Abstract
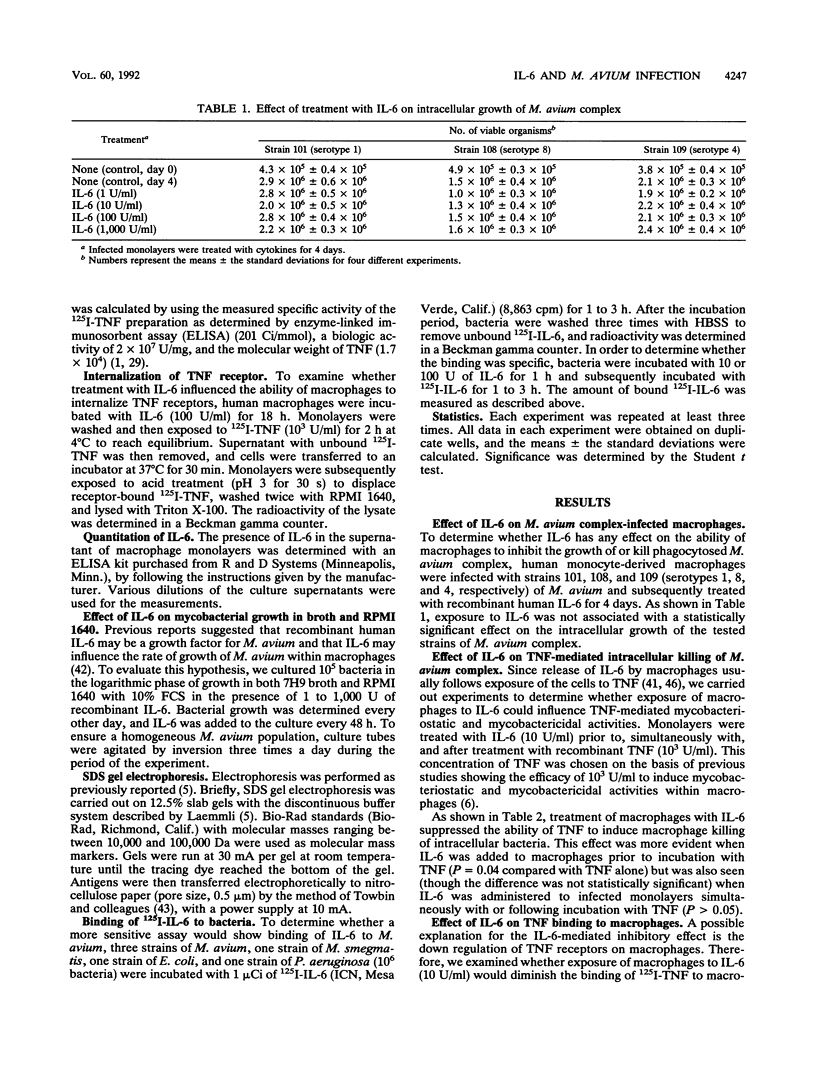
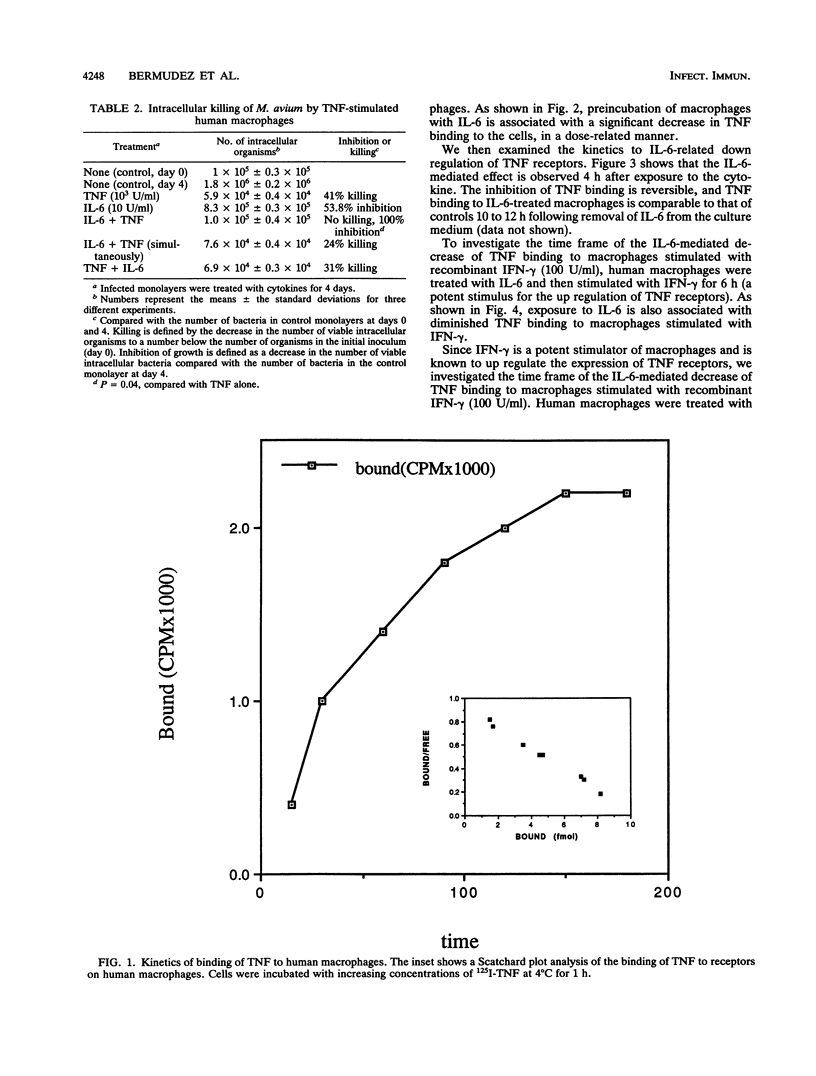
Interleukin-6 (IL-6) is a cytokine produced by a number of cells, including macrophages, and is directly involved in the inflammatory response. The production of IL-6 can be stimulated by monokines such as IL-1 and tumor necrosis factor (TNF). Mycobacterium avium complex organisms frequently cause disseminated disease in patients with AIDS. M. avium is an intracellular bacterium that that mainly infects macrophages. Treatment of M. avium-infected macrophage monolayers with recombinant IL-6 decreased the ability of TNF to activate cultured macrophages to inhibit growth of or kill intracellular M. avium (68% +/- 14% decrease in intracellular killing compared with that in monolayers not treated with IL-6). To further evaluate whether this effect was dependent on the down regulation of membrane receptors to TNF, we examined 125I-TNF binding to macrophages previously exposed to IL-6: the expression of TNF receptors was decreased by 78% +/- 9%. The effect of IL-6 on TNF receptors was observed after 4 h and was reversible. Infection of macrophages with different M. avium serovars was associated with release of IL-6, and IL-6 production peaked at 48 h after infection in concentrations ranging from 328 +/- 87 ng/10(5) cells to 907 +/- 224 ng/10(5) cells. IL-6 did not have any influence on the rate of growth of the tested strains of M. avium within or outside macrophages. These results suggest that release of IL-6 by M. avium-infected macrophages may influence the host's immune response and the outcome of the disease.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aggarwal B. B., Eessalu T. E., Hass P. E. Characterization of receptors for human tumour necrosis factor and their regulation by gamma-interferon. Nature. 1985 Dec 19;318(6047):665–667. doi: 10.1038/318665a0. [DOI] [PubMed] [Google Scholar]
- Bermudez L. E. Infection of "nonprofessional phagocytes" with Mycobacterium avium complex. Clin Immunol Immunopathol. 1991 Nov;61(2 Pt 1):225–235. doi: 10.1016/s0090-1229(05)80026-1. [DOI] [PubMed] [Google Scholar]
- Bermudez L. E., Stevens P., Kolonoski P., Wu M., Young L. S. Treatment of experimental disseminated Mycobacterium avium complex infection in mice with recombinant IL-2 and tumor necrosis factor. J Immunol. 1989 Nov 1;143(9):2996–3000. [PubMed] [Google Scholar]
- Bermudez L. E., Wu M., Enkel H., Young L. S. Naturally occurring antibodies against Mycobacterium avium complex. Ann Clin Lab Sci. 1989 Nov-Dec;19(6):435–443. [PubMed] [Google Scholar]
