Abstract
Background
It is unknown whether patients with nonleukemic myeloid sarcoma (MS) and those with acute myeloid leukemia (AML) have similar responses to anti-AML treatment. We addressed this question by matching MS patients with analogous AML patients and comparing their clinical outcomes.
Methods
We identified 23 consecutive MS and 1720 consecutive AML patients who presented at The University of Texas M. D. Anderson Cancer Center from 1990 to 2004. All AML patients and 16 MS patients received cytarabine plus idarubicin or fludarabine as induction remission therapy. We matched treated MS and AML patients according to cytogenetics, age, Zubrod performance status, and time of treatment. Event-free survival (EFS) and overall survival (OS) were compared using Kaplan-Meier analyses.
Results
Complete response rates were 69% in MS and 57% in AML (p=0.45). The respective 2-year EFS and OS rates were 32% and 18% (p=0.08) and 43% and 29% (p=0.11). Matches could be found for 14 MS patients, who were paired repeatedly with 91 AML patients to produce 94 matches (3 AML patients were matched twice). EFS was longer in 56 MS pair-mates, shorter in 26, and similar in 12 (p=0.01, Fisher exact test). OS analyses gave similar results.
Conclusions
Anti-AML therapy is highly effective in patients with non-leukemic MS. This study emphasizes the need to treat patients with non-leukemic MS with AML-type therapy.
Keywords: sarcoma, myeloid, chloroma, AML, therapy
INTRODUCTION
The term myeloid sarcoma (MS) is used to define an extramedullary mass composed of cells of myeloid lineage. Other terms used as synonyms for this process are chloroma, granulocytic sarcoma, myeloblastoma, and extramedullary myeloid cell tumor.1-4 The 2001 World Health Organization classification currently recommends the term MS for this disease.5 Patients with MS most often have evidence of concurrent acute myeloid leukemia (AML) involving blood and bone marrow or only bone marrow. Patients also may have a history of AML and relapse after therapy. Less commonly, myelodysplastic syndrome or chronic myeloproliferative disease can transform to MS. Very rarely, patients present with MS as an isolated mass, with no evidence of AML after extensive workup.6 MS can be characterized by granulocytic, monoblastic, or myelomonocytic differentiation and is often associated with distinctive cytogenetic and molecular abnormalities.7
One option for patients who present in such a fashion is anti-AML therapy, as if the patient had typical AML.8 However, there is little information comparing the effects of anti-AML treatment for MS patients without AML in the bone marrow with those for patients with typical AML. Here we address this question by matching MS patients with comparable AML patients according to age, performance status, year of treatment, and to the extent possible, cytogenetics.
PATIENTS AND METHODS
We searched The University of Texas M. D. Anderson Cancer Center leukemia database from 1990 to 2004 for patients who met the following three criteria: pathologically confirmed extramedullary MS, fewer than 5% bone marrow blasts, and no history of AML. Twenty-three patients with MS were identified. In 22 of 23 cases, markers were assessed to prove myeloid lineage.6 In one case, cytochemical studies were performed on touch imprints. In a second case, histochemical study for chloroacetate esterase was performed using histologic sections. In the remaining 20 cases, immunohistochemical analysis was performed using fixed, paraffin-embedded tissue sections and, in some of the earlier cases, frozen tissue sections. In the group assessed immunohistochemically, histochemical analysis for chloroacetate esterase was also performed in 7 cases, and flow cytometry immunophenotyping was performed in 3 cases. Sixteen of the 23 patients with MS received cytarabine plus idarubicin or fludarabine at M. D. Anderson Cancer Center, as previously described.9 Two patients had surgical resection only, while 5 were treated outside of M. D. Anderson. The 1720 patients with AML in the bone marrow (> 20% blasts and excluding patients with acute promyelocytic leukemia) presented during the same time period and received similar induction therapy; however, MS patients were less likely to receive post-complete response (CR) therapy (Table 1). All patients gave informed consent for treatment, which was carried out in accordance with the Declaration of Helsinki; the M. D. Anderson IRB approved the analyses described below.
Table 1.
