of both (Sorensen et al. 2008). In these animals, protein intake was strongly regulated, and imbalanced, carbohydrate-rich diets led to increased consumption and fat deposition (Sorensen et al. 2008). The prioritization of protein intake may partially explain obesity trends among human populations; levels of dietary protein negatively correlate with obesity prevalence in modern developed countries (Simpson & Raubenheimer 2005).
In most invertebrate systems, dietary restriction is applied somewhat non-traditionally in that food quality, rather than quantity, is manipulated through dilution of the nutritional components in the medium (Pletcher et al. 2005). This is in contrast to most rodent studies, where a fixed diet is provided to animals individually, and all of the food is consumed (Weindruch & Walford 1988). Moreover, different labs often employ different diet-restriction protocols involving different levels of nutrient dilution and alteration of dietary components. In several systems this has led to apparently conflicting results, and in C. elegans, different protocols seem to affect lifespan through different molecular mechanisms (Lee et al. 2006; Greer et al. 2007; Panowski et al. 2007). Finally, intermittent feeding, which extends mouse lifespan, has been less successfully applied in flies (Piper & Partridge 2007). All of this has fostered questions concerning which manipulations are relevant to the mammalian situation (Piper & Partridge 2007).
Given the multiple facets of diet composition, a detailed understanding of nutrient-dependent effects in a genetically tractable model system would provide direction for dissecting the mechanisms that link the dietary environment with disease and aging and clarify the extent to which they may be evolutionarily conserved. We therefore designed a set of experiments to critically assess the role of diet composition, as distinct from overall caloric content, on body composition and lifespan in Drosophila melanogaster. As adults, D. melanogaster are typically fed a standardized medium composed of sucrose and yeast suspended in agar (see Experimental Procedures for recipes). We manipulated the macronutrient composition of the food by varying levels of sucrose (carbohydrate) and yeast (protein) independently in a 5×5 factorial design. Detailed phenotypic analysis of flies maintained in each of 25 distinct nutritional regimes allowed us to assess the short-term effects of each nutritional component and their interactions on triglyceride (fat) levels, protein levels, and feeding rates as well as the long-term effects of the manipulations on age-related obesity and organism lifespan. Our studies revealed parallels between humans and flies in their physiological responses to a broad range of diets, suggesting evolutionarily conserved aspects of regulation by specific nutritional components.
Results
We investigated the impact of specific dietary components on a range of adult-specific physiological and health-related phenotypes. The experiments were designed to include five concentrations each of sucrose and brewer's yeast (2.5g·dl−1, 5g·dl−1, 10g·dl−1, 20g·dl−1 and 40g·dl−1). This strategy allows simultaneous estimation of the effect of yeast and sugar independently as well as the degree to which the two dietary components interact to determine physiological phenotypes. To avoid developmental effects and to isolate adult-specific phenotypes associated with obesity and lifespan, we reared all experimental larvae in a cornmeal-sugar-yeast media that was optimized for larval mobility. Following development, once-mated adult flies were transferred to one of the 25 different adult-specific food regimes, where they remained thereafter.
Triglyceride accumulation is promoted by dietary carbohydrates and suppressed by dietary yeast
We first determined the effect of diet composition on fat storage. Similar to mammals, fruit flies store energy from surplus calories in the form of triacylglycerol (TAG) in specialized lipid-droplet-containing cells, and they release this energy for utilization via lipolysis as free fatty acids (van der Horst et al. 2002). In our experiments, adult females and males were sacrificed at 13 days of adult age; following 10 days of exposure to a specific dietary regime. For each TAG measure, five females or eight males were homogenized, and TAG was determined by spectrophotometric analysis.
We estimated “Diet Response Surfaces,” which display the trends in the data that are supported by statistical analysis (see Experimental Procedures), and observed that diet composition dramatically altered TAG levels in the animals. The level of adiposity attained by the adult fly was strongly promoted by the amount of sugar in the diet (Figure 1A, p <0.0001, Table S2). In contrast, the concentration of dietary yeast was inversely related to the amount of fat stores (p <0.0001), suggesting that increased protein availability effectively suppressed fly adiposity. There was also a statistically significant interaction between sugar and yeast (p=0.04); yeast had a stronger suppressive effect on TAG storage in the presence of lower dietary sugar.
Figure 1. Diet Response Surfaces for metabolic phenotypes.
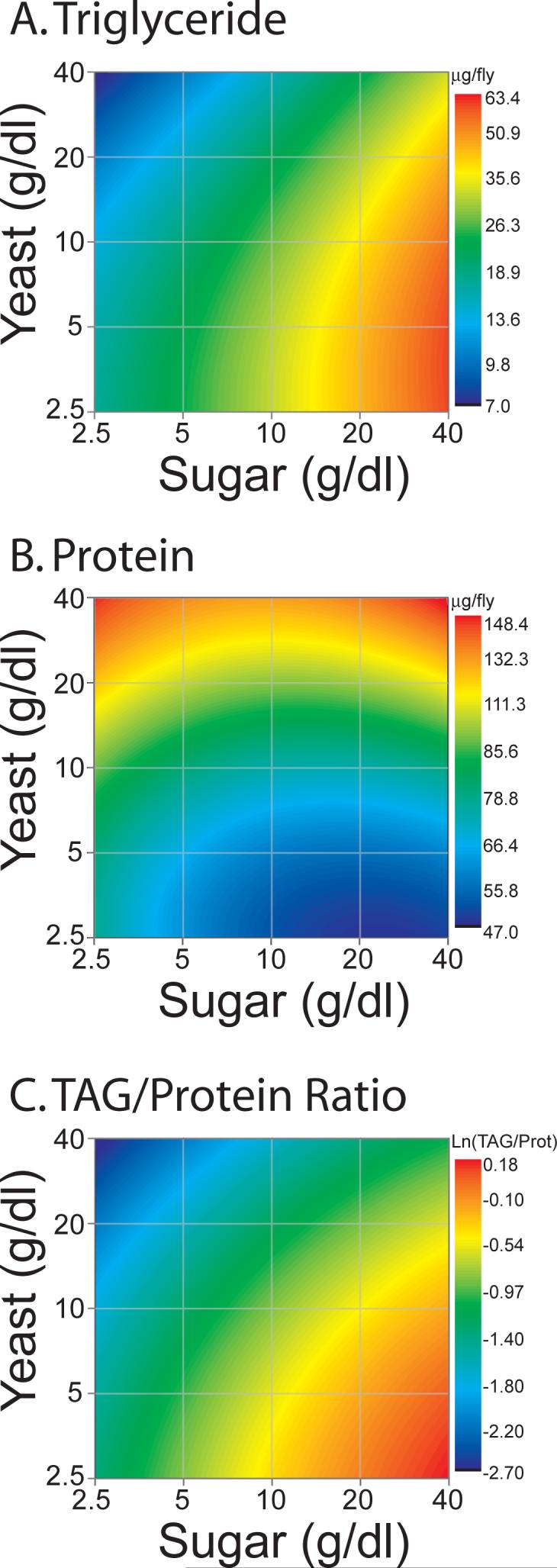
Levels (μg) of triglyceride (A) and protein (B) were measured per fly in each of 25 different diet regimes ranging from 2.5g·dl−1 to 40g·dl−1 sugar and yeast. For each diet, multiple replicates from each diet were used to estimate Diet Response Surfaces (see Experimental Procedures), which robustly characterize the impact of each dietary component on the phenotypes. A summary measure of body composition, which is based on the relative ratio of TAG to total protein is also examined (C). Dietary sugar promotes TAG storage, while dietary protein suppresses it. Actual mean values for each diet and appropriate statistical analyses are presented in the supplementary data (Tables S1 and S2). Female flies of the yw strain are represented here, but similar trends were observed in in males (Figure S1) and in females of the Canton-S (Figure S2) and w1118 (Figure S3) strains.
