Abstract
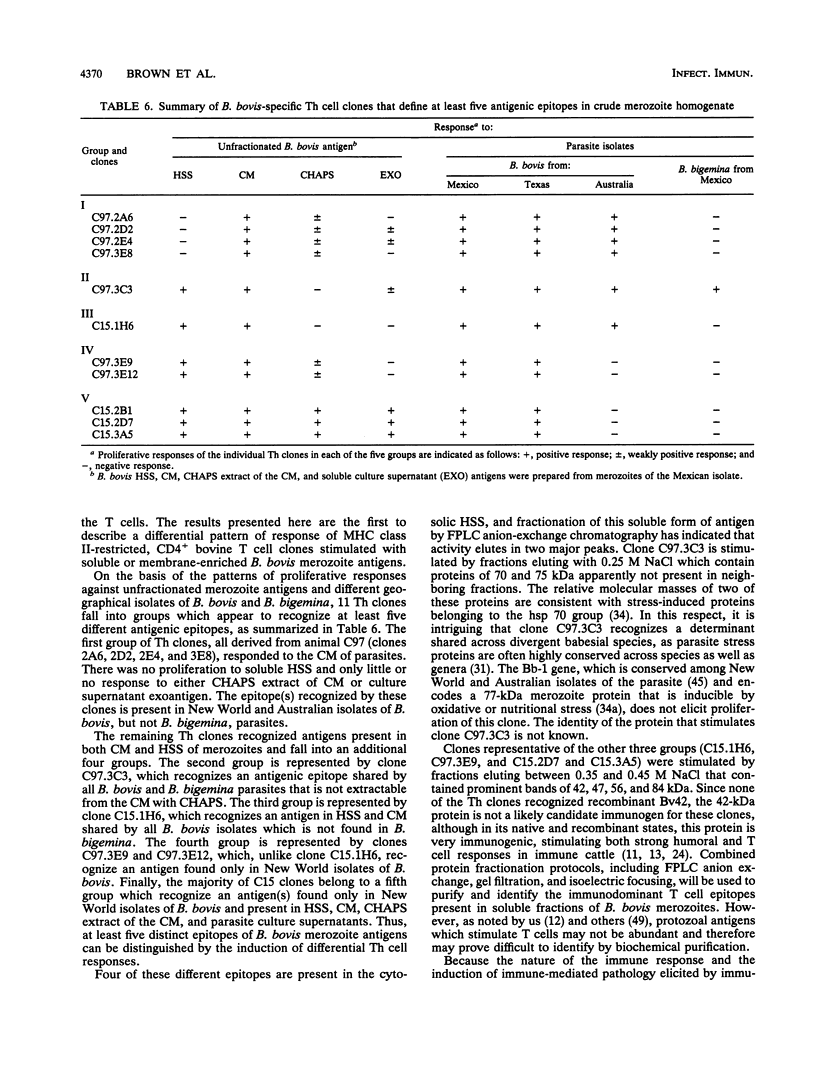
Helper T cell clones from two Babesia bovis-immune cattle were characterized for use in identification of potentially protective immunogens of B. bovis merozoites. Proliferation assays with 11 CD4+ clones revealed a differential pattern of response to soluble cytosolic antigen, membrane-enriched antigen, detergent extracts of the membrane-enriched antigen, soluble culture supernatant exoantigen, and different geographical isolates of B. bovis as well as Babesia bigemina parasites. When the data were combined, the clones could be grouped according to five different patterns of response. One group recognized only the membrane-enriched fraction of New World and Australian parasites. Four remaining groups recognized antigens found in the cytosolic as well as the membrane-enriched fraction, and clones representative of each group were used to identify cytosolic antigens fractionated by anion-exchange chromatography with the use of fast-performance liquid chromatography. One clone (C97.3C3), which responded to all B. bovis isolates and to B. bigemina, recognized a single peak of activity that eluted with 0.25 M NaCl and contained protein bands of 70 and 75 kDa. The remaining clones were stimulated by a second antigenic peak that eluted between 0.35 and 0.45 M NaCl and contained protein bands of 42, 47, 56, and 84 kDa. The majority of the clones produced interferon, whereas tumor necrosis factor alpha/tumor necrosis factor beta production was less frequent. These studies provide the basis for using helper T cell clones to identify potentially protective immunogens of B. bovis and delineate a minimum of five helper T cell epitopes recognized by two immune cattle.