Patient Characteristics
| Myeloid sarcoma N=23 (%) |
Acute myeloid leukemia N=1720 (%) |
|
|---|---|---|
| Age | ||
| Median | 57 | 60 |
| Range | 7-81 | 14-89 |
| Zubrod PS | ||
| 0 | 8 (35) | 158 (9) |
| 1 | 13 (57) | 1006 (58) |
| 2 | 1 (4) | 383 (22) |
| 3 | 1 (4) | 112 (7) |
| 4 | 0 (0) | 61 (4) |
| Cytogenetics | ||
| Inv 16 or t(8;21) | 2 (9) | 138 (8) |
| Normal | 11 (48) | 640 (37) |
| +8 | 5 (22)* | 127 (7) |
| -5, -7 | 1 (4) | 372 (22) |
| Abnormal 11 q or other ** | 3 (11) | 358 (21) |
| Insufficient | 0 | 76 (4) |
| Not done | 0 | 9 (0.005) |
| AHD | 3 (13) | 688 (40) |
| WBC count | ||
| Median | 6.7 | 10.1 |
| Range | 1.3 - 70.6 | 0.2 - 394 |
| Hemoglobin | ||
| Median | 13.5 | 8.0 |
| Range | 4.8 - 16.1 | 2.1 - 15.0 |
| Platelets | ||
| Median | 246 | 48 |
| Range | 110 - 534 | 2 - 2292 |
| Post-remission therapy | ||
| Idarubicin+AraC, % | 40 | 44 |
| Low-dose AraC, % | 8 | 0 |
| None, % | 24 | 0 |
| Other, % | 8 | 10 |
| Fludarabine+AraC, % | 8 | 0 |
| Topotecan+AraC +/- Cyclophosphamide, % | 8 | 14 |
| Stem cell transplantation, % | 0 | 1 |
| Therapy at 1st relapse | ||
| High-dose AraC, % | 25 | 7 |
| Fludarabine+AraC+Topotecan, % | 25 | 0 |
| Fludarabine+AraC+Idarubicin, % | 0 | 20 |
| Idarubicin+AraC, % | 25 | 17 |
| Topotecan+AraC+/- Cyclophosphamide, % | 0 | 12 |
| Decitabine + 5-azacytidine, % | 0 | 3 |
| Stem cell transplantation, % | 0 | 9 |
| Other, % | 25 | 27 |
| Unknown, % | 0 | 5 |
AHD = Antecedent hematologic disorder; PS = performance status; WBC = white blood cells
One additional patient had 8q deletion.
These chromosomal abnormalities were del12 (1 patient) and dup1 (1 patient).
Statistical methods
The Fisher exact test was used in univariate analyses. Survival curves were estimated using the Kaplan-Meier method, and survival between groups was compared using the two-sided log-rank test. The multivariate Cox proportional hazards regression model was used to examine risk factors related to survival or EFS after adjusting for other factors, including diagnosis (MS vs. AML).
We were principally interested in event-free survival (EFS) (with an “event” defined as relapse, death, or failure to achieve CR) and overall survival (OS). For patients with AML, the criteria for a CR were as defined previously, whereas the criterion for a CR for patients with MS was complete radiologic disappearance of disease. Differences in EFS and OS between patients with MS and AML were quantified with the log-rank test. To reduce the possibility that any differences merely reflected a better inherent prognosis in the MS (or the AML) group, we attempted to find as many prognostically comparable matches (“pair-mates”) as possible for each MS patient from among the 1720 patients with AML. Criteria for matching were as follows: cytogenetics, as described below; age (within ± 3 years); Zubrod performance status (0-2 vs. >2); and time of treatment (1990-1997 vs. 1998-2004). Matches fell into three categories: (a) an event had occurred in the MS patient and the patient’s AML pair-mate, (b) an event had occurred in either the patient or the pair-mate, or (c) an event had occurred in neither the patient nor the pair-mate. If, in cases (a) and (b), EFS was longer in the patient with MS than in the AML pair-mate, the patient with MS was considered to be the “winner,” whereas if EFS was longer in the AML pair-mate, the MS patient was considered to be the “loser”. Cases in category (c) were considered “ties.” The numbers of wins and losses for patients with MS were summed. If EFS in patients with MS and AML was equivalent, the number of wins would be expected to equal the number of losses.
OS and EFS in matched MS and AML patients were compared using Kaplan-Meier methodology. Statistical analyses were carried out using S Plus 2000 (Insightful Corp., Seattle, WA). P values were derived from two-sided tests and were significant if <.05.
RESULTS
The median age of the 23 patients with MS was 57 years (range, 7-81 years); 1 patient (4%) had a Zubrod performance status greater than 2 (Table 1). The biopsy specimens were obtained from the skin (n=10), lymph node (n=5), dura (n=2), breast + skin (n=1), bladder (n=1), widespread involvement of the gynecologic tract (n=1), pleura + chest wall (n=1), retroperitoneum (n=1), and small intestine (n=1). In each case, histological findings were consistent with the diagnosis of MS. The antibodies used for immunohistochemical analysis were highly variable, but in all cases the neoplastic cells were positive for one or more myeloid antigens and were negative for T- and B-cell antigens. Myeloperoxidase was positive in 13/14 cases, lysozyme in 7/8, CD13 in 5/5, CD33 in 4/4, CD34 in 6/8, CD68 in 5/7, and CD43 in 4/4. Six of 9 cases assessed for chloroacetate esterase were positive, including two cases that were not assessed by immunohistochemical analysis. The one neoplasm in this study not assessed by either immunohistochemistry of cytochemistry was histologically well-differentiated, with obvious eosinophilic differentiation.
