Abstract
Worry is thought to involve a strategy of cognitive avoidance, in which internal verbalization acts to suppress threatening emotional imagery. We tested the hypothesis that worry-prone individuals would exhibit patterns of between-hemisphere communication that reflect cognitive avoidance. Specifically, we predicted slower transfer of threatening images from the left to the right hemisphere among worriers. ERP measures of interhemispheric transfer time supported this prediction. Left-to-right hemisphere transfer times for angry faces were relatively slower for individuals scoring high in self-reported worry compared to those scoring low, while transfer of happy and neutral faces did not differ between groups. These results suggest that altered interhemispheric communication may constitute one mechanism of cognitive avoidance in worry.
Keywords: anxiety, avoidance, interhemispheric communication, corpus callosum
To uncover the neural correlates of anxiety, numerous researchers have focused on comparing levels of activity between left and right hemisphere brain regions (e.g., Coan & Allen, 2003; Heller, Koven, & Miller, 2003). Yet, the two hemispheres are massively interconnected by the corpus callosum, which transfers and filters information that is exchanged between the two sides of the brain. Prior research indicates that the dynamics of interhemispheric communication can yield insights about information processing that may not be evident by studying levels of activity in either hemisphere alone (e.g., Banich, 2003). For example, interhemispheric communication contributes to selective attention, presumably by facilitating the transfer of task-relevant information and inhibiting the transfer of task-irrelevant information (e.g., Mikels & Reuter-Lorenz, 2004; Weissman & Banich, 1999). Studying interhemispheric dynamics could therefore shed light on information processing in anxiety, particularly because anxiety is characterized by biases in selective attention (e.g. MacLeod & Rutherford, 2004; Mineka, Rafaeli, & Yovel, 2003). The present study specifically aims to investigate how altered communication between the hemispheres could contribute to cognitive avoidance in anxiety.
Understanding the neural underpinnings of anxiety requires first understanding the characteristics of different subtypes of anxiety. Researchers have identified two main subtypes or dimensions, namely anxious arousal and anxious apprehension (e.g., Heller & Nitschke, 1998; Nitschke, Heller, & Miller, 2000). Anxious arousal refers to a state of somatic arousal and is associated with dizziness, racing heart, autonomic arousal, and hypervigilance. It is most closely tied to the panic sensations of anxiety. In contrast, anxious apprehension describes the cognitive symptoms of verbal preoccupation with anticipated threats and concerns, and is most closely tied to the phenomenon of worry.
Research has shown that anxious arousal and anxious apprehension have different neural correlates, as we might expect given their different cognitive-behavioral characteristics (see Nitschke et al., 2000, for review). Specifically, anxious arousal is associated with activity in the posterior right hemisphere, a region known to be involved in attentive vigilance. In contrast, anxious apprehension is associated with increased left hemisphere activity, particularly in the frontal lobe, consistent with the verbal nature of worry.
Several studies support this conception of the different neural substrates for anxious apprehension and arousal. For example, Engels and colleagues (2007) examined brain activity using fMRI while participants attempted to ignore distracting negative, positive, and neutral words. Participant with high anxious apprehension scores showed increased activity in Broca’s region in the left hemisphere during the negative-word condition. In contrast, participants scoring high in anxious arousal showed enhanced activity in the right inferior temporal lobe. EEG studies have also provided results consistent with left-frontal activation in anxious apprehension, namely increased activity measured at left versus right frontal scalp recording sites (e.g., Carter, Johnson, & Borkovec, 1986; Heller, Nitschke, Etienne, & Miller, 1997; Hofmann et al., 2005). Right posterior activation in anxious arousal has been supported by behavioral and neuroimaging studies (e.g., Asbjornsen, Hugdahl, & Bryden, 1992; Reiman, Raichle, Butler, Herscovitch, & Robins, 1984).
