Abstract
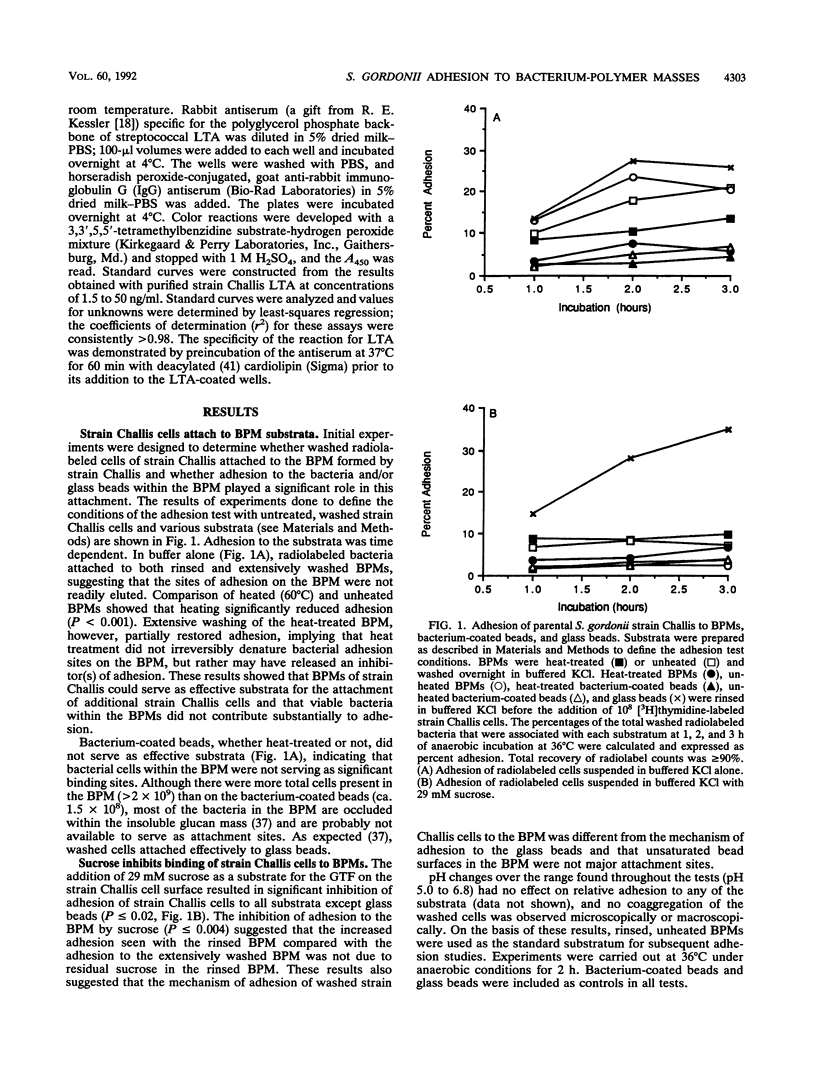
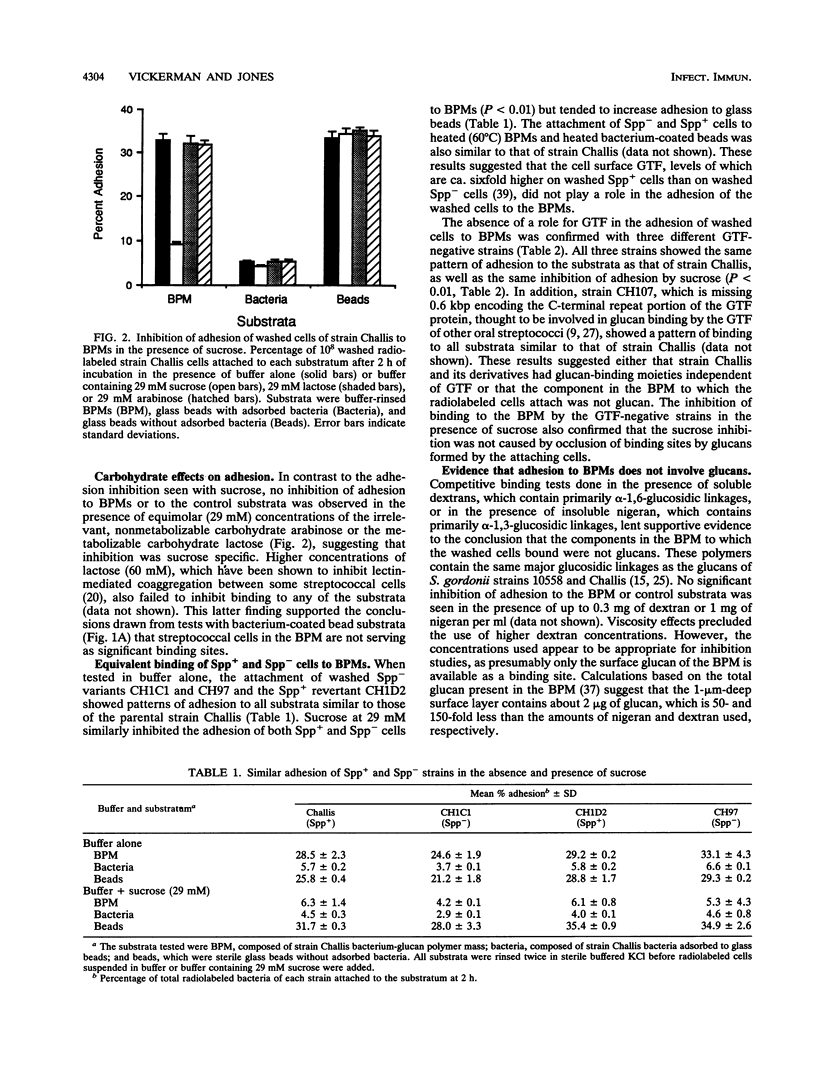
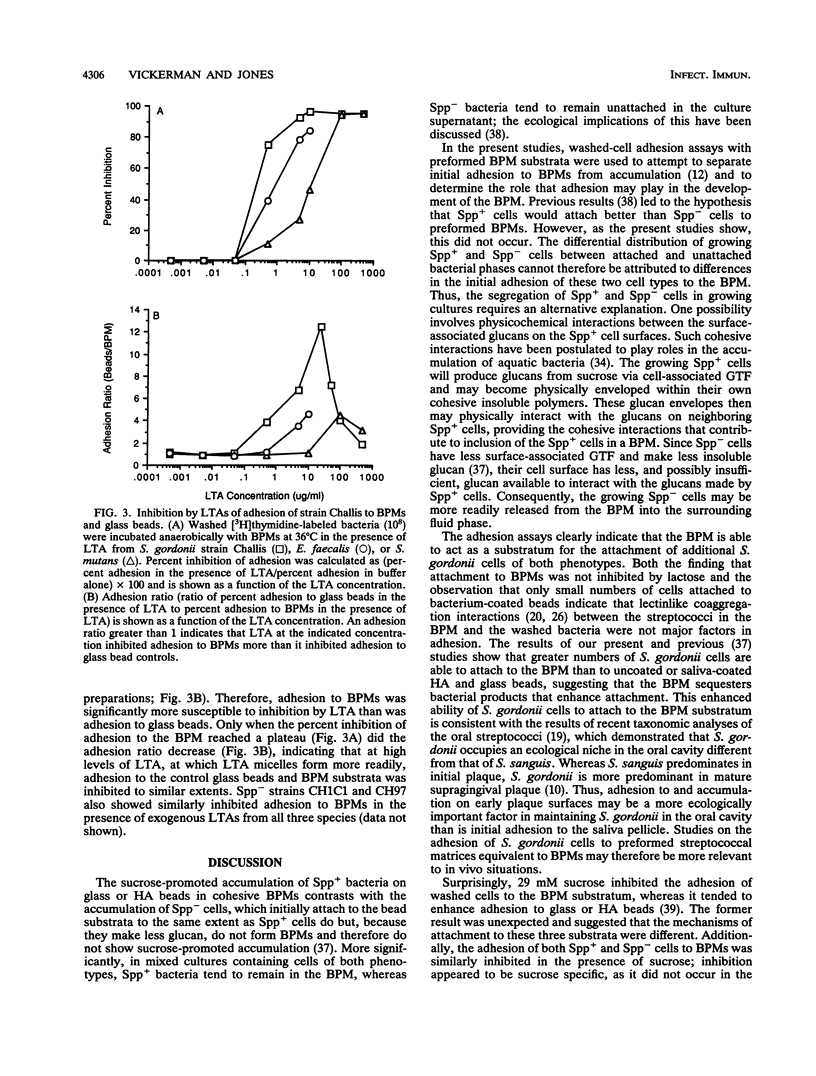
Growing Streptococcus gordonii Spp+ phase variants, which have normal levels of glucosyltransferase (GTF) activity, use sucrose to promote their accumulation on surfaces by forming a cohesive bacterium-insoluble glucan polymer mass (BPM). Spp- phase variants, which have lower levels of GTF activity, do not form BPMs and do not remain in BPMs formed by Spp+ cells when grown in mixed cultures. To test the hypothesis that segregation of attached Spp+ and unattached Spp- cells was due to differences in adhesiveness, adhesion between washed, [3H]thymidine-labeled cells and preformed BPM substrata was measured. Unexpectedly, the results showed that cells of both phenotypes, as well as GTF-negative cells, attached equally well to preformed BPMs, indicating that attachment to BPMs was independent of cell surface GTF activity. Initial characterization of this binding interaction suggested that a protease-sensitive component on the washed cells may be binding to lipoteichoic acids sequestered in the BPM, since exogenous lipoteichoic acid inhibited adhesion. Surprisingly, the adhesion of both Spp+ and Spp- cells was markedly inhibited in the presence of sucrose, which also released lipoteichoic acid from the BPM. These in vitro findings suggest that, in vivo, sucrose and lipoteichoic acid may modify dental plaque development by enhancing or inhibiting the attachment of additional bacteria.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appelbaum B., Golub E., Holt S. C., Rosan B. In vitro studies of dental plaque formation: adsorption of oral streptococci to hydroxyaptite. Infect Immun. 1979 Aug;25(2):717–728. doi: 10.1128/iai.25.2.717-728.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeau G., McBride B. C. Dextran-mediated interbacterial aggregation between dextran-synthesizing streptococci and Actinomyces viscosus. Infect Immun. 1976 Apr;13(4):1228–1234. doi: 10.1128/iai.13.4.1228-1234.