Full text
PDF


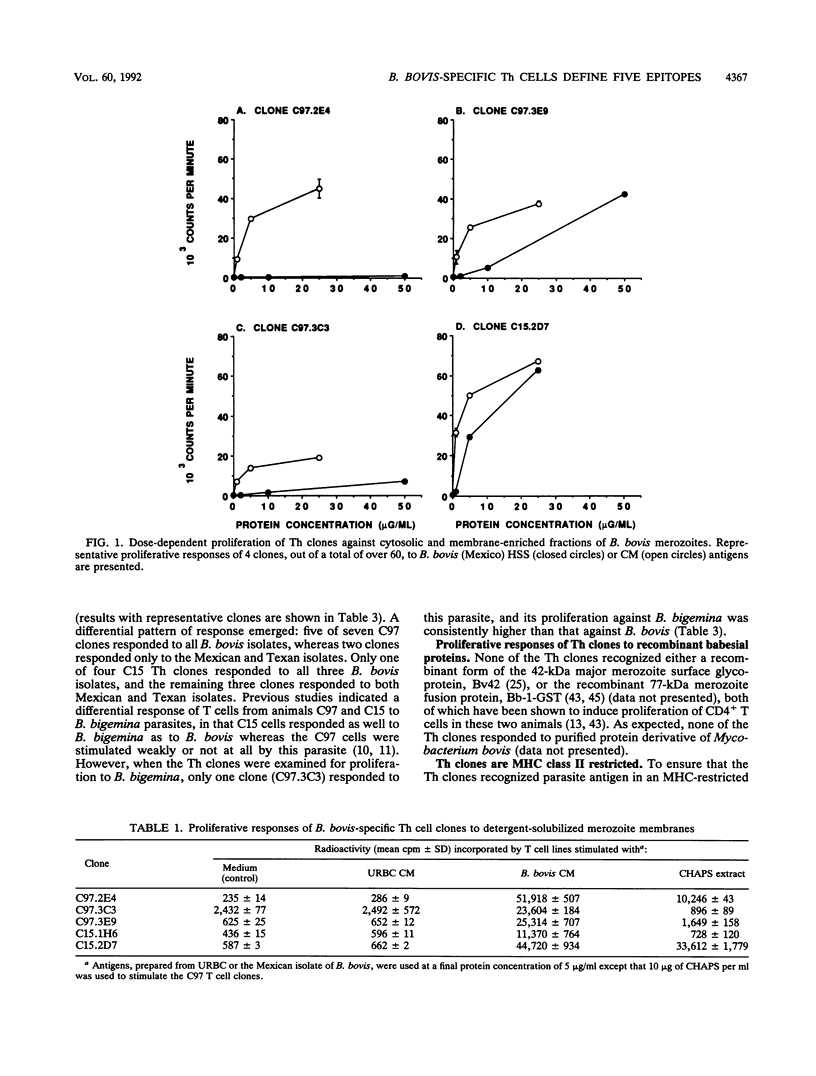
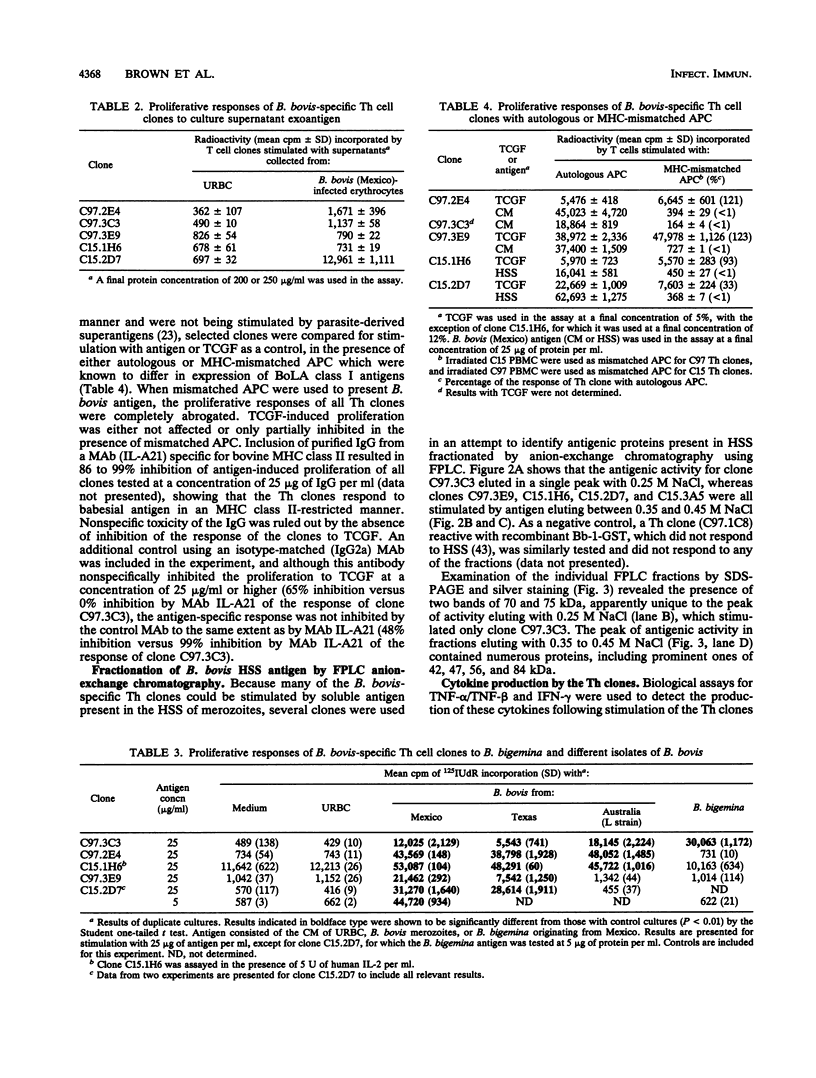
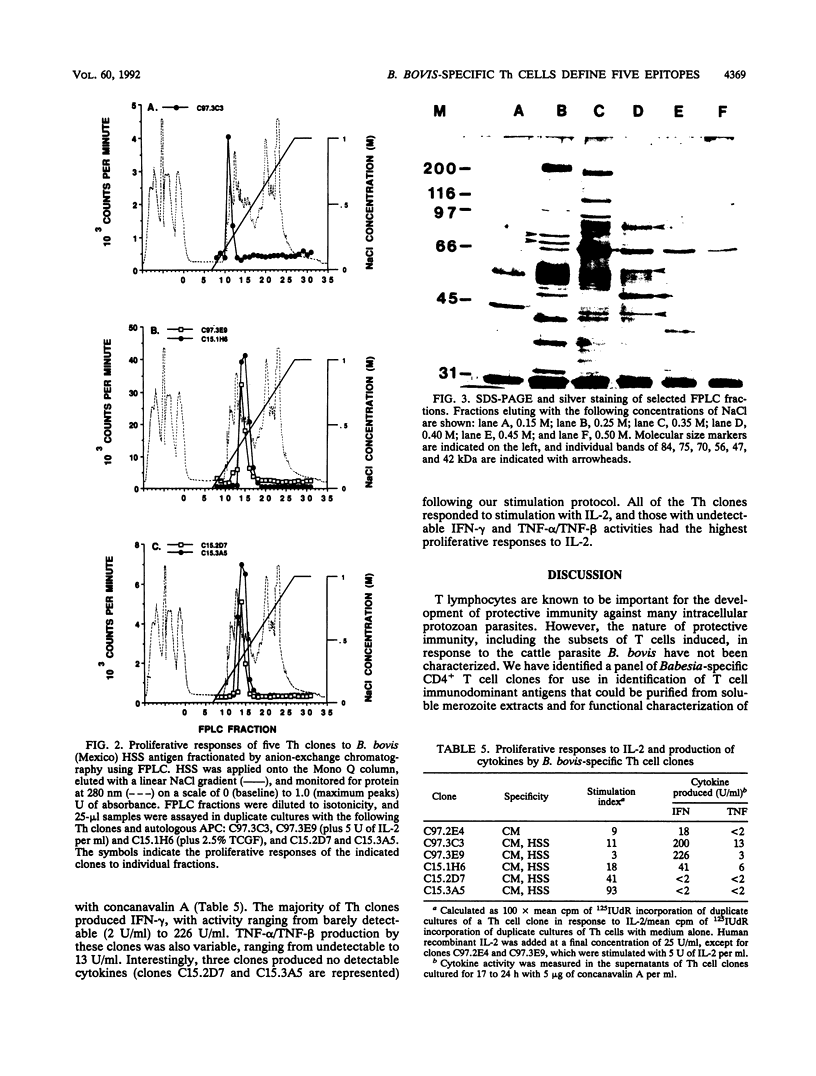


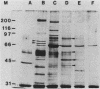
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arese P., Turrini F., Ginsburg H. Erythrophagocytosis in malaria: Host defence or menace to the macrophage? Parasitol Today. 1991 Jan;7(1):25–28. doi: 10.1016/0169-4758(91)90082-y. [DOI] [PubMed] [Google Scholar]
- Baldwin C. L., Machugh N. D., Ellis J. A., Naessens J., Newson J., Morrison W. I. Monoclonal antibodies which react with bovine T-lymphocyte antigens and induce blastogenesis: tissue distribution and functional characteristics of the target antigens. Immunology. 1988 Mar;63(3):439–446. [PMC free article] [PubMed] [Google Scholar]
- Bate C. A., Taverne J., Davé A., Playfair J. H. Malaria exoantigens induce T-independent antibody that blocks their ability to induce TNF. Immunology. 1990 Jul;70(3):315–320. [PMC free article] [PubMed] [Google Scholar]
- Bate C. A., Taverne J., Playfair J. H. Malarial parasites induce TNF production by macrophages. Immunology. 1988 Jun;64(2):227–231. [PMC free article] [PubMed] [Google Scholar]
- Bate C. A., Taverne J., Playfair J. H. Soluble malarial antigens are toxic and induce the production of tumour necrosis factor in vivo. Immunology. 1989 Apr;66(4):600–605. [PMC free article] [PubMed] [Google Scholar]
- Beutler B., Cerami A. Cachectin--Tumour necrosis factor: a cytokine that mediates injury initiated by invasive parasites. Parasitol Today. 1987 Nov;3(11):345–346. doi: 10.1016/0169-4758(87)90119-0. [DOI] [PubMed] [Google Scholar]
- Brake D. A., Long C. A., Weidanz W. P. Adoptive protection against Plasmodium chabaudi adami malaria in athymic nude mice by a cloned T cell line. J Immunol. 1988 Mar 15;140(6):1989–1993. [PubMed] [Google Scholar]
- Brown W. C., Grab D. J. Biological and biochemical characterization of bovine interleukin 2. Studies with cloned bovine T cells. J Immunol. 1985 Nov;135(5):3184–3190. [PubMed] [Google Scholar]
- Brown W. C., Logan K. S. Babesia bovis: bovine helper T cell lines reactive with soluble and membrane antigens of merozoites. Exp Parasitol. 1992 Mar;74(2):188–199. doi: 10.1016/0014-4894(92)90046-d. [DOI] [PubMed] [Google Scholar]