Among the 9 MS patients older than 60, 1 had cytogenetics assessed in the tumor sample (this patient had a 12p deletion), and 4 had a +8 abnormality in the bone marrow despite no excess blasts. The remaining 4 older MS patients had normal bone marrow cytogenetics but, of course, no excess blasts. Three of the 14 MS patients younger than 60 had cytogenetics assessed in the tumor sample: 1 had an 11q deletion in a complex karyotype, 1 had a deletion of 3 in a complex karyotype, and 1 had an 8q deletion. An additional 4 MS patients younger than 60 had cytogenetic abnormalities in the bone marrow, despite the absence of excess blasts: inv(16) in 2 patients, +8 in 1, and -7 in 1. The remaining 8 MS patients younger than 60 had normal cytogenetics in the bone marrow.
The median age of the 1720 patients with AML was 60 years (range, 14-89 years), and 173 patients (11%) had a Zubrod performance status of 3 or 4 (Table 1). The proportion of patients with a +8 abnormality was much lower in the AML group than in the MS group (127 of 1720 vs. 5 of 23; Fisher exact, p = 0.02).
Response, Event-Free Survival, and Overall Survival
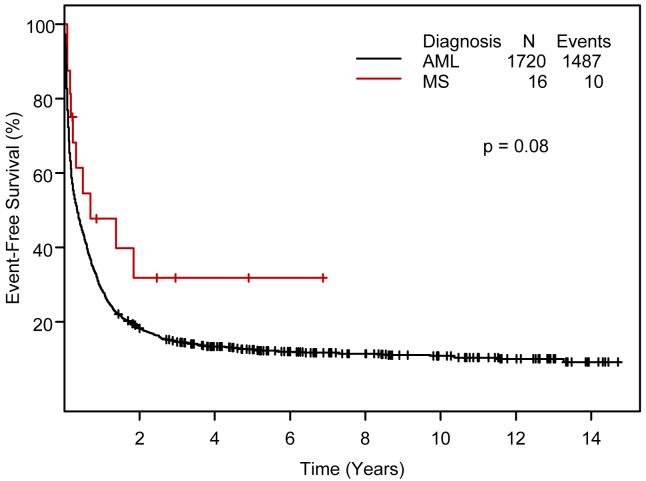
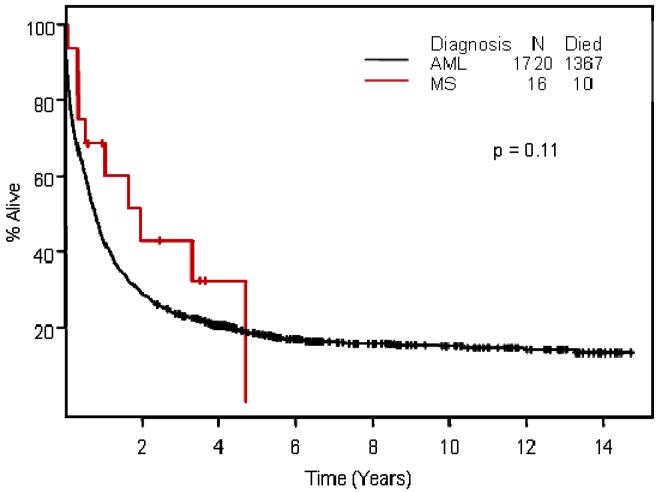
We focused on the 16 MS patients treated at M. D. Anderson with cytarabine combined with idarubicin or fludarabine. Eleven of these 16 patients (69%) entered CR, as did 57% of the similarly treated AML patients (p=0.45). Median follow-up times for patients remaining alive in CR were 3.5 years for MS and 5.3 years for AML. EFS was longer in MS (p=0.08;Figure 1); OS differences were less marked (p = 0.11, Figure 2).
Figure 1.
Comparison of event-free survival in patients with myeloid sarcoma and acute myeloid leukemia by Kaplan-Meier methodology
Figure 2.
Overall survival in patients with myeloid sarcoma and acute myeloid leukemia by Kaplan-Meier methodology
Multivariate Cox analysis in Ara-C—treated patients
We then performed a multivariate analysis of EFS in ara-C—treated patients (MS, 16; AML, 1720). Independent factors predicting shorter EFS were poorer risk cytogenetics (p<0.0001), worse performance status (p<0.0001), history of antecedent hematologic disorder (AHD; history of a hemoglobin level less than 12 g/dL, a platelet count less than 150,000/μL, a neutrophil count less than 1,500/μL, or a WBC count greater than 20,000/μL for at least 1 month before M. D. Anderson presentation) (p<0.0001), and higher leukocyte counts (p=0.001). Diagnosis of MS. vs. AML was not a significant factor (p=0.85).
Similarly, independent factors predicting shorter OS were poorer risk cytogenetics (p<0.0001), worse performance status (p<0.0001), history of AHD (p<0.0001), and higher leukocyte counts (p=0.01), but diagnosis was not significant (p=0.75).