On the cognitive level, an influential theory of anxious apprehension proposes that worry acts as a strategy of threat avoidance (Borkovec, Alcaine, & Behar, 2004; Borkovec, Ray, & Stöber, 1998). According to this theory, people engage in verbally-mediated worry in order to stave off the processing of threatening images. Several lines of evidence support this view. First, worry-prone people report that they worry in order to avoid thinking about more troubling possibilities (Borkovec & Roemer, 1995). Second, episodes of worry tend to be dominated by verbal thoughts rather than images (Behar, Zuellig, & Borkovec, 2005). Third, engaging in worry suppresses autonomic responses to threatening images (Borkovec & Hu, 1990; Borkovec, Lyonfields, Wiser, & Deihl, 1993; Peasley-Milkus & Vrana, 2000).
Recast in neural terms, cognitive avoidance by worriers may be seen as a tendency to favor left-hemisphere verbal processing over right-hemisphere image-based processing, particularly when threatening information is present. As reviewed earlier, anxious apprehension is correlated with enhanced left hemisphere activity, particularly in Broca’s region (e.g., Engels et al., 2007), consistent with a predominance of verbal activity in worry. In addition, because the right hemisphere is specialized for control of autonomic arousal (Craig, 2005; Dalton, Kalin, Grist, & Davidson, 2005; Wittling, 1995; Wittling, Block, Schweiger, & Genzel, 1998), reduced autonomic arousal as a consequence of worry could be due to a relatively less active right hemisphere. But how exactly might enhanced left-hemisphere activity result in reduced processing of threatening images?
One possibility is that in anxious apprehension, the active left hemisphere tends to inhibit threatening images from being transferred to the right hemisphere. While direct evidence supporting this possibility is difficult to obtain, indirect evidence supports it. In a study using behavioral methods (Compton, Wilson, & Wolf, 2004), we examined performance on a task that required matching angry, happy, and neutral faces between the left and right visual fields, that is between the right and left hemispheres. We found that people scoring high in anxious apprehension had significantly lower accuracy on trials in which the left hemisphere had to share angry face information with the right hemisphere in order for the match to be recognized correctly. We interpreted the results as consistent with Borkovec’s threat-avoidance model of worry, speculating that the left-hemisphere dominated verbal processing mode disrupted the interhemispheric transfer of the threatening images to the right hemisphere in worry-prone people.
The purpose of the present study was to test more directly the hypothesis that interhemispheric exchange of threatening information is disrupted in people with high levels of anxious apprehension. In this study, we used an ERP method of assessing interhemispheric transfer. Due to the anatomical organization of the visual pathways, an image presented to the left visual field (LVF) reaches the right hemisphere directly, and is then relayed to the left hemisphere via the corpus callosum, the fiber bundle that connects the two hemispheres (e.g., Hellige, 1993; Zaidel & Iacoboni, 2003). Likewise, information in the right visual field (RVF) reaches the left hemisphere directly and is then shared with the right hemisphere via callosal transfer. Researchers have investigated interhemispheric transfer by studying the timing of event-related potential (ERP) peaks evoked over each hemisphere following stimulation of either visual field. Numerous studies have demonstrated that ERP peaks-- such as the N170, a negative-going brain potential occurring about 170 ms after stimulus presentation—occur with a shorter latency over the hemisphere contralateral to the stimulus (the directly-receiving hemisphere), compared to the hemisphere ipsilateral to the stimulus (the indirectly-receiving hemisphere; Brown & Jeeves, 1993; Rugg, Lines, & Milner, 1984; Saron & Davidson, 1989). The time difference in the latency of the ipsilateral and contralateral peaks is taken as a measure of interhemispheric transfer time, that is the time taken for information to cross from the directly stimulated hemisphere across the callosum to the other hemisphere.
In this study, we tested the hypothesis that interhemispheric transfer times would be influenced by individual differences in anxious apprehension. In particular, based on the findings from our earlier study (Compton et al., 2004), we expected transfer times to be slower when the left hemisphere is required to communicate threatening images to the right hemisphere in worry-prone individuals. We used pictures of facial expressions in this study, as in our earlier study, because faces have ecological validity as emotional images in everyday life and because they elicit reliable N170 peaks over the temporal lobes (e.g., Bentin, Allison, Puce, Perez, & McCarthy, 1996). We predicted that transfer times for angry faces would be slower when those faces were presented to the left hemisphere (i.e., the RVF) in individuals high in anxious apprehension.