- Brown W. C., Logan K. S., Wagner G. G., Tetzlaff C. L. Cell-mediated immune responses to Babesia bovis merozoite antigens in cattle following infection with tick-derived or cultured parasites. Infect Immun. 1991 Jul;59(7):2418–2426. doi: 10.1128/iai.59.7.2418-2426.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W. C., Lonsdale-Eccles J. D., DeMartini J. C., Grab D. J. Recognition of soluble Theileria parva antigen by bovine helper T cell clones: characterization and partial purification of the antigen. J Immunol. 1990 Jan 1;144(1):271–277. [PubMed] [Google Scholar]
- Cavacini L. A., Parke L. A., Weidanz W. P. Resolution of acute malarial infections by T cell-dependent non-antibody-mediated mechanisms of immunity. Infect Immun. 1990 Sep;58(9):2946–2950. doi: 10.1128/iai.58.9.2946-2950.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark I. A., Allison A. C. Babesia microti and Plasmodium berghei yoelii infections in nude mice. Nature. 1974 Nov 22;252(5481):328–329. doi: 10.1038/252328a0. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Chaudhri G. Tumour necrosis factor may contribute to the anaemia of malaria by causing dyserythropoiesis and erythrophagocytosis. Br J Haematol. 1988 Sep;70(1):99–103. doi: 10.1111/j.1365-2141.1988.tb02440.x. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Cowden W. B., Butcher G. A., Hunt N. H. Possible roles of tumor necrosis factor in the pathology of malaria. Am J Pathol. 1987 Oct;129(1):192–199. [PMC free article] [PubMed] [Google Scholar]
- Ellis J. A., Baldwin C. L., MacHugh N. D., Bensaid A., Teale A. J., Goddeeris B. M., Morrison W. I. Characterization by a monoclonal antibody and functional analysis of a subset of bovine T lymphocytes that express BoT8, a molecule analogous to human CD8. Immunology. 1986 Jul;58(3):351–358. [PMC free article] [PubMed] [Google Scholar]
- Goodger B. V., Commins M. A., Wright I. G., Mirre G. B., Waltisbuhl D. J., White M. Babesia bovis: vaccination trial with a dominant immunodiffusion antigen in splenectomised calves. Z Parasitenkd. 1986;72(6):715–722. doi: 10.1007/BF00925093. [DOI] [PubMed] [Google Scholar]
- Grau G. E., Fajardo L. F., Piguet P. F., Allet B., Lambert P. H., Vassalli P. Tumor necrosis factor (cachectin) as an essential mediator in murine cerebral malaria. Science. 1987 Sep 4;237(4819):1210–1212. doi: 10.1126/science.3306918. [DOI] [PubMed] [Google Scholar]