Storage of protein is largely independent of dietary carbohydrates
Unlike TAG levels, the protein content of flies was primarily determined by the concentration of yeast in the medium (Figure 1B, p <0.0001, Table S2). Increased dietary yeast resulted in higher levels of protein storage. Dietary carbohydrate had a much smaller effect, although it did promote a slight reduction in steady state protein levels. The effect of dietary sugar was less extensive in yeast-rich diets. The weight of the flies mirrored protein levels, with high levels of dietary yeast leading to heavier flies and increased carbohydrate availability leading to lighter flies (data not presented).
Together with the TAG measurements, these data establish that diet composition dramatically alters the body composition of flies. Following normalization to total protein, TAG levels were maximized in a low-protein/high-carbohydrate diet and minimized in a high-protein/low-carbohydrate diet (Figure 1C). Generally speaking, yeast availability suppressed TAG storage and dietary sugar suppressed protein storage. Thus, the sugar/yeast ratio of the diet, more so than its caloric content, robustly determined the amount of TAG and protein the flies maintained (Figure1C). We observed similar trends in multiple replicates, in two additional genetic backgrounds (Figures S2, S3), and in males (Figure S1), suggesting that costs associated with reproduction or egg-laying are not the primary determinant of these trends. Notably, a slight decrease in the animals' protein level was observed when sugar was added to the least nutritious diet (2.5 g·dl−1 sugar and protein; compare Figure 1B bottom left vs. bottom right corner), and likewise a decrease in TAG level was observed when protein was added. These data coordinately suggest that our least caloric diet is above that which is minimally required to sustain the daily energetic requirements of the fly. The reduced lifespan that is often observed in these conditions (see below) may therefore not be due to starvation or general nutrient deprivation.
Fecundity is primarily determined by dietary yeast while a balanced diet promotes longevity
Alterations in fecundity can have a significant impact on physiology and are often observed in association with diet manipulation (Piper et al. 2005). We measured egg production during the first two weeks of life, which is thought to precede the manifestation of reproductive senescence (Novoseltsev et al. 2003). As with all of our assays, females were exposed to males for 72 hours following eclosion, after which they were maintained in single-sex vials with the appropriate experimental diet. Our results establish the concentration of yeast in the diet as the primary determinant of egg production (Figure 2A, p <2.2e−16, Table S2), while increased sugar was found to moderately suppress egg-laying rates (p <1.81e−07, Table S2). We observed a large range of reproductive output throughout the experiment; in the high-protein/low-sugar regimes, flies laid more than one egg per hour during peak reproductive periods, while those in low-protein/high-sugar regimes often laid less than one egg per day (Table S1).
Figure 2. Diet Response Surfaces for reproductive and longevity phenotypes.
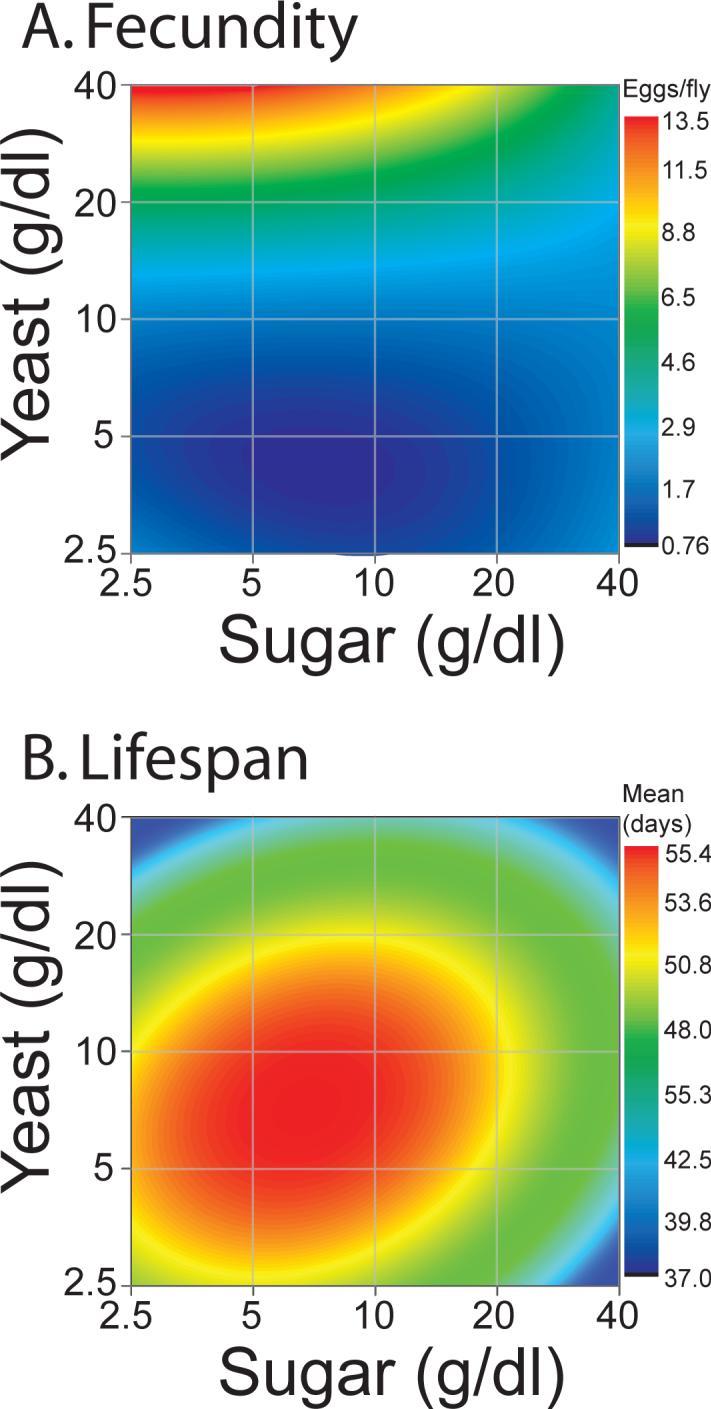
Fecundity (A) and lifespan (B) were measured in female flies maintained on each of 25 different diet regimes ranging from 2.5g·dl−1to 40g·dl−1 sugar and yeast. The data were used to estimate Diet Response Surfaces (see Experimental Procedures), which robustly characterize the impact of each dietary component on the phenotypes. Actual mean values for each diet and appropriate statistical analyses are presented in the supplementary data (Tables S1 and S2). Higher levels of dietary protein promote reproduction, while flies have the longest lifespan on a balanced diet. Fecundity is expressed as the eggs/day for each fly averaged over the two weeks (aged 3−17 days).
To characterize the impact of each dietary component on fly longevity, we measured the lifespan of female flies that were maintained on each of the 25 different food treatments throughout their adult lives. Most dietary restriction experiments in flies and worms limit animals’ access to all nutrient components. Accordingly, we observed a response to simultaneous manipulation of both sugar and yeast (Figure 2B, focus on the diagonal from bottom left to top right) that corresponded well with published results (Magwere et al. 2004; Libert et al. 2007); longevity was maximized near 10 /10g·dl−1 yeast/sucrose and reduced at higher (e.g., 40 /40g·dl−1) and lower (e.g., 5 /5g·dl−1) levels of yeast and sucrose. Surprisingly, however, lifespan was more severely attenuated when either of the two single-nutrient concentrations was altered individually. While we confirmed that yeast manipulation alone was sufficient to modulate longevity (Mair et al. 2005), this effect was enhanced at higher levels of sucrose: yeast level had a relatively small effect on lifespan when sugar was kept at a low concentration (e.g., 2.5g·dl−1). In some dietary conditions, we observed an effect of sugar on lifespan that nearly equaled that of yeast (Figure 2B). The effect of sugar is much greater in high-yeast conditions, which may explain why previous reports failed to document an effect of similar magnitude. Similar trends were observed by measuring lifespan in a second genetic background (Figure S2).
