Abstract
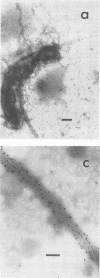
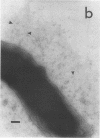
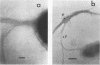
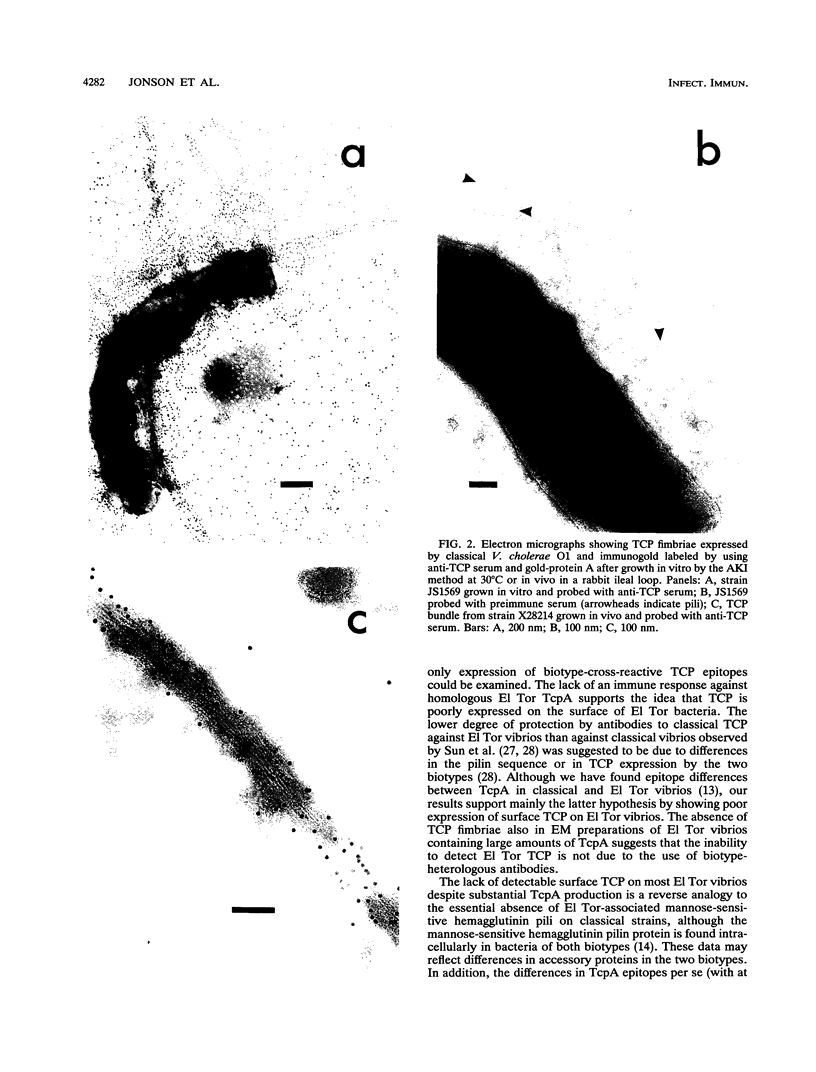
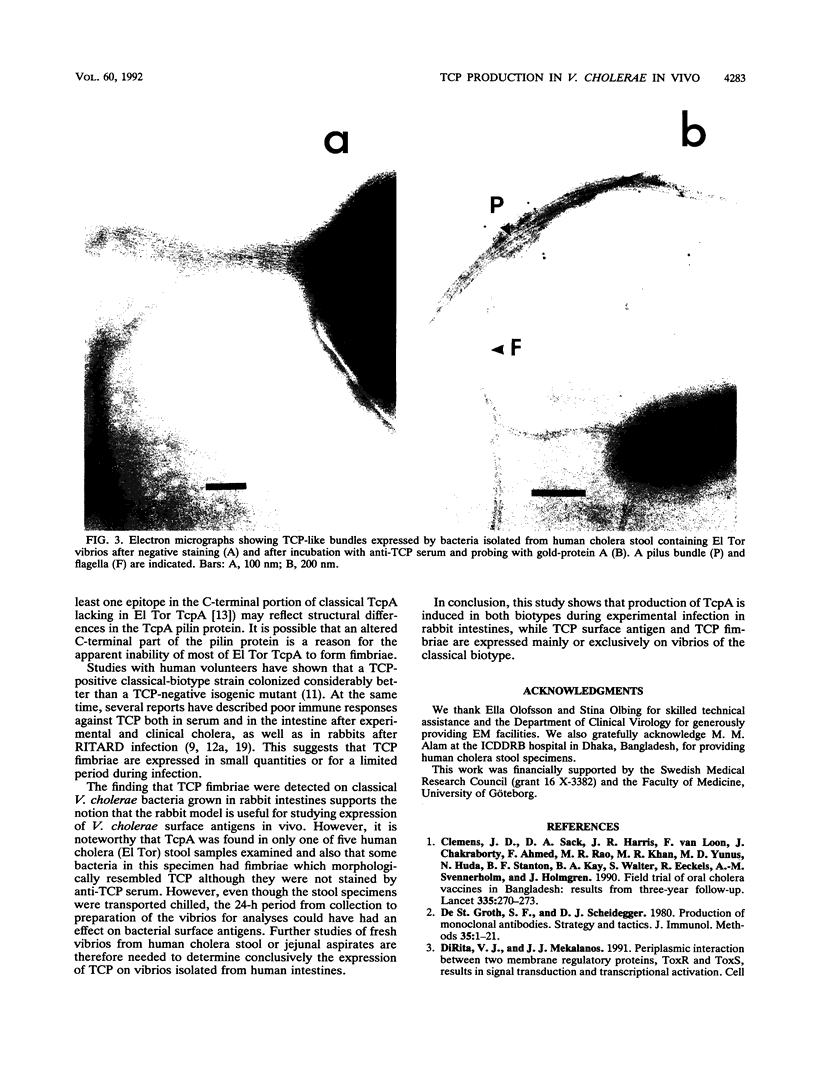
The expression of toxin-coregulated pili (TCP) and their structural subunit TcpA was compared in 20 strains of Vibrio cholerae of the classical and El Tor biotypes. Bacteria were isolated from the intestines of rabbits with experimental cholera and compared with the same strains grown under optimal TCP expression conditions in vitro. Immunoblotting revealed that TcpA production was induced in both biotypes after vibrios entered the intestinal milieu; TcpA-negative inocula gave rise to TcpA-positive vibrios after multiplication in the gut. The levels of TcpA expressed during growth in the intestine were, for most strains, comparable to those attained under optimal growth conditions in vitro. Of 11 classical strains tested, 10 expressed TCP antigen on the bacterial surface at levels comparable to or exceeding those seen after growth in vitro as determined by an inhibition enzyme-linked immunosorbent assay. In contrast, only one of the nine El Tor strains studied produced detectable amounts of TCP surface antigen in vivo and no fimbriae or surface antigen reacting with anti-TCP serum was found on El Tor vibrios from human cholera stools. Distinct TCP fimbriae were observed by immunoelectron microscopy on classical-biotype vibrios grown either in rabbit intestines or in vitro but were not detected on El Tor vibrios. The results show that TCP is expressed on V. cholerae O1 of the classical biotype but not on V. cholerae O1 of the El Tor biotype in the intestines of rabbits with experimental cholera infection.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clemens J. D., Sack D. A., Harris J. R., Van Loon F., Chakraborty J., Ahmed F., Rao M. R., Khan M. R., Yunus M., Huda N. Field trial of oral cholera vaccines in Bangladesh: results from three-year follow-up. Lancet. 1990 Feb 3;335(8684):270–273. doi: 10.1016/0140-6736(90)90080-o. [DOI] [PubMed] [Google Scholar]