Matching of MS with AML patients
Although cytogenetics is a major prognostic factor in AML, the 12 MS patients for whom tumor cytogenetics were not assessed and who had normal bone marrow cytogenetics were cytogenetically not informative. We addressed this problem as follows. Given that 4 of the 5 MS patients at least 60 years old with known cytogenetic abnormalities had +8 abnormalities (see above), we matched similarly aged MS patients in whom cytogenetics were not assessed in MS and with normal marrow with AML patients who had +8 abnormalities. Analogously, the distribution of cytogenetic abnormalities in MS patients younger than 60 for whom cytogenetics were evaluated in MS or known to be abnormal in bone marrow (2 prognostically favorable [inv 16], 2 prognostically intermediate [+8, 8q-], and 3 prognostically unfavorable [11q, complex del 3q , -7]) led us to attempt to pair each MS patient younger than 60 with normal bone marrow cytogenetics and unknown MS cytogenetics with an equal number of AML patients with favorable, intermediate, and unfavorable cytogenetics, as generally considered.10
Details regarding matching of MS and AML patients are shown in Table 2. Matches were found for 14 of the 16 patients with MS. One MS patient had no AML match because he was 7 years old (all the AML patients in our database were older than 14). The second MS patient had a performance status of 4, for which there was no match among the AML patients.
Table 2.
Matching of Patients with Myeloid Sarcoma with Patients with Acute Myeloid Leukemia Treated with Cytarabine-Containing Therapies
| Patient | Cytogenetics | Age | Zubrod PS |
Year of Treatment |
Event-free Survival (weeks) |
Overall Survival (weeks) |
|---|---|---|---|---|---|---|
| MS1 | +8 | 64 | 2 | 1990 | 8 | 18 |
| M1 for MS1 | +8 | 67 | 1 | 1991 | 2 | 2 |
| M2 for MS1 | +8 | 67 | 1 | 1991 | 5 | 5 |
| M3 for MS1 | +8 | 67 | 1 | 1992 | 19 | 34 |
| M4 for MS1 | +8 | 65 | 2 | 1994 | 9 | 9 |
| M5 for MS1 | +8 | 65 | 0 | 1994 | 45 | 68 |
| M6 for MS1 | +8 | 66 | 1 | 1995 | 44 | 60 |
| M7 for MS1 | +8 | 62 | 0 | 1995 | 10 | 10 |
| M8 for MS1 | +8 | 61 | 1 | 1997 | 39 | 43 |
| M9 for MS1 | +8 | 64 | 1 | 1997 | 12 | 12 |
| M10 for MS1 * | +8 | 67 | 2 | 1996 | 3 | 3 |
| M11 for MS1 ** | +8 | 67 | 1 | 1996 | 6 | 178 |
| MS2 | Normal | 53 | 1 | 1991 | 16 | 22 |
| M1 for MS2 | 11Q | 52 | 1 | 1991 | 5 | 5 |
| M2 for MS2 | INV 16 | 50 | 0 | 1994 | 572 | 572+ |
| MS3 | +8 | 57 | 0 | 1991 | 26 | 42+ |
| M1 for MS3 | +8 | 58 | 1 | 1995 | 545 | 545+ |
| M2 for MS3 | +8 | 58 | 2 | 1995 | 63 | 127 |
| M3 for MS3 | +8 | 59 | 2 | 1996 | 159 | 263 |
| M4 for MS3 | +8 | 57 | 1 | 1998 | 47 | 65 |
| MS4 | +8 | 70 | 1 | 1992 | 9 | 9+ |
| M1 for MS4 | +8 | 70 | 1 | 1993 | 655 | 655+ |
| M2 for MS4 | +8 | 71 | 1 | 1995 | 0 | 0 |
| M3 for MS4 | +8 | 69 | 1 | 1993 | 183 | 258 |
| M4 for MS4 | +8 | 68 | 1 | 1993 | 31 | 40 |
| M5 for MS4 | +8 | 73 | 1 | 1995 | 29 | 51 |
| M6 for MS4 | +8 | 72 | 0 | 1996 | 68 | 85 |
| M7 for MS4 | +8 | 73 | 2 | 1997 | 2 | 2 |
| M8 for MS4 | +8 | 68 | 1 | 1998 | 10 | 25 |
| M9 for MS4 | +8 | 73 | 2 | 1998 | 6 | 223 |