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney H. S., Simpson W. A., Beachey E. H. Relationship of critical micelle concentrations of bacterial lipoteichoic acids to biological activities. Infect Immun. 1986 Feb;51(2):414–418. doi: 10.1128/iai.51.2.414-418.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan M. M., Parrish K., Kessler R. E., Pyle C., Jr, Taylor K. G., Ciardi J. E., Doyle R. J. Glucan-binding factor in saliva. Infect Immun. 1988 Nov;56(11):2912–2917. doi: 10.1128/iai.56.11.2912-2917.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Stoppelaar J. D., Van Houte J., Backer DIRKS O. The effect of carbohydrate restriction on the presence of Streptococcus mutans, Streptococcus sanguis and iodophilic polysaccharide-producing bacteria in human dental plaque. Caries Res. 1970;4(2):114–123. doi: 10.1159/000259633. [DOI] [PubMed] [Google Scholar]
- Drake D., Taylor K. G., Bleiweis A. S., Doyle R. J. Specificity of the glucan-binding lectin of Streptococcus cricetus. Infect Immun. 1988 Aug;56(8):1864–1872. doi: 10.1128/iai.56.8.1864-1872.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder B. L., Fives-Taylor P. Characterization of monoclonal antibodies specific for adhesion: isolation of an adhesin of Streptococcus sanguis FW213. Infect Immun. 1986 Nov;54(2):421–427. doi: 10.1128/iai.54.2.421-427.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferretti J. J., Gilpin M. L., Russell R. R. Nucleotide sequence of a glucosyltransferase gene from Streptococcus sobrinus MFe28. J Bacteriol. 1987 Sep;169(9):4271–4278. doi: 10.1128/jb.169.9.4271-4278.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganeshkumar N., Song M., McBride B. C. Cloning of a Streptococcus sanguis adhesin which mediates binding to saliva-coated hydroxyapatite. Infect Immun. 1988 May;56(5):1150–1157. doi: 10.1128/iai.56.5.1150-1157.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Cohen L., Hay D. I. Strains of Streptococcus mutans and Streptococcus sobrinus attach to different pellicle receptors. Infect Immun. 1986 May;52(2):555–561. doi: 10.1128/iai.52.2.555-561.