Caloric intake is insufficient to explain diet-induced changes in adult physiology and lifespan
It is of interest to note that trends in adult body composition, fecundity, and longevity show little direct correspondence to differences in the caloric content of the food. As previously reported, per unit mass, the caloric content of our brewer's yeast is nearly equivalent to sucrose (Mair et al. 2005). Therefore, assuming that accessibility in the food is roughly equivalent for each nutrient, a 40g·dl−1 yeast/5g·dl−1 sucrose diet is calorically equivalent to a 5g·dl−1 yeast/40g·dl−1sucrose diet. The longevity and physiology associated with these two diets, however, are significantly different (P = 1.5e−9, log rank test).
At least two testable hypotheses might account for these observations. In a calorie-central hypothesis, one might maintain that the caloric characteristic of the diet is the important factor influencing our observed phenotypes, but this relationship is obscured because nutrient availability does not translate directly into caloric uptake. For instance, some diets may be unpalatable, may alter feeding behavior, or may interact with nutrient absorption (Tatar 2007). In a composition-central hypothesis, diet composition (i.e., the ratio of dietary components) rather than caloric content may be the critical factor that determines physiology. For example, a low protein/carbohydrate ratio may drive increased consumption; certain biological processes may be induced in the presence of excess carbohydrates, regardless of net caloric intake; or the smell, taste, or texture of the diet, as determined by diet composition, might be a critical factor.
To distinguish these hypotheses we measured feeding rates, which allowed us to interpret our findings in terms of estimated energy consumption. The feeding rate of flies was determined by the addition of an inert dye (FD&C Blue No.1) to each of the 25 food treatments (Tanimura et al. 1988). Adult flies were acclimated to their food type for 10 days then transferred to vials containing the same food type except with 0.5% blue dye added. After 6 hours of daytime feeding, the flies were sampled and homogenized. Intake was then measured spectrophotometricaily. Previous work in our laboratory has shown that the absorbance of the homogenate at 630nm with 675nm as a reference wavelength is proportional to the amount of food consumed, and it agrees well with alternative measures of food intake such as proboscis extension rates (Libert et al. 2007).
We determined that both dietary sugar and yeast had an impact on consumption. In agreement with previous studies (Edgecomb et al. 1994), we found that flies tended to consume slightly more when presented with increasingly carbohydrate-rich food (Figure 3A, p <0.0001). In contrast, we observed that dietary yeast had a pronounced suppressive effect on feeding. Food consumption decreased precipitously as flies were exposed to increasingly yeast-rich diets (Figure 3A, p <0.0001) also in agreement with some previous reports (Ja et al. 2007). Notably, the counteracting effects of dietary yeast and sucrose resulted in roughly equivalent rates of feeding on all diets where the sugar- to-yeast ratio was one, which were achieved through simultaneous dilution of both sucrose and yeast (e.g., minor differences were observed along the diagonal in Figure 3A).
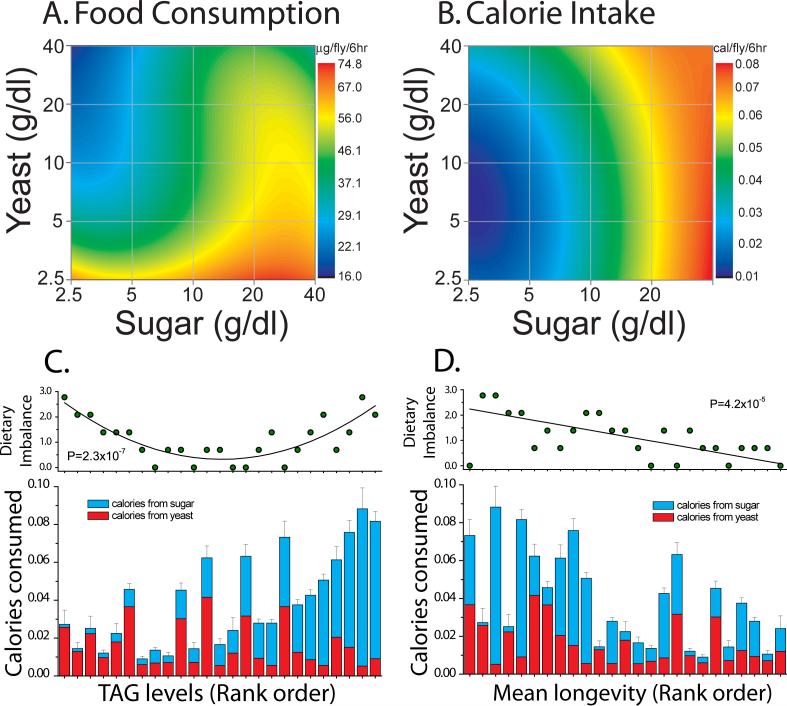
Figure 3. Drosophila obesity and lifespan are strongly affected by diet composition.
(A) Food intake was measured in female flies maintained on each of 25 different diet regimes ranging from 2.5g·dl−1to 40g·dl−1 sugar and yeast. (B) Total caloric intake was estimated based on feeding rate and the nutritional parameters of each diet. The data were used to estimate a Diet Response Surface for total caloric intake. (C and D) For flies maintained on each of the 25 diets, average triglyceride levels (C) and lifespan (D) were rank ordered from smallest to largest values. In the bottom panels the caloric intake on each diet was plotted as a stacked bar chart in terms of calories from sugar (top portion of each bar) and calories from yeast (bottom portion of each bar). In the upper panels, the dietary imbalance was plotted for the same rank order. Dietary imbalance was measured as abs(ln(sugar calories/yeast calories)). It ranges from 0 (balanced diet) to near 3 (severely imbalanced sugar- or yeast-rich diet). Sugar calories and yeast calories promoted obesity and leanness, respectively, while a balanced diet promoted longer life.
Variation in feeding rates across the remainder of the diets prompted an examination of the physiology and lifespan data in terms of caloric intake. Based on our feeding data, flies were estimated to consume the most calories in a high-carbohydrate, low-protein diet (Figure 3B). For a fixed level of sugar in the diet, increased dietary yeast generally resulted in equal or slightly increased caloric intake, such that a greater proportion of calories were coming from yeast than from sugar without grossly affecting the overall calories consumed. Interestingly, we estimated that flies most often consumed the least calories in a medium-protein, low-carbohydrate diet, which itself is comparatively rich in caloric value.
When specific sources of consumed calories are taken into account, we find that dietary composition plays a critical role in determining physiology and lifespan in Drosophila. When diet treatments were ranked according to their propensity to promote TAG storage, it was clear that the flies became increasingly fat as a greater portion of their diet was composed of sugar (Figure 3C). Diets composed largely of calories from yeast produced lean flies, and leanness was preserved in these yeast-rich diets even in cases where total caloric intake was substantial. Indeed, for a given level of estimated calories consumed, diets rich in carbohydrates always resulted in significantly higher fat storage (Figure 3C). As a result, there was a strong quadratic relationship between the degree of dietary imbalance and the rank order of TAG storage (Figure 3C, top panel, p=2.3e−07). Severe dietary imbalance led to very lean (protein-rich diets) or excessively fat (sugar- rich diets) animals, and balanced diets produced flies of intermediate body composition. Finally, flies from diets of equal sugar/yeast composition (Figure 3C, top panel, dietary imbalance = 0) exhibited relatively little variation in TAG levels despite large differences in caloric intake. In total, these observations are consistent with a composition-central hypothesis: triglyceride storage is strongly determined by dietary composition.