Methods
Participants
Participants were selected on the basis of a screening questionnaire. The questionnaire was advertised on the college’s electronic bulletin board and drew approximately 700 respondents. Items included the Edinburgh Handedness Inventory (Oldfield, 1971), the Penn State Worry Questionnaire (PSWQ; Meyer, Miller, Metzger, & Borkovec, 1990), and questions related to exclusionary criteria (prior neurological history, uncorrected vision problems, learning disability, regular use of psychoactive substances).
Questionnaire respondents were invited to participate in the lab session if they were right-handed in writing and drawing, did not indicate presence of exclusionary criteria listed above, and had PSWQ scores that were either less than the 25th percentile in the sample (Low Worry Group) or greater than the 75th percentile (High Worry Group). Thirty participants (24 females) completed the lab session and are included in the present analyses. Mean PSWQ scores were 30.8 for the Low Worry Group (n = 16; 11 women) and 71.7 for the High Worry Group (n = 14; 13 women).
At the end of the lab session, participants also completed the state version of the State-Trait Anxiety Inventory (STAI; Spielberger, 1968) and the brief version of the Fear of Negative Evaluation scale (FNE; Leary, 1983). The FNE is intended to tap concerns about being negatively evaluated by other people. The 12 items include statements such as “I am afraid that others will not approve of me” and “I am usually worried about what kind of impression I make” (for psychometric information, see Collins, Westra, Dozois, & Stewart, 2005; Rodebaugh et al., 2004). We included the FNE because it is closely related to symptoms of social phobia, which in turn may be especially related to processing of angry faces (e.g., Kolassa & Miltner, 2006; Mogg, Philippot, & Bradley, 2004).
Compared to participants in the Low Worry Group, those in the High Worry Group had higher scores on the STAI (M = 42.9 vs. 34.3; t(28) = 2.93, p<.01) and the FNE (M = 44.3 vs. 24.6, t(28) = 9.44, p<.001).
Faces Task
Stimuli were 18 black-and-white photographs from the Ekman and Friesen (1976) set. The selected photographs included six posers (three male and three female) each depicting three facial expressions (angry, happy, neutral). Each photograph was cropped to a visual angle of 4° × 6°, and stimuli were presented unilaterally with the medial edge at 3° of eccentricity. Stimuli were presented by E-prime software (v. 1.0) running on a Dell Dimension desktop.
The task consisted of 432 trials, divided into six blocks of 72 trials each. On each trial, the participant was expected to indicate by a keypress whether the photograph depicted a male or female. Half of the participants used the left index finger on the “g” key to indicate female and the right index finger on the “h” key to indicate male, and half of the participants used the reverse mapping (counterbalanced across worry groups). Emotional expression was varied across blocks, such that two blocks included only angry faces, two included only happy faces, and two included only neutral faces. The order of the six blocks was randomized. Within each block, trials included half male and half female faces, and half LVF and half RVF presentations. Trial types were fully counterbalanced, such that each photograph appeared 6 times in the LVF and 6 times in the RVF in a given block. Trial types within each block were randomly intermixed.
Trial events began with a 500-ms black fixation point against a gray background. The face stimulus was then presented against the gray background to either the LVF or the RVF for 14 ms, synchronized with the monitor’s 75-Hz refresh cycle. Following the stimulus presentation, a fixation point remained on the screen until the participant’s keypress or for a maximum of 1000 ms. After the keypress, the fixation point remained visible for a variable duration of 500–1500 ms prior to the beginning of the next trial. This variable inter-trial interval was intended to reduce anticipation of the next stimulus onset. At the onset of each stimulus, a digital trigger was sent via the parallel port to the EEG amplifier for event marking.