| M10 for MS4 | +8 | 68 | 1 | 1999 | 128 | 168+ |
| M11 for MS4 | +8 | 72 | 2 | 1999 | 4 | 4 |
| M12 for MS4 * | +8 | 67 | 2 | 1996 | 3 | 3 |
| M13 for MS4 ** | +8 | 67 | 1 | 1996 | 6 | 178 |
| MS5 | Normal | 45 | 0 | 1994 | 36 | 36+ |
| M1 for MS5 | 11Q | 46 | 1 | 1998 | 3 | 58 |
| M2 for MS5 | T(8,21) | 45 | 1 | 1996 | 6 | 6 |
| M3 for MS5 | +8 | 46 | 1 | 1999 | 3 | 3 |
| MS6 | Normal | 31 | 1 | 1994 | 350 | 350+ |
| M1 for MS6 | T(8,21) | 30 | 1 | 1996 | 105 | 170 |
| M2 for MS6 | 11Q | 30 | 1 | 1997 | 15 | 15 |
| M3 for MS6 | +8 | 34 | 1 | 1998 | 5 | 50 |
| MS7 | DEL 12 | 69 | 0 | 1995 | 71 | 71 |
| M1 for MS7 | MISC | 67 | 1 | 1999 | 148 | 323 |
| M2 for MS7 | MISC | 72 | 1 | 1996 | 103 | 272 |
| M3 for MS7 | MISC | 71 | 1 | 1996 | 3 | 3 |
| M4 for MS7 | MISC | 69 | 1 | 1997 | 5 | 5 |
| M5 for MS7 | MISC | 68 | 2 | 1997 | 3 | 3 |
| M6 for MS7 | MISC | 72 | 0 | 1997 | 37 | 64 |
| M7 for MS7 | MISC | 72 | 1 | 1998 | 6 | 51 |
| M8 for MS7 | MISC | 68 | 2 | 1999 | 5 | 43 |
| M9 for MS7 | MISC | 70 | 1 | 1999 | 23 | 23 |
| M10 for MS7 | MISC | 70 | 2 | 1999 | 1 | 1 |
| M11 for MS7 | MISC | 72 | 2 | 2000 | 168 | 168 |
| M12 for MS7 | MISC | 67 | 2 | 2000 | 22 | 24 |
| M13 for MS7 | MISC | 69 | 1 | 2000 | 3 | 35 |
| M14 for MS7 | MISC | 66 | 1 | 2000 | 3 | 29 |
| M15 for MS7 | MISC | 69 | 1 | 2000 | 4 | 4 |
| M16 for MS7 | MISC | 71 | 1 | 2002 | 7 | 24 |
| M17 for MS7 | MISC | 68 | 1 | 2001 | 12 | 24 |
| M18 for MS7 | MISC | 67 | 0 | 2002 | 166 | 166+ |
| M19 for MS7 | MISC | 66 | 1 | 2002 | 8 | 21 |
| M20 for MS7 | MISC | 70 | 1 | 2002 | 9 | 25 |
| M21 for MS7 | MISC | 68 | 1 | 2002 | 31 | 161+ |
| MS8 | INV 16 | 57 | 0 | 1995 | 357+ | 357+ |
| M1 for MS8 | INV 16 | 55 | 1 | 2001 | 245+ | 245+ |
| M2 for MS8 | T(8,21) | 56 | 1 | 2001 | 182+ | 182+ |
| M3 for MS8 | INV 16 | 59 | 1 | 2002 | 79 | 147+ |
| MS9 | Normal | 48 | 1 | 1995 | 255+ | 255+ |
| M1 for MS9 | 11Q | 49 | 1 | 1998 | 23 | 23 |
| M2 for MS9 | +8 | 50 | 1 | 1998 | 61 | 63 |
| M3 for MS9 | T(8,21) | 51 | 1 | 2001 | 46 | 46 |
| MS10 | Normal | 59 | 1 | 1997 | 4 | 4 |
| M1 for MS10 | INV 16 | 59 | 1 | 2003 | 125 | 125+ |
| M2 for MS10 *** | +8 | 59 | 0 | 2003 | 42 | 90 |
| MS11 | Normal | 60 | 0 | 1998 | 154+ | 155+ |
| M1 for MS11 | +8 | 62 | 1 | 2003 | 4 | 4 |
| M2 for MS11 | +8 | 57 | 2 | 2001 | 8 | 8 |
| M3 for MS11 | +8 | 57 | 1 | 2002 | 92 | 134+ |
| M4 for MS11 *** | +8 | 59 | 0 | 2003 | 42 | 90 |
| MS12 | Normal | 25 | 1 | 1998 | 44+ | 45+ |
| M1 for MS12 | 11Q | 25 | 1 | 1995 | 529+ | 529+ |
| M2 for MS12 | INV 16 | 23 | 1 | 2002 | 59 | 117 |
| MS13 | INV 16 | 47 | 0 | 1998 | 128+ | 128+ |
| M1 for MS13 | INV 16 | 44 | 2 | 2001 | 27 | 81 |
| M2 for MS13 | INV 16 | 44 | 1 | 2001 | 216+ | 216+ |
| M3 for MS13 | INV 16 | 49 | 0 | 2001 | 201+ | 201+ |
| M4 for MS13 | INV 16 | 44 | 1 | 2002 | 156+ | 156+ |
| M5 for MS13 | T(8,21) | 47 | 1 | 2002 | 37 | 85 |
| M6 for MS13 | T(8,21) | 47 | 0 | 2004 | 38+ | 38+ |
| M7 for MS13 | T(8,21) | 48 | 2 | 2004 | 66 | 118+ |
| M8 for MS13 | T(8,21) | 49 | 1 | 2001 | 334+ | 334+ |
| M9 for MS13 | T(8,21) | 50 | 1 | 2001 | 81 | 222+ |
| M10 for MS13 | T(8,21) | 50 | 1 | 2002 | 18 | 71 |