When longevity was examined in the same manner as TAG, where diet treatments were ranked in terms of increasing lifespan, there was no obvious relationship between rank longevity and caloric input. Neither calories consumed specifically from yeast nor those consumed specifically from sugar correlated well with increased lifespan (Figure 3D). There was a trend of marginal significance linking increased longevity with reduced total caloric intake (p<0.05), however, a much stronger relationship was observed between dietary imbalance and lifespan. Contrary to TAG storage, there was a linear relationship between dietary imbalance and longevity; animals experiencing severe imbalance, regardless of whether it was due to a sugar- or yeast-enriched diet, were short-lived, while flies that were estimated to consume equal calories from sugar and yeast were the longest-lived (Figure 3D, top panel, p=4.2e−05). The 40/40 g·dl−1 yeast/sucrose diet was an exception to this rule. Flies maintained in this diet exhibited the shortest-lifespan, suggesting that extremely high-nutrient diets may induce pathology. In summary, Drosophila lifespan is compromised under conditions of excessive nutritional imbalance. Sugar and yeast appear to interact in such a way that addition of the limiting component may alleviate the deleterious effects of the nutrient in excess, regardless of the overall caloric content of the food.
Dietary composition influences age-dependent obesity in Drosophila
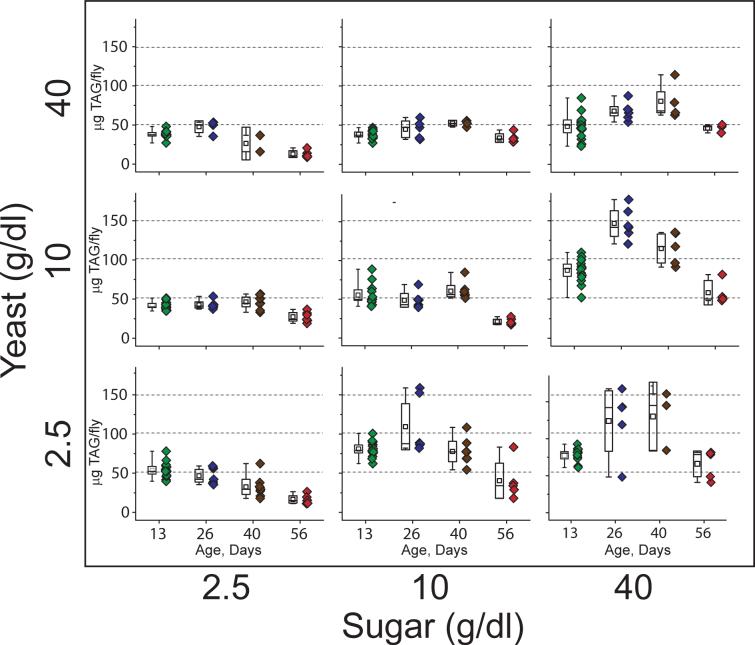
The impact of diet on human health has a strong age-dependent component, as alterations in physiology lead to increased accumulation and redistribution of adipose tissue and enhanced risk of cardiovascular and other aging-related diseases (Chumlea et al. 2002). Having observed deleterious effects on lifespan associated with consumption of unbalanced diets, we next asked whether these diets induce long-term changes in overall TAG levels. Due to practical considerations we were unable to measure age- dependent changes in all 25 dietary regimes. We therefore chose the most extreme treatments and subjected flies to a 3x3 matrix of varied sugar and yeast concentrations throughout their lifespan (yeast and sugar concentrations of 2.5g·dl−1, 10g·dl−1, and 40g·dl−1). Females were harvested after 13, 26, 40, and 52 or 56 days of age. TAG and protein levels were measured as previously described.
The most striking age-dependent pattern was observed in flies maintained in high-sugar diets, where the propensity for fat storage was magnified by age. While normal-to-low-nutrient concentrations resulted in a roughly constant loss of TAG with age, flies maintained on a sucrose-rich diet (2.5g·dl−1 yeast/40g·dl−1 sugar) exhibited nearly two-fold increase in TAG stores at 13 days of age and a further two-fold increase by 40 days (Figure 4). This effect contrasts with that previously observed (and replicated here) in dietary restriction experiments by food dilution where flies maintained on a balanced diet experienced age-induced weight and TAG loss (Johnson & Butterworth 1985). We also observed that very old flies (52 or 56 days of age) lost significant amounts of TAG during the latter part of their lives, even on the pro-obesity diets, during a time when they also tend to lose body weight (Bross et al. 2005).
Figure 4. Diet composition influences aging-related obesity in Drosophila.
Female flies were aged on nine different diets and sampled at 4 ages to assess changes in levels of triglyceride (TAG), the primary lipid storage molecule in Drosophila. Each point represents a single TAG measure obtained from 5 females. Corresponding box plots represent the mean (square symbol), 99% confidence intervals on the mean (box), and range (whiskers) of the data. Therefore, boxes that do not overlap are statistically significantly different at the 1% level. Diets that promoted triglyceride storage early in life (age 13 days) induced dramatic age-dependent increases and resulted in a strong middle-age obesity phenotype.
Discussion
Our data suggest that alterations in dietary sugar, yeast or both modulate normal lifespan and physiology in Drosophila in a manner that is largely independent of their caloric content. Carbohydrates enhanced consumption in flies, while increased protein intake seemed to induce satiation. Alterations in ingestion alone, however, were not sufficient to compensate for the patterns of increased TAG and protein accumulation, both of which were dependent on diet composition. Severely unbalanced diets seemed to induce a disease-like pathological state reminiscent of human obesity. For example, flies maintained on high sugar diets were generally obese even when they consumed a modest number of calories. They also displayed a shorter than expected lifespan and reduced fecundity, suggesting that longevity can be modulated by diet under nonstarvation conditions without necessarily increasing reproduction. Yeast-rich diets suppressed feeding and overall caloric intake, yet they also resulted in reduced lifespan. These flies are highly fecund (Figure 2A), indicating that this food is not overtly toxic.
Flies exposed to a high-glycemic diet exhibited enhanced fat storage when young and dramatic age-associated obesity. Under most circumstances, the body composition of aging flies tended to remain relatively constant, with protein and fat levels holding steady or modestly decreasing throughout life (Johnson & Butterworth 1985). Transcriptional changes with age in these conditions support the notions that extremely old flies experience moderate to severe starvation and that aging in Drosophila is accompanied by a loss in metabolic efficiency, feeding rate, or both (Pletcher et al. 2005). In hyperglycemic diets, however, flies experienced an age-dependent increase in TAG levels of up to 200−300% (Figure 4), which appeared to result from a combination of physiological and behavioral changes.
The complex effects that nutritional components have on body composition, behavior, and lifespan in flies provide a broad perspective for investigating the mechanisms of dietary restriction. Dietary restriction extends lifespan in species as diverse as yeast (Jiang et al. 2000), nematode worms (Braeckman et al. 2001) and flies (Pletcher et al. 2002), and it is the most powerful modulator of the aging process known in mammals (Masoro 2005). With a few exceptions, dietary restriction has been applied in flies by concomitant manipulation of both sucrose and yeast (Pletcher et al. 2005) or by modulation of yeast at a single level of sucrose (Mair et al. 2005; Min & Tatar 2006b). Based on the data presented here, these manipulations merely scratch the surface of the biological changes that are induced by diet. The integrative nature and opposing effects of dietary sugar and yeast suggest the possibility that the two dietary components may modulate lifespan and physiology through distinct mechanisms. Modulation of longevity by manipulation of dietary yeast does not require the transcription factor FOXO (Giannakou et al. 2008; Min et al. 2008), while components of the TOR pathway may be important. It will be interesting to assess whether these relationships hold true for modulation of lifespan by sucrose, which under certain conditions can have effects similar to that of yeast.
One hypothesis for the phenotypes observed in this study revolves around the primacy of ingested dietary protein. Diets with a low protein/carbohydrate ratio may stimulate increased feeding, and therefore increased energy uptake, in order to satisfy a basic requirement for protein-based energy (Sorensen et al. 2008). Such a model would correctly predict several of our observed trends including enhanced fat deposition on high carbohydrate diets, which is enhanced by aging, and reduced feeding on high-protein diets. Such a model does not fully account for the tendency of dietary sugar to modulate phenotypes independently of protein or for animals to experience maximum longevity on diets with an intermediate ratio of dietary components regardless of that diet's total nutritional and caloric content. Nevertheless, given the established importance of strict protein regulation in many species, this mechanism is likely contributing to the trends in the reported data (Simpson et al. 2003).