EEG Data Acquisition and Signal Processing
Electrodes were applied using an elastic cap (Quik-Caps) fitted with sintered Ag/AgCl electrodes. Positioning of the cap was confirmed by measurements from nasion and inion, and left-right alignment of the cap was confirmed by ensuring that midline electrodes were positioned halfway between the two ears. Data were recorded continuously from 15 scalp sites: Fz, FCz, Cz, C3/C4, CP3/CP4, P3/P4, PO1/PO2, O1/O2, and T5/T6. Data were referenced on-line to the right mastoid, and were digitally re-referenced off-line to the average of left and right mastoids. Eye movements were monitored by electrodes placed above and below the left eye and at the outer canthus of each eye. Recordings from these four sites were used to compute bipolar horizontal and vertical EOG channels off-line. Analyses here focus on the T5 and T6 sites, where the N170 peak is most reliably observed.
Signals were amplified by a NuAmps amplifier controlled by Neuroscan software, with a sampling rate of 1000 Hz and a bandpass of 0.1–70 Hz (−3 dB). Off-line, signals were low-pass filtered with a 60-Hz cut-off (48 dB/oct) to remove additional noise attributable to the 75-Hz monitor refresh rate.
Artifacts were addressed off-line in three steps. First, upon visual inspection, portions of the EEG record with large non-blink artifacts were manually excluded. Second, the effect of blinks was reduced using the Neuroscan software’s regression-based algorithm for ocular artifact reduction. Finally, remaining artifacts in the EEG were identified using a +/− 150 μV threshold, and corresponding epochs were rejected.
Signal averaging was carried out separately for each trial type and encompassed a window around the stimulus event marker from −100 to 500 ms. Prior to averaging, epochs were baseline-corrected using the 100-ms pre-stimulus interval as the baseline.
Results
Behavioral Data
Mean accuracy was 83.3% correct. An ANOVA on percent correct with repeated measures factors Visual Field (VF; LVF vs. RVF), Emotion (angry, happy, neutral), and Worry Group (Low, High) found no significant effects. Accuracy tended to be slightly higher for LVF stimuli (M = 84.6%) than for RVF stimuli (M = 82.0%), but this effect was only evident at a trend level (F(1,28) = 3.67, p <.07). In a parallel ANOVA on reaction time data, there was a tendency for slightly faster responses to LVF than RVF stimuli (M = 598 vs. 603 ms), but this effect was not significant (p >.15). There were no significant effects involving Emotion or Worry Group.