- DiRita V. J., Parsot C., Jander G., Mekalanos J. J. Regulatory cascade controls virulence in Vibrio cholerae. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5403–5407. doi: 10.1073/pnas.88.12.5403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey R. S., Lindblad M., Holmgren J. Purification of El Tor cholera enterotoxins and comparisons with classical toxin. J Gen Microbiol. 1990 Sep;136(9):1839–1847. doi: 10.1099/00221287-136-9-1839. [DOI] [PubMed] [Google Scholar]
- Ehara M., Ishibashi M., Ichinose Y., Iwanaga M., Shimotori S., Naito T. Purification and partial characterization of fimbriae of Vibrio cholerae O1. Vaccine. 1987 Dec;5(4):283–288. doi: 10.1016/0264-410x(87)90153-8. [DOI] [PubMed] [Google Scholar]
- Finkelstein R. A., Boesman-Finkelstein M., Holt P. Vibrio cholerae hemagglutinin/lectin/protease hydrolyzes fibronectin and ovomucin: F.M. Burnet revisited. Proc Natl Acad Sci U S A. 1983 Feb;80(4):1092–1095. doi: 10.1073/pnas.80.4.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn T. M., Reiser J., Germanier R., Cryz S. J., Jr Cell-associated hemagglutinin-deficient mutant of Vibrio cholerae. Infect Immun. 1987 Apr;55(4):942–946. doi: 10.1128/iai.55.4.942-946.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall R. H., Losonsky G., Silveira A. P., Taylor R. K., Mekalanos J. J., Witham N. D., Levine M. M. Immunogenicity of Vibrio cholerae O1 toxin-coregulated pili in experimental and clinical cholera. Infect Immun. 1991 Jul;59(7):2508–2512. doi: 10.1128/iai.59.7.2508-2512.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall R. H., Vial P. A., Kaper J. B., Mekalanos J. J., Levine M. M. Morphological studies on fimbriae expressed by Vibrio cholerae 01. Microb Pathog. 1988 Apr;4(4):257–265. doi: 10.1016/0882-4010(88)90086-1. [DOI] [PubMed] [Google Scholar]
- Herrington D. A., Hall R. H., Losonsky G., Mekalanos J. J., Taylor R. K., Levine M. M. Toxin, toxin-coregulated pili, and the toxR regulon are essential for Vibrio cholerae pathogenesis in humans. J Exp Med. 1988 Oct 1;168(4):1487–1492. doi: 10.1084/jem.168.4.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwanaga M., Yamamoto K., Higa N., Ichinose Y., Nakasone N., Tanabe M. Culture conditions for stimulating cholera toxin production by Vibrio cholerae O1 El Tor. Microbiol Immunol. 1986;30(11):1075–1083. doi: 10.1111/j.1348-0421.1986.tb03037.x. [DOI] [PubMed] [Google Scholar]
- Jonson G., Holmgren J., Svennerholm A. M. Epitope differences in toxin-coregulated pili produced by classical and El Tor Vibrio cholerae O1. Microb Pathog. 1991 Sep;11(3):179–188. doi: 10.1016/0882-4010(91)90048-f. [DOI] [PubMed] [Google Scholar]