- Bermudez L. E., Young L. S. Natural killer cell-dependent mycobacteriostatic and mycobactericidal activity in human macrophages. J Immunol. 1991 Jan 1;146(1):265–270. [PubMed] [Google Scholar]
- Bermudez L. E., Young L. S. Oxidative and non-oxidative intracellular killing of Mycobacterium avium complex. Microb Pathog. 1989 Oct;7(4):289–298. doi: 10.1016/0882-4010(89)90047-8. [DOI] [PubMed] [Google Scholar]
- Bermudez L. E., Young L. S. Recombinant granulocyte-macrophage colony-stimulating factor activates human macrophages to inhibit growth or kill Mycobacterium avium complex. J Leukoc Biol. 1990 Jul;48(1):67–73. doi: 10.1002/jlb.48.1.67. [DOI] [PubMed] [Google Scholar]
- Bermudez L. E., Young L. S. Tumor necrosis factor, alone or in combination with IL-2, but not IFN-gamma, is associated with macrophage killing of Mycobacterium avium complex. J Immunol. 1988 May 1;140(9):3006–3013. [PubMed] [Google Scholar]
- Bertram M. A., Inderlied C. B., Yadegar S., Kolanoski P., Yamada J. K., Young L. S. Confirmation of the beige mouse model for study of disseminated infection with Mycobacterium avium complex. J Infect Dis. 1986 Jul;154(1):194–195. doi: 10.1093/infdis/154.1.194. [DOI] [PubMed] [Google Scholar]
- Beutler B., Cerami A. Cachectin: more than a tumor necrosis factor. N Engl J Med. 1987 Feb 12;316(7):379–385. doi: 10.1056/NEJM198702123160705. [DOI] [PubMed] [Google Scholar]
- Beutler B., Milsark I. W., Cerami A. C. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985 Aug 30;229(4716):869–871. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- Birx D. L., Redfield R. R., Tencer K., Fowler A., Burke D. S., Tosato G. Induction of interleukin-6 during human immunodeficiency virus infection. Blood. 1990 Dec 1;76(11):2303–2310. [PubMed] [Google Scholar]
- Black C. M., Bermudez L. E., Young L. S., Remington J. S. Co-infection of macrophages modulates interferon gamma and tumor necrosis factor-induced activation against intracellular pathogens. J Exp Med. 1990 Sep 1;172(3):977–980. doi: 10.1084/jem.172.3.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard D. K., Djeu J. Y., Klein T. W., Friedman H., Stewart W. E., 2nd Protective effects of tumor necrosis factor in experimental Legionella pneumophila infections of mice via activation of PMN function. J Leukoc Biol. 1988 May;43(5):429–435. doi: 10.1002/jlb.43.5.429. [DOI] [PubMed] [Google Scholar]
- Blanchard D. K., Michelini-Norris M. B., Pearson C. A., Freitag C. S., Djeu J. Y. Mycobacterium avium-intracellulare induces interleukin-6 from human monocytes and large granular lymphocytes. Blood. 1991 May 15;77(10):2218–2224. [PubMed] [Google Scholar]