ERP Data
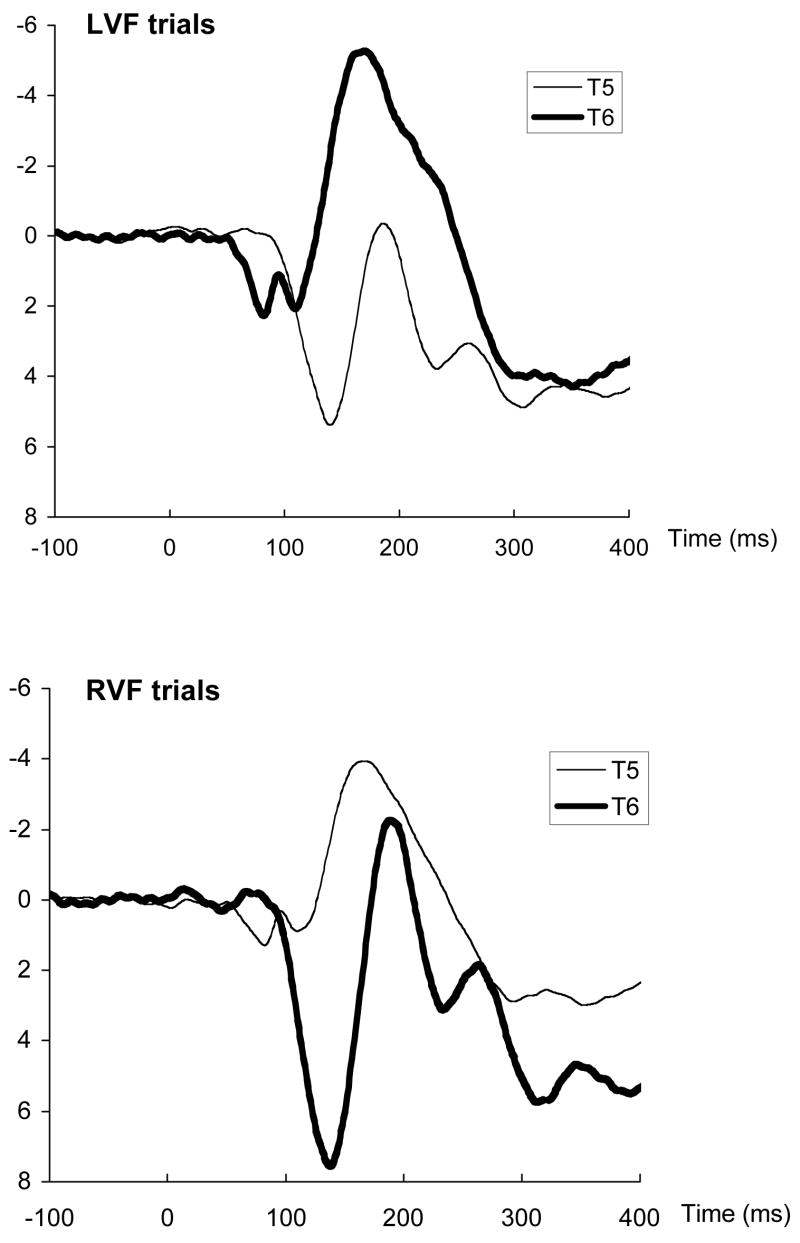
Grand-average waveforms are illustrated in Figure 1. As seen in the waveforms, the N170 peak occurred earlier over the contralateral (directly-stimulated) hemisphere compared to the ipsilateral (callosally-stimulated) hemisphere, consistent with expectations based on the anatomy of the visual system. To examine this effect statistically, peak latencies were extracted from the averaged waveforms for each participant and trial type, with the N170 peak defined as the most negative point between 130 and 250 ms post-stimulus.
Figure 1.
Grand-average waveforms at T5 (left temporal) and T6 (right temporal) sites following stimulus presentation to the left visual field (LVF) or right visual field (RVF).
N170 peak latencies were submitted to a three-way ANOVA, with VF (LVF, RVF), Hemisphere (T5/left, T6/right), and Emotion (angry, happy, neutral) as repeated-measures factors. As expected, the VF x Hemisphere interaction was significant, F(1,26) = 44.75, p<.0001. Means, displayed in Figure 2, indicate that peak latencies were shorter over the directly-stimulated hemisphere, compared to the transcallosally stimulated hemisphere. This effect did not further interact with Emotion (F < 1), indicating that callosal transfer time did not vary significantly across angry, happy, and neutral faces in the group as a whole. No other effects in the ANOVA were significant.
Figure 2.
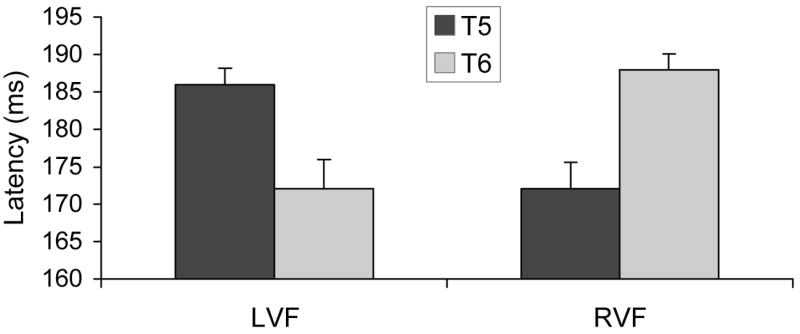
Peak latencies of the N170 component following stimulus presentation to the left (LVF) or right (RVF) visual field. Components were recorded at T5 (left temporal) and T6 (right temporal) electrode sites.
Mean estimates of transfer time, calculated as the ipsilateral latency minus the contralateral latency, were 17 ms for LVF stimuli and 13 ms for RVF stimuli. These values are similar to transfer times reported in prior studies (e.g., Brown & Jeeves, 1993; Saron & Davidson, 1989; Terasaki & Okazaki, 2002). Transfer times did not differ between LVF and RVF presentations (F < 1).