- Grau G. E., Heremans H., Piguet P. F., Pointaire P., Lambert P. H., Billiau A., Vassalli P. Monoclonal antibody against interferon gamma can prevent experimental cerebral malaria and its associated overproduction of tumor necrosis factor. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5572–5574. doi: 10.1073/pnas.86.14.5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L. M., Reade J. L., Ware C. F. Rapid colorimetric assay for cell viability: application to the quantitation of cytotoxic and growth inhibitory lymphokines. J Immunol Methods. 1984 May 25;70(2):257–268. doi: 10.1016/0022-1759(84)90190-x. [DOI] [PubMed] [Google Scholar]
- Herman A., Kappler J. W., Marrack P., Pullen A. M. Superantigens: mechanism of T-cell stimulation and role in immune responses. Annu Rev Immunol. 1991;9:745–772. doi: 10.1146/annurev.iy.09.040191.003525. [DOI] [PubMed] [Google Scholar]
- Hines S. A., McElwain T. F., Buening G. M., Palmer G. H. Molecular characterization of Babesia bovis merozoite surface proteins bearing epitopes immunodominant in protected cattle. Mol Biochem Parasitol. 1989 Nov;37(1):1–9. doi: 10.1016/0166-6851(89)90096-0. [DOI] [PubMed] [Google Scholar]
- Kumar S., Good M. F., Dontfraid F., Vinetz J. M., Miller L. H. Interdependence of CD4+ T cells and malarial spleen in immunity to Plasmodium vinckei vinckei. Relevance to vaccine development. J Immunol. 1989 Sep 15;143(6):2017–2023. [PubMed] [Google Scholar]
- Miller K. L., Silverman P. H., Kullgren B., Mahlmann L. J. Tumor necrosis factor alpha and the anemia associated with murine malaria. Infect Immun. 1989 May;57(5):1542–1546. doi: 10.1128/iai.57.5.1542-1546.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montealegre F., Levy M. G., Ristic M., James M. A. Growth inhibition of Babesia bovis in culture by secretions from bovine mononuclear phagocytes. Infect Immun. 1985 Nov;50(2):523–526. doi: 10.1128/iai.50.2.523-526.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newport G., Culpepper J., Agabian N. Parasite heat-shock proteins. Parasitol Today. 1988 Nov;4(11):306–312. doi: 10.1016/0169-4758(88)90111-1. [DOI] [PubMed] [Google Scholar]