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Hay D. I., Schlesinger D. H. Delineation of a segment of adsorbed salivary acidic proline-rich proteins which promotes adhesion of Streptococcus gordonii to apatitic surfaces. Infect Immun. 1991 Sep;59(9):2948–2954. doi: 10.1128/iai.59.9.2948-2954.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Houte J. V. Bacterial adherence in oral microbial ecology. Annu Rev Microbiol. 1975;29:19–44. doi: 10.1146/annurev.mi.29.100175.000315. [DOI] [PubMed] [Google Scholar]
- Haisman R. J., Jenkinson H. F. Mutants of Streptococcus gordonii Challis over-producing glucosyltransferase. J Gen Microbiol. 1991 Mar;137(3):483–489. doi: 10.1099/00221287-137-3-483. [DOI] [PubMed] [Google Scholar]
- Kessler R. E., Shockman G. D. Precursor-product relationship of intracellular and extracellular lipoteichoic acids of Streptococcus faecium. J Bacteriol. 1979 Feb;137(2):869–877. doi: 10.1128/jb.137.2.869-877.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R. E., Thivierge B. H. Effects of substitution on polyglycerol phosphate-specific antibody binding to lipoteichoic acids. Infect Immun. 1983 Aug;41(2):549–555. doi: 10.1128/iai.41.2.549-555.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolenbrander P. E. Intergeneric coaggregation among human oral bacteria and ecology of dental plaque. Annu Rev Microbiol. 1988;42:627–656. doi: 10.1146/annurev.mi.42.100188.003211. [DOI] [PubMed] [Google Scholar]
- Landale E. C., McCabe M. M. Characterization by affinity electrophoresis of an alpha-1,6-glucan-binding protein from Streptococcus sobrinus. Infect Immun. 1987 Dec;55(12):3011–3016. doi: 10.1128/iai.55.12.3011-3016.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesche W. J. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986 Dec;50(4):353–380. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrina F. L., Evans R. P., Tobian J. A., Hartley D. L., Clewell D. B., Jones K. R. Novel shuttle plasmid vehicles for Escherichia-Streptococcus transgeneric cloning. Gene. 1983 Nov;25(1):145–150. doi: 10.1016/0378-1119(83)90176-2. [DOI] [PubMed] [Google Scholar]