- Jonson G., Holmgren J., Svennerholm A. M. Identification of a mannose-binding pilus on Vibrio cholerae El Tor. Microb Pathog. 1991 Dec;11(6):433–441. doi: 10.1016/0882-4010(91)90039-d. [DOI] [PubMed] [Google Scholar]
- Jonson G., Sanchez J., Svennerholm A. M. Expression and detection of different biotype-associated cell-bound haemagglutinins of Vibrio cholerae O1. J Gen Microbiol. 1989 Jan;135(1):111–120. doi: 10.1099/00221287-135-1-111. [DOI] [PubMed] [Google Scholar]
- Jonson G., Svennerholm A. M., Holmgren J. Vibrio cholerae expresses cell surface antigens during intestinal infection which are not expressed during in vitro culture. Infect Immun. 1989 Jun;57(6):1809–1815. doi: 10.1128/iai.57.6.1809-1815.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levine M. M., Kaper J. B., Herrington D., Losonsky G., Morris J. G., Clements M. L., Black R. E., Tall B., Hall R. Volunteer studies of deletion mutants of Vibrio cholerae O1 prepared by recombinant techniques. Infect Immun. 1988 Jan;56(1):161–167. doi: 10.1128/iai.56.1.161-167.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lycke N., Svennerholm A. M., Holmgren J. Strong biotype and serotype cross-protective antibacterial and antitoxic immunity in rabbits after cholera infection. Microb Pathog. 1986 Aug;1(4):361–371. doi: 10.1016/0882-4010(86)90068-9. [DOI] [PubMed] [Google Scholar]
- Miller V. L., Mekalanos J. J. Genetic analysis of the cholera toxin-positive regulatory gene toxR. J Bacteriol. 1985 Aug;163(2):580–585. doi: 10.1128/jb.163.2.580-585.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsot C., Mekalanos J. J. Expression of ToxR, the transcriptional activator of the virulence factors in Vibrio cholerae, is modulated by the heat shock response. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9898–9902. doi: 10.1073/pnas.87.24.9898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez J., Holmgren J. Recombinant system for overexpression of cholera toxin B subunit in Vibrio cholerae as a basis for vaccine development. Proc Natl Acad Sci U S A. 1989 Jan;86(2):481–485. doi: 10.1073/pnas.86.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma D. P., Stroeher U. H., Thomas C. J., Manning P. A., Attridge S. R. The toxin-coregulated pilus (TCP) of Vibrio cholerae: molecular cloning of genes involved in pilus biosynthesis and evaluation of TCP as a protective antigen in the infant mouse model. Microb Pathog. 1989 Dec;7(6):437–448. doi: 10.1016/0882-4010(89)90024-7. [DOI] [PubMed] [Google Scholar]