- Palmer G. H., McElwain T. F., Perryman L. E., Davis W. C., Reduker D. R., Jasmer D. P., Shkap V., Pipano E., Goff W. L., McGuire T. C. Strain variation of Babesia bovis merozoite surface-exposed epitopes. Infect Immun. 1991 Sep;59(9):3340–3342. doi: 10.1128/iai.59.9.3340-3342.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picot S., Peyron F., Vuillez J. P., Barbe G., Marsh K., Ambroise-Thomas P. Tumor necrosis factor production by human macrophages stimulated in vitro by Plasmodium falciparum. Infect Immun. 1990 Jan;58(1):214–216. doi: 10.1128/iai.58.1.214-216.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polla B. S. Heat shock proteins in host-parasite interactions. Immunol Today. 1991 Mar;12(3):A38–A41. doi: 10.1016/S0167-5699(05)80011-8. [DOI] [PubMed] [Google Scholar]
- Ruddle N. H., Bergman C. M., McGrath K. M., Lingenheld E. G., Grunnet M. L., Padula S. J., Clark R. B. An antibody to lymphotoxin and tumor necrosis factor prevents transfer of experimental allergic encephalomyelitis. J Exp Med. 1990 Oct 1;172(4):1193–1200. doi: 10.1084/jem.172.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruebush M. J., Hanson W. L. Thymus dependence of resistance to infection with Babesia microti of human origin in mice. Am J Trop Med Hyg. 1980 Jul;29(4):507–515. doi: 10.4269/ajtmh.1980.29.507. [DOI] [PubMed] [Google Scholar]
- Schofield L., Ferreira A., Altszuler R., Nussenzweig V., Nussenzweig R. S. Interferon-gamma inhibits the intrahepatocytic development of malaria parasites in vitro. J Immunol. 1987 Sep 15;139(6):2020–2025. [PubMed] [Google Scholar]
- Schofield L., Villaquiran J., Ferreira A., Schellekens H., Nussenzweig R., Nussenzweig V. Gamma interferon, CD8+ T cells and antibodies required for immunity to malaria sporozoites. Nature. 1987 Dec 17;330(6149):664–666. doi: 10.1038/330664a0. [DOI] [PubMed] [Google Scholar]
- Stevenson M. M., Ghadirian E. Human recombinant tumor necrosis factor alpha protects susceptible A/J mice against lethal Plasmodium chabaudi AS infection. Infect Immun. 1989 Dec;57(12):3936–3939. doi: 10.1128/iai.57.12.3936-3939.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taverne J., Bate C. A., Kwiatkowski D., Jakobsen P. H., Playfair J. H. Two soluble antigens of Plasmodium falciparum induce tumor necrosis factor release from macrophages. Infect Immun. 1990 Sep;58(9):2923–2928. doi: 10.1128/iai.58.9.2923-2928.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taverne J., Tavernier J., Fiers W., Playfair J. H. Recombinant tumour necrosis factor inhibits malaria parasites in vivo but not in vitro. Clin Exp Immunol. 1987 Jan;67(1):1–4. [PMC free article] [PubMed] [Google Scholar]