Most individual participants displayed transfer times consistent with anatomical constraints, that is positive transfer-time values due to shorter contralateral than ipsilateral latencies. Table 1 lists the percentages of participants with anatomically-predicted transfer times for each Emotion × Visual Field trial type separately. The percentage of participants with anatomically-predicted scores was greater than expected by chance for each of these trial types, according to the sign test (z’s > 2.55, p’s <.02). These percentages are similar to those reported by other researchers using similar paradigms (Saron & Davidson, 1989).
Table 1.
Percentage of participants (n = 30) who displayed transfer times in the anatomically-predicted direction.
| Emotion Type
|
|||
|---|---|---|---|
| Visual Field | Angry | Happy | Neutral |
| Left | 83% | 83% | 83% |
| Right | 73% | 83% | 77% |
Group differences in interhemispheric transfer
To address the question of anxiety-group differences in transfer time, we examined transfer times for each emotion type separately, considering only subjects with positive transfer times for both visual fields for that emotion type. The reason for selecting this analysis strategy was to exclude data that are logically erroneous. Participants with anatomically-impossible (i.e., negative) transfer times, while a minority of the sample, can add substantially to error variance in between-subjects comparisons and therefore make genuine individual differences related to anxiety difficult to detect. Therefore, while the preceding analyses included all participants—in order to demonstrate reliable interhemispheric transfer patterns in the group as a whole—the individual differences analyses included only participants whose transfer times met the logical criterion of being anatomically possible.
Transfer times were examined separately for each emotion type with VF (LVF, RVF) and Worry Group (Low, High) as factors. For angry faces, the VF x Worry Group effect was significant (F(1,16) = 5.23, p <.04). As indicated by the interaction means in Figure 3, High Worry participants had slower transfer times than Low Worry participants for angry faces presented to the RVF (simple effect, F(1,16) = 4.74, p<.05). When angry faces were presented to the LVF, the groups did not differ (F < 1). These results are consistent with the prediction that anxious participants would have slower transfer of angry face information from the left to the right hemisphere. Parallel analyses that focused on happy and neutral face types found no significant effects related to anxiety group (F’s <1; see Table 2 for means).
Figure 3.
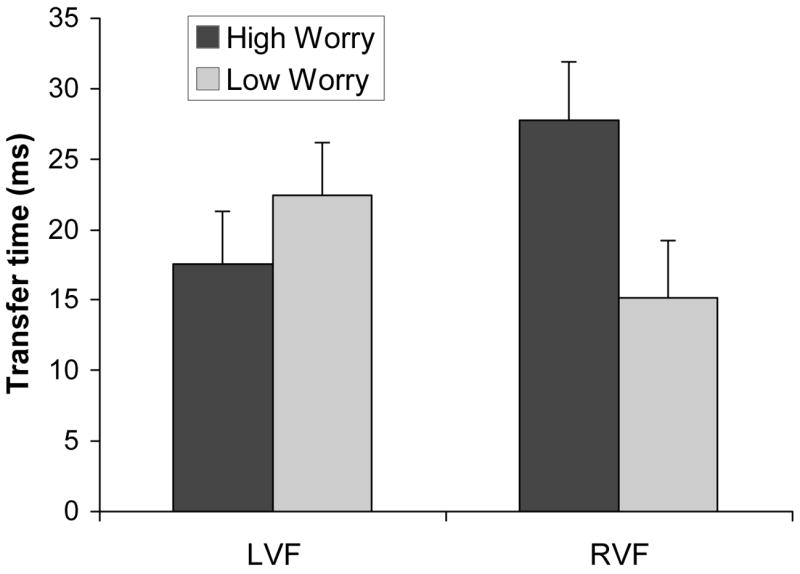
Interhemispheric transfer time for angry faces as a function of anxiety group and visual field of presentation (LVF = left visual field, RVF = right visual field).
Table 2.