| M11 for MS13 | T(8,21) | 50 | 1 | 2003 | 121+ | 121+ |
| MS14 | Normal | 71 | 1 | 2000 | 12 | 17 |
| M1 for MS14 | +8 | 69 | 1 | 2003 | 12 | 77 |
| M2 for MS14 | +8 | 69 | 2 | 2000 | 3 | 5 |
| M3 for MS14 | +8 | 71 | 1 | 2001 | 4 | 44 |
| M4 for MS14 | +8 | 74 | 1 | 2001 | 3 | 4 |
| M5 for MS14 | +8 | 69 | 0 | 2003 | 6 | 12 |
| M6 for MS14 | +8 | 70 | 1 | 2001 | 33 | 64 |
| M7 for MS14 | +8 | 74 | 0 | 2002 | 4 | 18 |
| M8 for MS14 | +8 | 72 | 1 | 2002 | 20 | 20 |
| M9 for MS14 | +8 | 69 | 1 | 2003 | 12 | 37 |
| M10 for MS14 | +8 | 73 | 2 | 2003 | 2 | 2 |
| M11 for MS14 | +8 | 68 | 0 | 2003 | 6 | 86 |
| M12 for MS14 | +8 | 74 | 1 | 2004 | 4 | 11 |
Abbreviations: MS=myeloid sarcoma, M = match (with acute myeloid leukemia)
This AML patient matches two different MS patients.
This AML patient matches two different MS patients.
This AML patient matches two different MS patients.
Ninety-four matches, representing 91 patients with AML, were found, with 3 AML patients each found to be matches for 2 separate MS patients. For 3 MS patients younger than 60, only AML patients with favorable and intermediate cytogenetics could be found as matches; the absence of an unfavorable AML match would presumably tend to make the MS group appear less favorable. Among the 11 AML matches for the first MS patient (MS1), 7 had longer EFS and 4 had shorter EFS compared with MS1 (Table 2). Proceeding in this fashion, we found 56 matches favoring MS, 26 favoring AML, and 12 inconclusive matches (Table 3). If EFS duration in MS and AML was identical, one would expect MS cases to have an equal number of “wins” and “losses,” i.e., 47 wins and 47 losses. Using the Fisher exact test comparing 56 of 82 and 47 of 94, the p-value was 0.01.
Table 3.
Event-free Survival in Patients with Myeloid Sarcoma and Matched Patients with Acute Myeloid Leukemia
| MS Patient |
Number of matches |
Matches in which EFS was longer in MS patient |
Matches in which EFS was longer in AML patient |
Inconclusive match (see text) |
Matches in which OS was longer in MS patient |
Matches in which OS was longer in AML patient |
Inconclusive match (see text) |
|---|---|---|---|---|---|---|---|
| 1 | 11 | 4 | 7 | 0 | 6 | 5 | 0 |
| 2 | 2 | 1 | 1 | 0 | 1 | 1 | 0 |
| 3 | 4 | 0 | 4 | 0 | 0 | 0 | 4 |
| 4 | 13 | 7 | 6 | 0 | 4 | 0 | 9 |
| 5 | 3 | 3 | 0 | 0 | 2 | 0 | 1 |
| 6 | 3 | 3 | 0 | 0 | 3 | 0 | 0 |
| 7 | 21 | 17 | 4 | 0 | 16 | 5 | 0 |
| 8 | 3 | 1 | 0 | 2 | 0 | 0 | 3 |
| 9 | 3 | 3 | 0 | 0 | 3 | 0 | 0 |
| 10 | 2 | 0 | 2 | 0 | 0 | 2 | 0 |
| 11 | 4 | 4 | 0 | 0 | 3 | 0 | 1 |
| 12 | 2 | 0 | 0 | 2 | 0 | 0 | 2 |
| 13 | 11 | 5 | 0 | 6 | 3 | 0 | 8 |
| 14 | 12 | 8 | 2 | 2 | 5 | 7 | 0 |
| Totals | 94 | 56 | 26 | 12 | 46 | 20 | 28 |
When analysis was limited to MS patients with informative cytogenetics (patients MS1, MS3, MS4, MS7, MS8, and MS13), the observed 32 wins for MS vs. 26 wins for AML, with 5 ties, translated into a 71% probability of longer EFS in patients with MS.
When OS was similarly compared in matched patients, 46 matches favored MS, 20 favored AML, and 28 were inconclusive (Tables 2 and 3). Using the Fisher exact test comparing 46 of 66 and 47 of 94, the p-value was 0.01.