- Carswell E. A., Old L. J., Kassel R. L., Green S., Fiore N., Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowle A. J., Dahl R., Ross E., May M. H. Evidence that vesicles containing living, virulent Mycobacterium tuberculosis or Mycobacterium avium in cultured human macrophages are not acidic. Infect Immun. 1991 May;59(5):1823–1831. doi: 10.1128/iai.59.5.1823-1831.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desiderio J. V., Kiener P. A., Lin P. F., Warr G. A. Protection of mice against Listeria monocytogenes infection by recombinant human tumor necrosis factor alpha. Infect Immun. 1989 May;57(5):1615–1617. doi: 10.1128/iai.57.5.1615-1617.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djeu J. Y., Blanchard D. K., Halkias D., Friedman H. Growth inhibition of Candida albicans by human polymorphonuclear neutrophils: activation by interferon-gamma and tumor necrosis factor. J Immunol. 1986 Nov 1;137(9):2980–2984. [PubMed] [Google Scholar]
- Fong Y., Moldawer L. L., Marano M., Wei H., Tatter S. B., Clarick R. H., Santhanam U., Sherris D., May L. T., Sehgal P. B. Endotoxemia elicits increased circulating beta 2-IFN/IL-6 in man. J Immunol. 1989 Apr 1;142(7):2321–2324. [PubMed] [Google Scholar]
- Fràter-Schröder M., Risau W., Hallmann R., Gautschi P., Böhlen P. Tumor necrosis factor type alpha, a potent inhibitor of endothelial cell growth in vitro, is angiogenic in vivo. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5277–5281. doi: 10.1073/pnas.84.15.5277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauldie J., Richards C., Harnish D., Lansdorp P., Baumann H. Interferon beta 2/B-cell stimulatory factor type 2 shares identity with monocyte-derived hepatocyte-stimulating factor and regulates the major acute phase protein response in liver cells. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7251–7255. doi: 10.1073/pnas.84.20.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good R. C. Opportunistic pathogens in the genus Mycobacterium. Annu Rev Microbiol. 1985;39:347–369. doi: 10.1146/annurev.mi.39.100185.002023. [DOI] [PubMed] [Google Scholar]
- Hawkins C. C., Gold J. W., Whimbey E., Kiehn T. E., Brannon P., Cammarata R., Brown A. E., Armstrong D. Mycobacterium avium complex infections in patients with the acquired immunodeficiency syndrome. Ann Intern Med. 1986 Aug;105(2):184–188. doi: 10.7326/0003-4819-105-2-184. [DOI] [PubMed] [Google Scholar]
- Hirano T., Yasukawa K., Harada H., Taga T., Watanabe Y., Matsuda T., Kashiwamura S., Nakajima K., Koyama K., Iwamatsu A. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature. 1986 Nov 6;324(6092):73–76. doi: 10.1038/324073a0. [DOI] [PubMed] [Google Scholar]