- Mattingly S. J., Johnston B. P. Comparative analysis of the localization of lipoteichoic acid in Streptococcus agalactiae and Streptococcus pyogenes. Infect Immun. 1987 Oct;55(10):2383–2386. doi: 10.1128/iai.55.10.2383-2386.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer R. M. Dextransucrase: a glucosyltransferase from Streptococcus sanguis. Methods Enzymol. 1987;138:649–661. doi: 10.1016/0076-6879(87)38059-0. [DOI] [PubMed] [Google Scholar]
- McIntire F. C., Vatter A. E., Baros J., Arnold J. Mechanism of coaggregation between Actinomyces viscosus T14V and Streptococcus sanguis 34. Infect Immun. 1978 Sep;21(3):978–988. doi: 10.1128/iai.21.3.978-988.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooser G., Wong C. Isolation of a glucan-binding domain of glucosyltransferase (1,6-alpha-glucan synthase) from Streptococcus sobrinus. Infect Immun. 1988 Apr;56(4):880–884. doi: 10.1128/iai.56.4.880-884.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray P. A., Prakobphol A., Lee T., Hoover C. I., Fisher S. J. Adherence of oral streptococci to salivary glycoproteins. Infect Immun. 1992 Jan;60(1):31–38. doi: 10.1128/iai.60.1.31-38.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosan B., Baker C. T., Nelson G. M., Berman R., Lamont R. J., Demuth D. R. Cloning and expression of an adhesin antigen of Streptococcus sanguis G9B in Escherichia coli. J Gen Microbiol. 1989 Mar;135(3):531–538. doi: 10.1099/00221287-135-3-531. [DOI] [PubMed] [Google Scholar]
- Russell R. R. Glucan-binding proteins of Streptococcus mutans serotype c. J Gen Microbiol. 1979 May;112(1):197–201. doi: 10.1099/00221287-112-1-197. [DOI] [PubMed] [Google Scholar]
- Rølla G., Oppermann R. V., Bowen W. H., Ciardi J. E., Knox K. W. High amounts of lipoteichoic acid in sucrose-induced plaque in vivo. Caries Res. 1980;14(4):235–238. doi: 10.1159/000260459. [DOI] [PubMed] [Google Scholar]
- Sutherland I. W. Microbial exopolysaccharides -- their role in microbial adhesion in aqueous systems. Crit Rev Microbiol. 1983;10(2):173–201. doi: 10.3109/10408418209113562. [DOI] [PubMed] [Google Scholar]