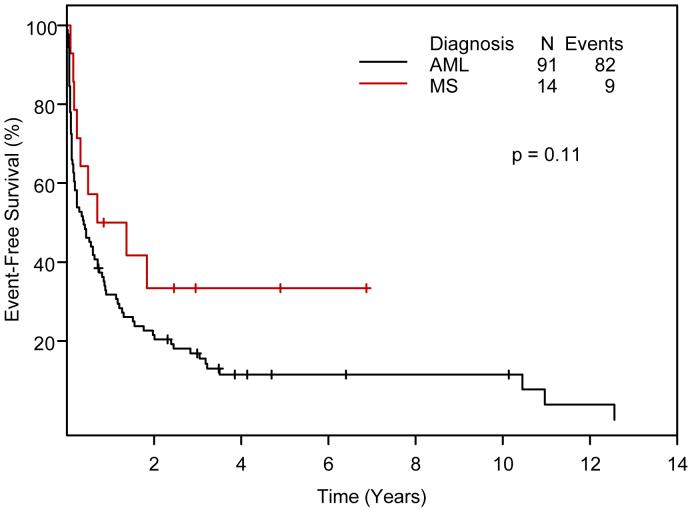
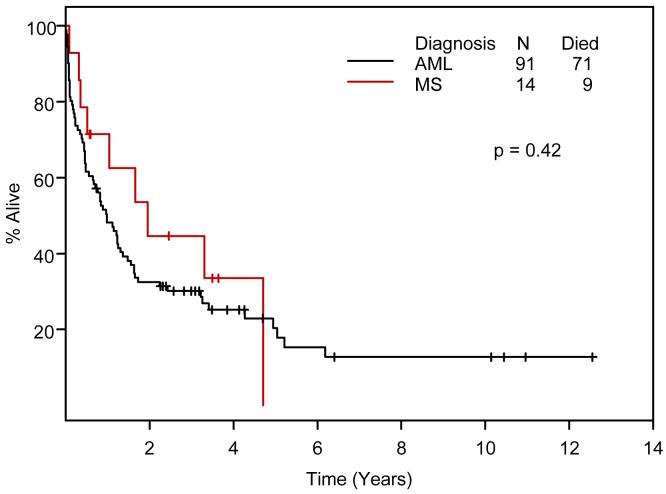
EFS and OS of 14 MS patients and 91 AML control patients by Kaplan-Meier methodology are compared in Figures 3 and 4.
Figure 3.
Event-free survival in 14 Ara-C-treated patients with myeloid sarcoma and 91 matched patients with acute myeloid leukemia (Kaplan-Meier).
Figure 4.
Overall survival in 14 Ara-C-treated patients with myeloid sarcoma and 91 matched patients with acute myeloid leukemia (Kaplan-Meier).
Outcomes in the 2 unmatched MS patients
At the time of this writing, the 7-year-old MS patient is alive in a first CR 11 months after beginning treatment, and the MS patient with Zubrod performance status 4 died 4 months into treatment without achieving a CR.
DISCUSSION
The current study demonstrates that anti-AML therapy is highly effective in patients with non-leukemic MS and is associated with higher rates of EFS and OS in MS than in AML after matching MS patients with comparable typical AML patients according to age, performance status, year of treatment, and to the extent possible, conventional cytogenetics.
This is the first study to suggest that EFS is longer in patients with MS than in patients with AML (Figure 1). However the data in Figure 1 do not address the issue of comparability between the MS and AML patients, i.e., how do we know that the results can be attributed primarily to a different diagnosis (MS vs. AML), rather than to differences in treatment, follow-up time, or prognostic covariates? Regarding treatment, patients with MS and patients with AML each received cytarabine-containing induction therapy, although the MS patients were less likely to receive post-remission therapy (Table 1). Follow-up was similar in the two groups, and median follow-up was > 3 years, which is relevant given previous suggestions that the risk of relapse declines 3 years after the CR date and such patients can be considered potentially cured.
The cytogenetically noninformative status (cytogenetics not done on MS samples and normal in bone marrow) of 8 of the 14 MS patients used in our matching analysis made it difficult to assess comparability with AML patients. It is, of course, possible that patients for whom cytogenetics were not evaluated in MS tissue and were normal in bone marrow were, in fact, cytogenetically normal in the MS tissue, i.e., these cases were cytogenetically informative. Arguing against this possibility is the incidence of cytogenetic abnormalities (4 of 4) in patients for whom cytogenetics were investigated in MS tissue. The possibility that MS patients with normal bone marrow cytogenetics would have been cytogenetically abnormal if MS tissue had been examined led us to match these patients with AML patients who had abnormal cytogenetics, with the AML patients chosen on the basis of the distribution of cytogenetic abnormalities observed in the informative MS patients.