Mean transfer times (ms) following LVF and RVF presentation of angry, happy, and neutral faces.
| Visual Field | |||
|---|---|---|---|
| Worry Group | LVF | RVF | Asymmetry
(LVF – RVF) |
| Angry | |||
| High (n = 9) | 18 | 28 | −10 |
| Low (n = 9) | 24 | 15 | 9 |
| Difference
(High – Low) |
−6 | 13 | 19 |
| Happy | |||
| High (n = 10) | 32 | 28 | 4 |
| Low (n = 9) | 26 | 23 | 3 |
| Difference
(High – Low) |
6 | 5 | 1 |
| Neutral | |||
| High (n = 10) | 22 | 23 | −1 |
| Low (n = 9) | 28 | 19 | 9 |
| Difference
(High – Low |
−6 | 4 | 10 |
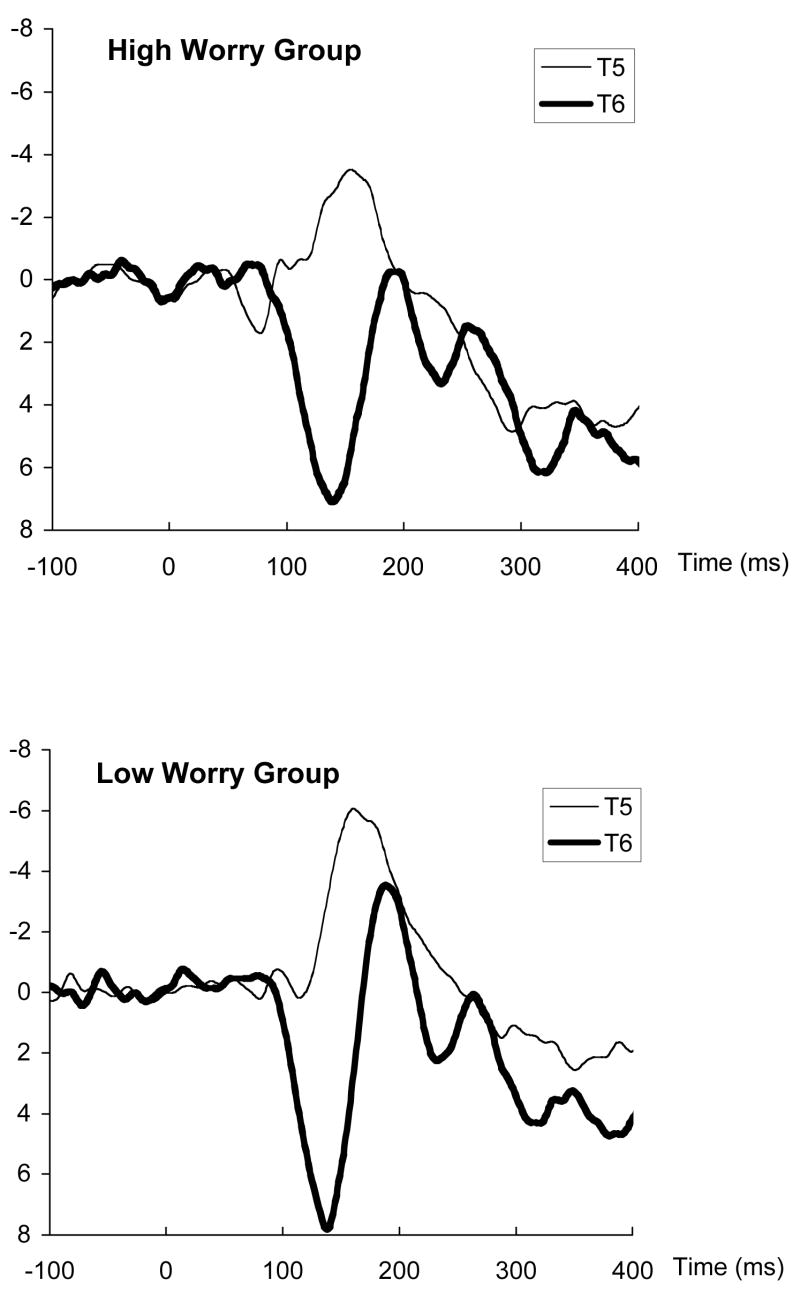
Figure 4 illustrates the waveforms for angry RVF trials separately for High and Low Worry groups. Visual inspection of the waveforms confirms the statistical account, as the difference between contralateral (T5) and ipsilateral (T6) peaks is greater for High than Low Worry groups.