- Tardif G., Sulavik M. C., Jones G. W., Clewell D. B. Spontaneous switching of the sucrose-promoted colony phenotype in Streptococcus sanguis. Infect Immun. 1989 Dec;57(12):3945–3948. doi: 10.1128/iai.57.12.3945-3948.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terleckyj B., Willett N. P., Shockman G. D. Growth of several cariogenic strains of oral streptococci in a chemically defined medium. Infect Immun. 1975 Apr;11(4):649–655. doi: 10.1128/iai.11.4.649-655.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickerman M. M., Clewell D. B., Jones G. W. Ecological implications of glucosyltransferase phase variation in Streptococcus gordonii. Appl Environ Microbiol. 1991 Dec;57(12):3648–3651. doi: 10.1128/aem.57.12.3648-3651.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickerman M. M., Clewell D. B., Jones G. W. Glucosyltransferase phase variation in Streptococcus gordonii modifies adhesion to saliva-coated hydroxyapatite surfaces in a sucrose-independent manner. Oral Microbiol Immunol. 1992 Apr;7(2):118–120. doi: 10.1111/j.1399-302x.1992.tb00521.x. [DOI] [PubMed] [Google Scholar]
- Vickerman M. M., Clewell D. B., Jones G. W. Sucrose-promoted accumulation of growing glucosyltransferase variants of Streptococcus gordonii on hydroxyapatite surfaces. Infect Immun. 1991 Oct;59(10):3523–3530. doi: 10.1128/iai.59.10.3523-3530.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicken A. J., Evans J. D., Knox K. W. Critical micelle concentrations of lipoteichoic acids. J Bacteriol. 1986 Apr;166(1):72–77. doi: 10.1128/jb.166.1.72-77.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson S. G. Glycosyl diglycerides from Pseudomonas rubescens. Biochim Biophys Acta. 1968 Oct 22;164(2):148–156. doi: 10.1016/0005-2760(68)90141-0. [DOI] [PubMed] [Google Scholar]
- YEMM E. W., WILLIS A. J. The estimation of carbohydrates in plant extracts by anthrone. Biochem J. 1954 Jul;57(3):508–514. doi: 10.1042/bj0570508. [DOI] [PMC free article] [PubMed] [Google Scholar]