The matching analysis suggested a 99% probability that EFS and OS are longer in MS patients, after accounting for age, performance status, year of treatment, and cytogenetics. Limiting our analysis to matches involving cytogenetically informative MS patients, we reached a qualitatively similar conclusion, i.e., a diagnosis of MS is associated per se with longer EFS. It remains possible that any conclusion is influenced by selection bias; specifically, only 16 of 23 MS patients received cytarabine-containing therapy, in contrast to >90% of the AML patients. More generally, our MS patients may not be representative of MS patients in the general population; for example, in the current study, the median age of our MS patients (57 years) was higher than the median age (37 years) in a previous review of 74 MS patients.11
The better outcomes of the MS patients in our study may be explained by earlier-stage disease, with less of a tumor load associated with infiltration of extramedullary sites by leukemic blasts compared with AML. It is also conceivable that the better outcomes simply reflect the fact that, even if untreated, MS patients may not die until they develop AML. This would indicate the presence of a lead-time advantage for the MS patients. Even if this is the case, we are not prepared to recommend that the treatment of MS patients be postponed until AML develops.
This study emphasizes the need to treat patients with non-leukemic MS with AML-type therapy. Patients with non-leukemic MS should be enrolled in clinical trials for AML and may benefit from risk-adapted therapies that account for age, performance status, and cytogenetics.
Acknowledgments
Grant support: E Estey: National Cancer Institute grants P01CA108631, P01CA55164-, U01HL069334, R01CA085843, R01CA092115, R01CA083932, P50CA100632, P50CA100632; AM Tsimberidou: American Society of Clinical Oncology: Career Development Award
References
- 1.Neiman RS, Barcos M, Berard C. Granulocytic sarcoma: a clinicopathologic study of 61 biopsied cases. Cancer. 1981;48:1426–1437. doi: 10.1002/1097-0142(19810915)48:6<1426::aid-cncr2820480626>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 2.Muss HB, Moloney WC. Chloroma and other myeloblastic tumors. Blood. 1973;42:721–728. [PubMed] [Google Scholar]
- 3.Menasce LP, Banerjee SS, Beckett E, Harris M. Extra-medullary myeloid tumour (granulocytic sarcoma) is often misdiagnosed: a study of 26 cases. Histopathology. 1999;34:391–398. doi: 10.1046/j.1365-2559.1999.00651.x. [DOI] [PubMed] [Google Scholar]
- 4.Davey FR, Olson S, Kurec AS, Eastman-Abaya R, Gottlieb AJ, Mason DY. The immunophenotyping of extramedullary myeloid cell tumors in paraffin-embedded tissue sections. Am J Surg Pathol. 1988;12:699–707. doi: 10.1097/00000478-198809000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Brunning RD, Matutes E, Flandrin G. Acute myeloid leukaemia not otherwise categorised. In: Jaffe ES, Harris NL, Stein H, Vardiman JW, editors. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of Heamatopoietic and Lymphoid Tissues. Lyon: IARC Press: 2001. pp. 91–105. [Google Scholar]
- 6.Roth MJ, Medeiros LJ, Elenitoba-Johnson K, Kuchnio M, Jaffe ES, Stetler-Stevenson M. Extramedullary myeloid cell tumors. An immunohistochemical study of 29 cases using routinely fixed and processed paraffin-embedded tissue sections. Arch Pathol Lab Med. 1995;119:790–798. [PubMed] [Google Scholar]
- 7.Pileri SA, Ascani S, Cox MC. Myeloid sarcoma: clinico-pathologic, phenotypic and cytogenetic analysis of 92 adult patients. Leukemia. 2007;21:340–350. doi: 10.1038/sj.leu.2404491. [DOI] [PubMed] [Google Scholar]
- 8.Tsimberidou AM, Kantarjian HM, Estey E. Outcome in patients with nonleukemic granulocytic sarcoma treated with chemotherapy with or without radiotherapy. Leukemia. 2003;17:1100–1103. doi: 10.1038/sj.leu.2402958. [DOI] [PubMed] [Google Scholar]
- 9.Estey E, de Lima M, Tibes R. Prospective feasibility analysis of reduced-intensity conditioning (RIC) regimens for hematopoietic stem cell transplantation (HSCT) in elderly patients with acute myeloid leukemia (AML) and high-risk myelodysplastic syndrome (MDS) Blood. 2007;109:1395–1400. doi: 10.1182/blood-2006-05-021907. [DOI] [PubMed] [Google Scholar]
- 10.Grimwade D, Walker H, Oliver F. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. The Medical Research Council Adult and Children’s Leukaemia Working Parties. Blood. 1998;92:2322–2333. [PubMed] [Google Scholar]
- 11.Yamauchi K, Yasuda M. Comparison in treatments of nonleukemic granulocytic sarcoma: report of two cases and a review of 72 cases in the literature. Cancer. 2002;94:1739–1746. doi: 10.1002/cncr.10399. [DOI] [PubMed] [Google Scholar]