It is also likely that nutrient-specific features of the dietary environment influence metabolism and lifespan. Highly concentrated diets may pose an osmotic challenge. Flies are typically able to withstand large variances in osmolarity by regulating water flux via the cuticle and spiracles (Pierce et al. 1999), so this is not likely to be the driving force in our data. Sensory cues emanating from specific nutrients may activate potent signaling cascades and specify metabolic decisions in response to the animals' dietary context. Odors from live yeast have been shown to shorten D. melanogaster lifespan, and reduced olfaction promoted longevity and adiposity (Libert et al. 2007). Such data are consistent with the tendency for low-protein diets to be associated with obesity and enhanced longevity.
We propose a working model in which the total energy available from food consumption is directed by nutrient-dependent signaling mechanisms into three sinks: (a) reproduction, (b) somatic maintenance, and (c) energy storage. Our data suggest that dietary yeast promotes reproduction and inhibits TAG storage, while dietary sugar inhibits egg-laying and promotes adiposity. It is well established that increased reproduction limits lifespan. While impact of adiposity per se on aging is currently of some debate, studies suggest that fat storage is a fitness cost and is under intense evolutionary pressure (Warbrick-Smith et al. 2006). Regardless, diversion of substantial resources to either reproduction or storage limits their availability for programs of somatic maintenance. In low-protein/high-carbohydrate diets overconsumption is driven by strict regulation of protein levels, reproduction is strongly suppressed, and the absence of cues from dietary yeast activates storage programs. Flies become obese and short-lived (Figure 5A). In high-protein/low-carbohydrate diets, dietary yeast stimulates high levels of reproduction, while reduced carbohydrates inhibits storage and de-represses reproduction, which draws the majority of resources away from maintenance. Flies are very lean but also short-lived (Figure 5B). The addition of carbohydrates to this diet--which essentially adds caloric value-- increases lifespan by suppressing reproduction, promoting moderate levels of storage, and freeing resources for somatic maintenance (Figure 5C).
Figure 5. Dietary components may influence life strategies through their energetic and signaling characteristics.
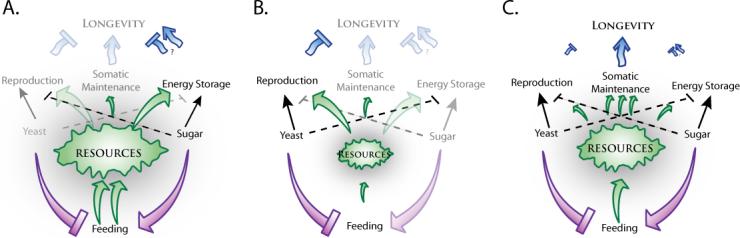
We propose a working model whereby the total energy available to an animal may be diverted towards reproduction, somatic maintenance and/or storage processes. Each of these three energy decisions interacts and harbors an inherent physiological impact on the organism that determines lifespan. Although both yeast and sugar consumption increase the total resources available, cues from high yeast diets drive reproduction and suppress mechanisms of somatic maintenance while cues from high sugar diets preferentially drive energy storage. (A) In a low-yeast/high-sugar environment resources are strongly diverted towards energy storage, resulting in obesity and reduced longevity. (B) In a high-yeast/low-sugar environment, reproduction is simultaneously stimulated (high protein) and de-repressed (low sugar), resulting in very high levels of reproduction that leave few resources for somatic maintenance. (C) Lifespan is optimized on a balanced diet as the interaction between cues from each dietary component maintain energy balance allowing sufficient resources for reproduction, energy storage, and somatic maintenance.
Testing such a model will be aided by continued development of methods to quantify feeding rates of individual flies and by ongoing discoveries regarding the mechanisms of energy homeostasis in Drosophila. Estimation of the absolute quantity of food uptake in D. melanogaster is difficult, and published results are conflicting (Bross et al. 2005)(Min & Tatar 2006a). We find little change in consumption across balanced diets, with large changes induced by unbalanced diets. Ja et al. (Ja et al. 2007), using the CAFÉ assay (a method involving liquid diet dispensed in capillary tubes) report greater uptake in all diets and a compensatory increase in feeding rate in diets of low nutrient concentration. Direct comparison between these techniques is difficult. The CAFÉ analysis provides accurate quantification. But suitable egg-laying surface is limited, and other important aspects of the dietary environment, such as olfactory and structural cues, are absent.
Flies present at least two lipocatabolic systems that influence energy storage—one reliant on adipokinetic hormone (Gronke et al. 2007) and the other on the adipose triglyceride lipase homolog brummer (Gronke et al. 2005)—while nutrient-specific signaling pathways, such as insulin signaling and TOR signaling, are known modulators of longevity (Tatar et al. 2003). It will be a priority to determine whether the diet-dependent effects that we observed are enacted by one or more of these systems. It is reasonable to predict that, as hypothesized for mammalian systems (Guarente & Picard 2005), longevity and its extension by dietary restriction may be mediated by fat metabolism. If so, we would predict that manipulation of lipolytic systems and the inputs that modulate them would reduce the costs of nutritional imbalance and increase health and lifespan when animals are exposed to an unhealthy diet.
Physiological changes induced in flies by alterations in dietary components mirror those seen in mammalian systems, including humans, and they suggest that flies may be a useful model system for studying mechanisms of diet-induced obesity. There was a pronounced increased fat deposition in flies in high-sugar diets that was suppressed by yeast, and largely decoupled from overall caloric content. Although flies were most lean when consuming a high-protein/low-carbohydrate diet, their longevity was severely curtailed. Indeed, for a given level of caloric intake, flies survived best when consuming a diet with intermediate levels of both nutrients. Thus, it seems plausible that strong adherence to diets rich in specific dietary components at the expense of others may be, regardless of their effects on body composition, detrimental to overall human health and longevity, a conclusion which has some empirical support (Lagiou et al. 2007). In the end, realizing the goal of genetic or pharmacologic interventions that alleviate the deleterious consequences of a sub-optimal diet or that mimic the beneficial effects of dietary restriction will require a more complete understanding of regulation of lifespan and associated metabolic phenotypes across a wide range of dietary regimes, including those of extreme nutrient imbalance.
Experimental Procedures
Fly husbandry& sampling
Wild-type flies were maintained at 25°C, 60% humidity in a 12h/12h dark/light cycle under conditions of controlled larval density in cornmeal-sugar-yeast “larval” media for at least 2 generations prior to experimentation. Adult flies were collected within 24 hours of emergence and placed on 10 g·dl−1 sugar/yeast food for 72 hours to mate. Mated flies were then collected under light CO2 anesthesia and placed into single-sex vials containing the experimental food type. Vials were changed every 2 or 3 days. For all experiments, flies were sampled by freezing (−20°C). For physiological measures each nutrient regime contained at least 3 independent vial replicates at a density of 20−25 females or 30−35 males. All samples were processed within 2 weeks of freezing time, the cohorts for age-dependent TAG measures were processed in 3 separate batches: young (day 13), middle-aged (days 26 & 40), and old (day 52 or 56) to prevent sample degradation over time.