- Hohmann H. P., Remy R., Brockhaus M., van Loon A. P. Two different cell types have different major receptors for human tumor necrosis factor (TNF alpha). J Biol Chem. 1989 Sep 5;264(25):14927–14934. [PubMed] [Google Scholar]
- Imamura K., Spriggs D., Kufe D. Expression of tumor necrosis factor receptors on human monocytes and internalization of receptor bound ligand. J Immunol. 1987 Nov 1;139(9):2989–2992. [PubMed] [Google Scholar]
- Kishimoto T. The biology of interleukin-6. Blood. 1989 Jul;74(1):1–10. [PubMed] [Google Scholar]
- Le J., Vilcek J. Tumor necrosis factor and interleukin 1: cytokines with multiple overlapping biological activities. Lab Invest. 1987 Mar;56(3):234–248. [PubMed] [Google Scholar]
- Luger T. A., Krutmann J., Kirnbauer R., Urbanski A., Schwarz T., Klappacher G., Köck A., Micksche M., Malejczyk J., Schauer E. IFN-beta 2/IL-6 augments the activity of human natural killer cells. J Immunol. 1989 Aug 15;143(4):1206–1209. [PubMed] [Google Scholar]
- Mayer B. K., Falkinham J. O., 3rd Superoxide dismutase activity of Mycobacterium avium, M. intracellulare, and M. scrofulaceum. Infect Immun. 1986 Sep;53(3):631–635. doi: 10.1128/iai.53.3.631-635.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawara A., Nathan C. F. A simple method for counting adherent cells: application to cultured human monocytes, macrophages and multinucleated giant cells. J Immunol Methods. 1983 Jan 28;56(2):261–268. doi: 10.1016/0022-1759(83)90418-0. [DOI] [PubMed] [Google Scholar]
- Nakane A., Minagawa T., Kato K. Endogenous tumor necrosis factor (cachectin) is essential to host resistance against Listeria monocytogenes infection. Infect Immun. 1988 Oct;56(10):2563–2569. doi: 10.1128/iai.56.10.2563-2569.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro S., Debili N., Bernaudin J. F., Vainchenker W., Doly J. Regulation of the expression of IL-6 in human monocytes. J Immunol. 1989 Jun 15;142(12):4339–4345. [PubMed] [Google Scholar]
- Old L. J. Tumor necrosis factor (TNF). Science. 1985 Nov 8;230(4726):630–632. doi: 10.1126/science.2413547. [DOI] [PubMed] [Google Scholar]
- Rook G. A., Attiyah R. A., Foley N. The role of cytokines in the immunopathology of tuberculosis, and the regulation of agalactosyl IgG. Lymphokine Res. 1989 Fall;8(3):323–328. [PubMed] [Google Scholar]
- Ruggiero V., Tavernier J., Fiers W., Baglioni C. Induction of the synthesis of tumor necrosis factor receptors by interferon-gamma. J Immunol. 1986 Apr 1;136(7):2445–2450. [PubMed] [Google Scholar]