- Templeton J. W., Tipton R. C., Garber T., Bondioli K., Kraemer D. C. Expression and genetic segregation of parental BoLA serotypes in bovine embryos. Anim Genet. 1987;18(4):317–322. doi: 10.1111/j.1365-2052.1987.tb00775.x. [DOI] [PubMed] [Google Scholar]
- Tetzlaff C. L., Rice-Ficht A. C., Woods V. M., Brown W. C. Induction of proliferative responses of T cells from Babesia bovis-immune cattle with a recombinant 77-kilodalton merozoite protein (Bb-1). Infect Immun. 1992 Feb;60(2):644–652. doi: 10.1128/iai.60.2.644-652.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timms P. Development of babesial vaccines. Trans R Soc Trop Med Hyg. 1989;83 (Suppl):73–79. doi: 10.1016/0035-9203(89)90608-1. [DOI] [PubMed] [Google Scholar]
- Tripp C. A., Wagner G. G., Rice-Ficht A. C. Babesia bovis: gene isolation and characterization using a mung bean nuclease-derived expression library. Exp Parasitol. 1989 Oct;69(3):211–225. doi: 10.1016/0014-4894(89)90068-4. [DOI] [PubMed] [Google Scholar]
- Waltisbuhl D. J., Goodger B. V., Wright I. G., Mirre G. B., Commins M. A. Babesia bovis: vaccination studies with three groups of high molecular weight antigens from lysate of infected erythrocytes. Parasitol Res. 1987;73(4):319–323. doi: 10.1007/BF00531085. [DOI] [PubMed] [Google Scholar]
- Weiss W. R., Mellouk S., Houghten R. A., Sedegah M., Kumar S., Good M. F., Berzofsky J. A., Miller L. H., Hoffman S. L. Cytotoxic T cells recognize a peptide from the circumsporozoite protein on malaria-infected hepatocytes. J Exp Med. 1990 Mar 1;171(3):763–773. doi: 10.1084/jem.171.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright I. G., Goodger B. V., Buffington G. D., Clark I. A., Parrodi F., Waltisbuhl D. J. Immunopathophysiology of babesial infections. Trans R Soc Trop Med Hyg. 1989;83 (Suppl):11–13. doi: 10.1016/0035-9203(89)90596-8. [DOI] [PubMed] [Google Scholar]
- Wright I. G., Goodger B. V., Clark I. A. Immunopathophysiology of Babesia bovis and Plasmodium falciparum infections. Parasitol Today. 1988 Aug;4(8):214–218. doi: 10.1016/0169-4758(88)90161-5. [DOI] [PubMed] [Google Scholar]