Metabolic Assays
For each replicate, frozen flies (5 female or 8 male) were placed into 2 ml, 96-well plates and homogenized in 300 μl PBS/0.05% Triton-X using an automated tissue processor. Samples were then filtered and used immediately for analysis. TAG assays were performed by combining 10 μl of fly homogenate with 200 μl of the Infinity Triglyceride Reagent (Thermo Electron Corp.) and incubated at 37°C for 15 minutes with constant agitation. Concentrations were estimated based on absorbance values (λ520 nm) and compared to glycerol standards. Although this assay measures all forms of glycerides (tri-, di-, and mono-), we assumed it accurately represented total TAG, considering it is estimated that over 90% of fat body lipids are in the form of TAG in insects (Arrese et al. 2006). Protein assays were performed according to the manufacturer's protocol using 5 μl of filtered fly homogenate combined with 200 μl of either the Bicinchoninic acid reagent (Sigma Aldrich) or the Bradford Reagent (BioRad) with BSA as the standard.
Lifespan Assays
A fixed volume of yw or Canton-S strain embryos (34μl) were washed with 70% ethanol and placed into bottles containing 30 ml of cornmeal-sugar-yeast “larval” media. Resulting progeny were collected within 24 hours of eclosion and placed into bottles containing 15 ml of 10 g·dL−1 sugar-yeast media for 72 hours to allow for mating. Once mated, female flies were then collected under CO2 anesthesia and placed into vials containing one of the 25 nutrient regimes at a density of 30 flies per vial. Vials were placed on their sides to allow access to both food and non-food surfaces at advanced ages when flying is impaired. Deaths were recorded and flies placed into fresh media every other day.
Feeding Assays
Feeding rates were determined by adding the inert brilliant blue R dye (0.05% w/v FD&C No.1) into the each of the 25 nutrient regimes. A 100x concentration of dye was prepared, combined with the tegosept/ethanol/propionic acid mixture and added to the food after autoclaving. Adult yw, w1118, or Canton-S strain flies were allowed to feed for 6 hours during the daytime, and then frozen for analysis. Canton-S flies were decapitated prior to analysis because the wild-type eye pigment was found to interfere with the feeding assay. 220 μl of filtered fly homogenates from five females or eight males per replicate were measured for their absorbance at 630nm, reference 675 nm.
Fecundity Assays
Fecundity was determined by counting the eggs laid by 72 hour-old mated females (as mentioned in “Fly husbandry & sampling”) that were placed on each of the 25 nutrient regimes. Vials were changed every day (two replicate vials per food type) and eggs counted every other day until the flies were two weeks old for a total of six consecutive measures. The experiment was repeated with another cohort using the same strain. Egg production between cohorts was not statistically significant (p= 0.74) and the results from each cohort were pooled. The only difference between the experimental groups was that the vials initially started with 20 or 25 flies each for cohorts 1 or 2, respectively. For estimation of Diet Response Surfaces, we summed the total eggs/fly laid per vial, and took the mean and standard error of the four independent vial replicates.
Diet Response Surface Estimation
To characterize the effects of different levels of dietary sugar and yeast on the phenotypic measures of interest we estimated Diet Response Surfaces (DRS). Response surface design and analysis strategy is based on least-squares or maximum likelihood estimation of low-order polynomial models (Kuehl 1994). Polynomial models often provide accurate approximation to the true surface, which is unknown. In this analysis we assume that the mean of the response, μy, is a polynomial function of the levels of sugar and yeast. For example, a first order model is:
a second order model is:
etc., where x1 is sugar level, x2is yeast level, and βi are the regression coefficients, which are estimated from the data. Polynomial models through forth order were examined in all instances, and the optimal model for a particular data set was determined by sequential regression coupled with likelihood ratio test and AIC test when appropriate (Venables & Ripley 1997). In no instance was the optimal model higher than third order and most were first or second-order (see experimental procedures). The predictor variables for our analysis (i.e., sugar and yeast) were ln-transformed to achieve equidistant spacing between treatments, which provides for more robust surface estimation (Kuehl 1994). For TAG and protein analyses the data were μg TAG/fly and μg Protein/fly, respectively, a nd these values were ln-transformed to achieve a normal distribution. Scaled TAG levels were represented as ln(μg TAG/μg Protein) and were found to be normally distributed. Egg counts were analyzed as total measured egg production over the period of observation, and these data were square-root- transformed to achieve normality. Total caloric intake was ln-transformed, and longevity analysis used age at death as t he response variable. Regression coefficients were obtained by ANOVA, while P values were obtained from bootstrap and randomization based on mean square and F values for each model tern. Relevant statistics are provided in the supplementary material.
The most efficient method of visualization for DRS is the contour plot, which we used extensively. Because they represent the best estimate of the true DRS, contour plots of the fitted DRS are presented in Figures 1, 2, 3, and in the supplementary material. These plots display the trends in the data that are supported by statistical analysis, and therefore they are expected to be a more accurate representation of the true surface than a discrete presentation of the mean response at each measured point. In all cases, summary statistics (mean, error, sample size, etc.) are provided in the supplementary data.
Diet Recipes
Treatment Media
Combine Water, Agar (Moorhead & Co.), Sucrose (SYSCO Corp.) and Brewer's Yeast (MP Biomedicals) in large Erlenmeyer flask. Mix well. Autoclave with sterilization set for 30 min at 121°C. Cool to appropriate temperature then add tegosept (Genesee Scientific) 20%w/v in ethanol and propionic acid (Fisher Scientific). Dispense into polystyrene 28.5x95 mm vials (Genesee Scientific) approx 8−10ml food/vial. Antibiotics (50 g/L kanamycin & 20 g/L tetracycline, Sigma-Aldrich) were added to the media to prevent bacterial growth.
| 2.5 g·dl−1 Yeast Media* | |||||
|---|---|---|---|---|---|
|
Sugar (g·dl−1) |
|||||
| Component | 2.5 | 5 | 10 | 20 | 40 |
| Water | 100 ml | 100 ml | 100 ml | 100 ml | 100 ml |
| Agar | 1.5 g | 1.5 g | 1.5 g | 1.5 g | 1.5 g |
| Sugar | 2.5 g | 5 g | 10 g | 20 g | 40 g |
| Yeast | 2.5 g | 2.5 g | 2.5 g | 2.5 g | 2.5 g |
| Tegosept (20%) | 1.5 ml | 1.5 ml | 1.5 ml | 1.5 ml | 1.5 ml |
| Propionic Acid | 0.3 ml | 0.3 ml | 0.3 ml | 0.3 ml | 0.3 ml |
Cool to 45°C before dispensing
| 5 g·dl−1 Yeast Media* | |||||
|---|---|---|---|---|---|
|
Sugar (g·dl−1) |
|||||
| Component | 2.5 | 5 | 10 | 20 | 40 |
| Water | 100 ml | 100 ml | 100 ml | 100 ml | 100 ml |
| Agar | 1.5 g | 1.5 g | 1.5 g | 1.5 g | 1.5 g |
| Sugar | 2.5 g | 5 g | 10 g | 20 g | 40 g |
| Yeast | 5 g | 5 g | 5 g | 5 g | 5 g |
| Tegosept (20%) | 1.5 ml | 1.5 ml | 1.5 ml | 1.5 ml | 1.5 ml |
| Propionic Acid | 0.3 ml | 0.3 ml | 0.3 ml | 0.3 ml | 0.3 ml |
Cool to 50°C before dispensing
| 10 g·dl−1 Yeast Media* | |||||
|---|---|---|---|---|---|
|
Sugar (g·dl−1) |
|||||
| Component | 2.5 | 5 | 10 | 20 | 40 |
| Water | 100 ml | 100 ml | 100 ml | 100 ml | 100 ml |
| Agar | 1.5 g | 1.5 g | 1.5 g | 1.5 g | 1.5 g |
| Sugar | 2.5 g | 5 g | 10 g | 20 g | 40 g |
| Yeast | 10 g | 10 g | 10 g | 10 g | 10 g |
| Tegosept (20%) | 1.5 ml | 1.5 ml | 1.5 ml | 1.5 ml | 1.5 ml |
| Propionic Acid | 0.3 ml | 0.3 ml | 0.3 ml | 0.3 ml | 0.3 ml |
Cool to 55°C before dispensing
| 20 g·dl−1 Yeast Media* | |||||
|---|---|---|---|---|---|
|
Sugar (g·dl−1) |
|||||
| Component | 2.5 | 5 | 10 | 20 | 40 |
| Water | 100 ml | 100 ml | 100 ml | 100 ml | 100 ml |
| Agar | 1.5 g | 1.5 g | 1.5 g | 1.5 g | 1.5 g |
| Sugar | 2.5 g | 5 g | 10 g | 20 g | 40 g |
| Yeast | 20 g | 20 g | 20 g | 20 g | 20 g |
| Tegosept (20%) | 1.5 ml | 1.5 ml | 1.5 ml | 1.5 ml | 1.5 ml |
| Propionic Acid | 0.3 ml | 0.3 ml | 0.3 ml | 0.3 ml | 0.3 ml |
Cool to 65°C before dispensing
| 40 g·dl−1 Yeast Media* | |||||
|---|---|---|---|---|---|
|
Sugar (g·dl−1) |
|||||
| Component | 2.5 | 5 | 10 | 20 | 40 |
| Water | 100 ml | 100 ml | 100 ml | 100 ml | 100 ml |
| Agar | 1.5 g | 1.5 g | 1.5 g | 1.5 g | 1.5 g |
| Sugar | 2.5 g | 5 g | 10 g | 20 g | 40 g |
| Yeast | 40 g | 40 g | 40 g | 40 g | 40 g |
| Tegosept (20%) | 1.5 ml | 1.5 ml | 1.5 ml | 1.5 ml | 1.5 ml |
| Propionic Acid | 0.3 ml | 0.3 ml | 0.3 ml | 0.3 ml | 0.3 ml |
Cool to 75°C before dispensing
Larval/Mating Media
In a large kettle combine water (1) and agar and mix well. Simmer (mix slowly) 40 min or slowly boil 15 min. Combine water (2), yeast, sucrose, dextrose (MP Biomedicals), and cornmeal (SYSCO Corp.) in separate container and mix well. Add yeast/sugar/cornmeal mixture to agar and increase mixing speed. Ensure the food boils for 15 min. Turn off heat, cool to 65°C then add tegosept and propionic acid. Mix well and dispense into 150 ml bottles.