- Sharma D. P., Thomas C., Hall R. H., Levine M. M., Attridge S. R. Significance of toxin-coregulated pili as protective antigens of Vibrio cholerae in the infant mouse model. Vaccine. 1989 Oct;7(5):451–456. doi: 10.1016/0264-410x(89)90161-8. [DOI] [PubMed] [Google Scholar]
- Spira W. M., Sack R. B., Froehlich J. L. Simple adult rabbit model for Vibrio cholerae and enterotoxigenic Escherichia coli diarrhea. Infect Immun. 1981 May;32(2):739–747. doi: 10.1128/iai.32.2.739-747.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D. X., Mekalanos J. J., Taylor R. K. Antibodies directed against the toxin-coregulated pilus isolated from Vibrio cholerae provide protection in the infant mouse experimental cholera model. J Infect Dis. 1990 Jun;161(6):1231–1236. doi: 10.1093/infdis/161.6.1231. [DOI] [PubMed] [Google Scholar]
- Svennerholm A. M. Experimental studies on cholera immunization. 4. The antibody response to formalinized Vibrio cholerae and purified endotoxin with special reference to protective capacity. Int Arch Allergy Appl Immunol. 1975;49(4):434–452. [PubMed] [Google Scholar]
- Svennerholm A. M., Strömberg G. J., Holmgren J. Purification of Vibrio cholerae soluble hemagglutinin and development of enzyme-linked immunosorbent assays for antigen and antibody quantitations. Infect Immun. 1983 Jul;41(1):237–243. doi: 10.1128/iai.41.1.237-243.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R. K., Miller V. L., Furlong D. B., Mekalanos J. J. Use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc Natl Acad Sci U S A. 1987 May;84(9):2833–2837. doi: 10.1073/pnas.84.9.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R., Shaw C., Peterson K., Spears P., Mekalanos J. Safe, live Vibrio cholerae vaccines? Vaccine. 1988 Apr;6(2):151–154. doi: 10.1016/s0264-410x(88)80019-7. [DOI] [PubMed] [Google Scholar]
- de StGroth S. F., Scheidegger D. Production of monoclonal antibodies: strategy and tactics. J Immunol Methods. 1980;35(1-2):1–21. doi: 10.1016/0022-1759(80)90146-5. [DOI] [PubMed] [Google Scholar]