- Schindler R., Mancilla J., Endres S., Ghorbani R., Clark S. C., Dinarello C. A. Correlations and interactions in the production of interleukin-6 (IL-6), IL-1, and tumor necrosis factor (TNF) in human blood mononuclear cells: IL-6 suppresses IL-1 and TNF. Blood. 1990 Jan 1;75(1):40–47. [PubMed] [Google Scholar]
- Shiratsuchi H., Johnson J. L., Ellner J. J. Bidirectional effects of cytokines on the growth of Mycobacterium avium within human monocytes. J Immunol. 1991 May 1;146(9):3165–3170. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto M., Yip Y. K., Vilcek J. Interferon-gamma enhances expression of cellular receptors for tumor necrosis factor. J Immunol. 1986 Apr 1;136(7):2441–2444. [PubMed] [Google Scholar]
- Urban J. L., Shepard H. M., Rothstein J. L., Sugarman B. J., Schreiber H. Tumor necrosis factor: a potent effector molecule for tumor cell killing by activated macrophages. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5233–5237. doi: 10.1073/pnas.83.14.5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Damme J., Opdenakker G., Simpson R. J., Rubira M. R., Cayphas S., Vink A., Billiau A., Van Snick J. Identification of the human 26-kD protein, interferon beta 2 (IFN-beta 2), as a B cell hybridoma/plasmacytoma growth factor induced by interleukin 1 and tumor necrosis factor. J Exp Med. 1987 Mar 1;165(3):914–919. doi: 10.1084/jem.165.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wankowicz Z., Megyeri P., Issekutz A. Synergy between tumour necrosis factor alpha and interleukin-1 in the induction of polymorphonuclear leukocyte migration during inflammation. J Leukoc Biol. 1988 Apr;43(4):349–356. doi: 10.1002/jlb.43.4.349. [DOI] [PubMed] [Google Scholar]
- Williams D. M., Magee D. M., Bonewald L. F., Smith J. G., Bleicker C. A., Byrne G. I., Schachter J. A role in vivo for tumor necrosis factor alpha in host defense against Chlamydia trachomatis. Infect Immun. 1990 Jun;58(6):1572–1576. doi: 10.1128/iai.58.6.1572-1576.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong G. G., Clark S. C. Multiple actions of interleukin 6 within a cytokine network. Immunol Today. 1988 May;9(5):137–139. doi: 10.1016/0167-5699(88)91200-5. [DOI] [PubMed] [Google Scholar]
- Yakrus M. A., Good R. C. Geographic distribution, frequency, and specimen source of Mycobacterium avium complex serotypes isolated from patients with acquired immunodeficiency syndrome. J Clin Microbiol. 1990 May;28(5):926–929. doi: 10.1128/jcm.28.5.926-929.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L. S., Inderlied C. B., Berlin O. G., Gottlieb M. S. Mycobacterial infections in AIDS patients, with an emphasis on the Mycobacterium avium complex. Rev Infect Dis. 1986 Nov-Dec;8(6):1024–1033. doi: 10.1093/clinids/8.6.1024. [DOI] [PubMed] [Google Scholar]