| Larval Media |
Mating Media |
|
|---|---|---|
| Component | Amount | Amount |
| Water (1) | 800 ml | 750 ml |
| Water (2) | 200 ml | 250 ml |
| Agar | 10 g | 20 g |
| Dextrose | 55 g | - |
| Corn Meal | 60 g | - |
| Sucrose | 30 g | 100 g |
| Yeast | 25 g | 100 g |
| 20% Tegosept | 15 ml | 15 ml |
| Propionic Acid | 3 ml | 3 ml |
Supplemental Material
Acknowledgments
We thank the Pletcher laboratory for help with Drosophila husbandry, metabolic assays, and critical comments on the manuscript. Thanks to S.Libert for technical help, M.A. Sawyer and L. Skorupa for editorial assistance. The research is supported by US National Institutes of Health (R01AG023166), Glenn Foundation, American Federation for Aging Research, and the Ellison Medical Foundation to S. P., and by US National Institutes of Health training grant award (R5T32AG000183-15) to D.S.
References
- Arrese EL, Patel RT, Soulages JL. The main triglyceride-lipase from the insect fat body is an active phospholipase A(1): identification and characterization. J Lipid Res. 2006;47:2656–2667. doi: 10.1194/jlr.M600161-JLR200. [DOI] [PubMed] [Google Scholar]
- Braeckman BP, Houthoofd K, Vanfleteren JR. Insulin-Like Signaling, Metabolism, Stress Resistance and Aging in Caenorhabditis Elegans. Mechanisms of Ageing and Development. 2001;122:673–693. doi: 10.1016/s0047-6374(01)00222-6. [DOI] [PubMed] [Google Scholar]
- Bross TG, Rogina B, Helfand SL. Behavioral, physical, and demographic changes in Drosophila populations through dietary restriction. Aging Cell. 2005;4:309–317. doi: 10.1111/j.1474-9726.2005.00181.x. [DOI] [PubMed] [Google Scholar]
- Chumlea WC, Guo SS, Kuczmarski RJ, Flegal KM, Johnson CL, Heymsfield SB, Lukaski HC, Friedl K, Hubbard VS. Body composition estimates from NHANES III bioelectrical impedance data. Int J Obes Relat Metab Disord. 2002;26:1596–1609. doi: 10.1038/sj.ijo.0802167. [DOI] [PubMed] [Google Scholar]
- Edgecomb RS, Harth CE, Schneiderman AM. Regulation of feeding behavior in adult Drosophila melanogaster varies with feeding regime and nutritional state. J Exp Biol. 1994;197:215–235. doi: 10.1242/jeb.197.1.215. [DOI] [PubMed] [Google Scholar]
- Giannakou ME, Goss M, Partridge L. Role of dFOXO in lifespan extension by dietary restriction in Drosophila melanogaster: not required, but its activity modulates the response. Aging Cell. 2008 doi: 10.1111/j.1474-9726.2007.00362.x. [DOI] [PubMed] [Google Scholar]
- Goff SA, Klee HJ. Plant volatile compounds: sensory cues for health and nutritional value? Science. 2006;311:815–819. doi: 10.1126/science.1112614. [DOI] [PubMed] [Google Scholar]
- Greer EL, Dowlatshahi D, Banko MR, Villen J, Hoang K, Blanchard D, Gygi SP, Brunet A. An AMPK-FOXO pathway mediates longevity induced by a novel method of dietary restriction in C. elegans. Curr Biol. 2007;17:1646–1656. doi: 10.1016/j.cub.2007.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronke S, Mildner A, Fellert S, Tennagels N, Petry S, Muller G, Jackle H, Kuhnlein RP. Brummer lipase is an evolutionary conserved fat storage regulator in Drosophila. Cell Metab. 2005;1:323–330. doi: 10.1016/j.cmet.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Gronke S, Muller G, Hirsch J, Fellert S, Andreou A, Haase T, Jackle H, Kuhnlein RP. Dual Lipolytic Control of Body Fat Storage and Mobilization in Drosophila. PLoS Biol. 2007;5:e137. doi: 10.1371/journal.pbio.0050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarente L, Picard F. Calorie restriction--the SIR2 connection. Cell. 2005;120:473–482. doi: 10.1016/j.cell.2005.01.029. [DOI] [PubMed] [Google Scholar]
- Ja WW, Carvalho GB, Mak EM, de la Rosa NN, Fang AY, Liong JC, Brummel T, Benzer S. Prandiology of Drosophila and the CAFE assay. Proc Natl Acad Sci U S A. 2007;104:8253–8256. doi: 10.1073/pnas.0702726104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang JC, Jaruga E, Repnevskaya MV, Jazwinski SM. An Intervention Resembling Caloric Restriction Prolongs Life Span and Retards Aging in Yeast. Faseb Journal. 2000;14:2135–2137. doi: 10.1096/fj.00-0242fje. [DOI] [PubMed] [Google Scholar]
- Johnson MB, Butterworth FM. Maturation and aging of adult fat body and oenocytes in Drosophila as revealed by light microscopic morphometry. Journal of morphology. 1985;184:51–59. doi: 10.1002/jmor.1051840106. [DOI] [PubMed] [Google Scholar]
- Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, Benzer S. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol. 2004;14:885–890. doi: 10.1016/j.cub.2004.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehl RO. Statistical Principles of Research Design and Analysis. Wadsworth Inc.; Belmont, CA: 1994. [Google Scholar]
- Lagiou P, Sandin S, Weiderpass E, Lagiou A, Mucci L, Trichopoulos D, Adami HO. Low carbohydrate-high protein diet and mortality in a cohort of Swedish women. Journal of internal medicine. 2007;261:366–374. doi: 10.1111/j.1365-2796.2007.01774.x. [DOI] [PubMed] [Google Scholar]
- Lee GD, Wilson MA, Zhu M, Wolkow CA, de Cabo R, Ingram DK, Zou S. Dietary deprivation extends lifespan in Caenorhabditis elegans. Aging Cell. 2006;5:515–524. doi: 10.1111/j.1474-9726.2006.00241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libert S, Zwiener J, Chu X, Vanvoorhies W, Roman G, Pletcher SD. Regulation of Drosophila life span by olfaction and food-derived odors. Science. 2007;315:1133–1137. doi: 10.1126/science.1136610. [DOI] [PubMed] [Google Scholar]
- Longo VD, Finch CE. Evolutionary medicine: from dwarf model systems to healthy centenarians? Science. 2003;299:1342–1346. doi: 10.1126/science.1077991. [DOI] [PubMed] [Google Scholar]
- Magwere T, Chapman T, Partridge L. Sex differences in the effect of dietary restriction on life span and mortality rates in female and male Drosophila melanogaster. J Gerontol A Biol Sci Med Sci. 2004;59:3–9. doi: 10.1093/gerona/59.1.b3. [DOI] [PubMed] [Google Scholar]
- Mair W, Piper MDW, Partridge L. Calories do not explain extension of lifespan by dietary restriction in Drosophila. PLoS Biol. 2005;3:e223. doi: 10.1371/journal.pbio.0030223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masoro EJ. Overview of caloric restriction and ageing. Mech Ageing Dev. 2005;126:913–922. doi: 10.1016/j.mad.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Mayntz D, Raubenheimer D, Salomon M, Toft S, Simpson SJ. Nutrient-specific foraging in invertebrate predators. Science. 2005;307:111–113. doi: 10.1126/science.1105493. [DOI] [PubMed] [Google Scholar]
- Miller RA, Buehner G, Chang Y, Harper JM, Sigler R, Smith-Wheelock M. Methionine-deficient diet extends mouse lifespan, slows immune and lens aging, alters glucose, T4, IGF-I and insulin levels, and increases hepatocyte MIF levels and stress resistance. Aging Cell. 2005;4:119–125. doi: 10.1111/j.1474-9726.2005.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min KJ, Tatar M. Drosophila diet restriction in practice: do flies consume fewer nutrients? Mech Ageing Dev. 2006a;127:93–96. doi: 10.1016/j.mad.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Min KJ, Tatar M. Restriction of amino acids extends lifespan in Drosophila melanogaster. Mech Ageing Dev. 2006b;127:643–646. doi: 10.1016/j.mad.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Min KJ, Yamamoto R, Buch S, Pankratz M, Tatar M. Drosophila life span control by dietary restriction independent of insulin-like signaling. Aging Cell. 2008 doi: 10.1111/j.1474-9726.2008.00373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novoseltsev VN, Novoseltseva JA, Yashin AI. What does a fly's individual fecundity pattern look like? The dynamics of resource allocation in reproduction and ageing. Mech Ageing Dev. 2003;124:605–617. doi: 10.1016/s0047-6374(03)00061-7. [DOI] [PubMed] [Google Scholar]
- Olshansky SJ, Passaro DJ, Hershow RC, Layden J, Carnes BA, Brody J, Hayflick L, Butler RN, Allison DB, Ludwig DS. A potential decline in life expectancy in the United States in the 21st century. The New England journal of medicine. 2005;352:1138–1145. doi: 10.1056/NEJMsr043743. [DOI] [PubMed] [Google Scholar]
- Panowski SH, Wolff S, Aguilaniu H, Durieux J, Dillin A. PHA-4/Foxa mediates diet-restriction-induced longevity of C. elegans. Nature. 2007 doi: 10.1038/nature05837. [DOI] [PubMed] [Google Scholar]
- Pierce VA, Mueller LD, Gibbs AG. Osmoregulation in Drosophila melanogaster selected for urea tolerance. J Exp Biol. 1999;202:2349–2358. doi: 10.1242/jeb.202.17.2349. [DOI] [PubMed] [Google Scholar]
- Piper MD, Partridge L. Dietary restriction in Drosophila: delayed aging or experimental artefact? PLoS genetics. 2007;3:e57. doi: 10.1371/journal.pgen.0030057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper MD, Skorupa D, Partridge L. Diet, metabolism and lifespan in Drosophila. Exp Gerontol. 2005;40:857–862. doi: 10.1016/j.exger.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Pletcher SD, Libert S, Skorupa D. Flies and their golden apples: the effect of dietary restriction on Drosophila aging and age-dependent gene expression. Ageing Res Rev. 2005;4:451–480. doi: 10.1016/j.arr.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Pletcher SD, Macdonald SJ, Marguerie R, Certa U, Stearns SC, Goldstein DB, Partridge L. Genome-Wide Transcript Profiles in Aging and Calorically Restricted Drosophila melanogaster. Curr Biol. 2002;12:712–723. doi: 10.1016/s0960-9822(02)00808-4. [DOI] [PubMed] [Google Scholar]
- Simpson SJ, Batley R, Raubenheimer D. Geometric analysis of macronutrient intake in humans: the power of protein? Appetite. 2003;41:123–140. doi: 10.1016/s0195-6663(03)00049-7. [DOI] [PubMed] [Google Scholar]
- Simpson SJ, Raubenheimer D. Obesity: the protein leverage hypothesis. Obes Rev. 2005;6:133–142. doi: 10.1111/j.1467-789X.2005.00178.x. [DOI] [PubMed] [Google Scholar]
- Simpson SJ, Raubenheimer D. Caloric restriction and aging revisited: the need for a geometric analysis of the nutritional bases of aging. J Gerontol A Biol Sci Med Sci. 2007;62:707–713. doi: 10.1093/gerona/62.7.707. [DOI] [PubMed] [Google Scholar]
- Sorensen A, Mayntz D, Raubenheimer D, Simpson SJ. Protein-leverage in Mice: The Geometry of Macronutrient Balancing and Consequences for Fat Deposition. Obesity (Silver Spring, Md. 2008 doi: 10.1038/oby.2007.58. [DOI] [PubMed] [Google Scholar]
- Swithers SE, Davidson TL. A role for sweet taste: Calorie predictive relations in energy regulation by rats. Behavioral Neuroscience. 2008 doi: 10.1037/0735-7044.122.1.161. Advanced Online Publication. [DOI] [PubMed] [Google Scholar]
- Tanimura T, Isono K, Yamamoto MT. Taste Sensitivity to Trehalose and Its Alteration by Gene Dosage in Drosophila Melanogaster. Genetics. 1988;119:399–406. doi: 10.1093/genetics/119.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatar M. Diet restriction in Drosophila melanogaster. Design and analysis. Interdisciplinary topics in gerontology. 2007;35:115–136. doi: 10.1159/000096559. [DOI] [PubMed] [Google Scholar]
- Tatar M, Bartke A, Antebi A. The endocrine regulation of aging by insulin-like signals. Science. 2003;299:1346–1351. doi: 10.1126/science.1081447. [DOI] [PubMed] [Google Scholar]
- van der Horst DJ, van Hoof D, van Marrewijk WJ, Rodenburg KW. Alternative lipid mobilization: the insect shuttle system. Mol Cell Biochem. 2002;239:113–119. [PubMed] [Google Scholar]
- Venables WN, Ripley BD. Modern Applied Statistics with S-Plus. Springer-Verlag; New York, NY: 1997. [Google Scholar]
- Warbrick-Smith J, Behmer ST, Lee KP, Raubenheimer D, Simpson SJ. Evolving resistance to obesity in an insect. Proc Natl Acad Sci U S A. 2006;103:14045–14049. doi: 10.1073/pnas.0605225103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weindruch R, Walford RL. The retardation of aging and disease by dietary restriction. Charles C. Thomas; Springfield: 1988. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.