Figure 4.
Waveforms for high and low worry groups following presentation of an angry face to the right visual field. Waveforms are shown for the left (T5) and right (T6) temporal lobe sites.
To examine the specificity of the worry aspect of anxiety in predicting transfer times, we calculated zero-order correlations and partial correlations between the three anxiety measures (PSWQ, STAI, and FNE) and transfer times. In zero-order correlations, angry RVF transfer times were significantly correlated with PSWQ scores (r = 0.54, p <.03) and with FNE scores (r = 0.54, p <.03), which were highly intercorrelated with one another (r = 0.85, p <.001). However, angry RVF transfer times were not correlated with STAI scores (r = −0.04, ns). Further, the relationship between PSWQ scores and angry RVF transfer times remained significant even once variance shared with STAI scores was partialed out (partial r = 0.60, p <.02). Likewise, the relationship between FNE scores and angry RVF transfer times remained significant once accounting for STAI scores (partial r = 0.58, p <.02). Together these results indicate that aspects of anxiety tapped by the worry and negative evaluation questionnaires predicted interhemispheric transfer times for angry RVF faces, but state anxiety did not. Transfer times following LVF presentation of angry faces were not correlated with any of the anxiety measures.
Discussion
The results of this study demonstrate that individuals with high levels of anxious apprehension tend to have slower transfer of angry face information from the left to the right hemisphere, compared to individuals with lower levels of anxious apprehension. The groups did not differ in interhemispheric transfer of happy or neutral faces. Further, state anxiety was uncorrelated with interhemispheric transfer times, indicating that the effect was specific to worry-related aspects of anxiety. These results support the idea that altered interhemispheric communication of threatening information may be one neural mechanism of cognitive avoidance in individuals prone to worry.
These results conceptually replicate and extend earlier findings of altered interhemispheric communication in worriers. An earlier study (Compton et al., 2004) used a behavioral paradigm that required participants to match pictures of facial expressions either within or between the hemispheres. Worriers tended to match angry faces less accurately in a condition in which the left hemisphere had to transfer the angry face information to the right hemisphere. Although behavioral performance in bilateral matching paradigms is correlated with interhemispheric transfer times (especially from the left to right hemisphere; Larson & Brown, 1997), the behavioral method is limited in its ability to assess interhemispheric dynamics. Interhemispheric communication is not measured directly in the behavioral paradigm, but rather inferred from patterns of within-and between-hemisphere matching performance. To address this limitation, the present study used an ERP method of measuring interhemispheric transfer time. Across the whole sample, peak N170 latencies occurred earlier over the contralateral than the ipsilateral hemisphere, replicating earlier data (e.g., Rugg et al., 1984; Saron & Davidson, 1989). Because ipsilateral peaks are typically absent in those with callosal sections or callosal agenesis (Bayard, Gosselin, Robert, & Lassonde, 2004; Brown, Jeeves, Dietrich, & Burnison, 1999; Rugg, Milner, & Lines, 1985), we can assume that ipsilateral peaks depend upon an intact callosum, and that time lags between contralateral and ipsilateral peaks reflect callosal transfer. Therefore, the individual differences reported in this study likely reflect individual differences in the timing of information transfer across the callosum.
These results suggest one possible neural mechanism for cognitive avoidance in people who are prone to worry. The cognitive avoidance hypothesis was originally developed based on the self-reported characteristics of worriers, and has received further support from studies showing that worry reduces autonomic responses to threats (Borkovec et al., 1998). Studies demonstrating enhanced left frontal activity in those with high anxious apprehension (Carter et al., 1986; Engels et al., 2007; Heller et al., 1997; Hofmann et al., 2005) are consistent with the verbal nature of cognitive avoidance in anxious apprehension. The present results complement and extend these prior findings by demonstrating that it is not just regional brain activity levels, but also communication of information between brain regions that differs between worriers and non-worriers. Reduced left-to-right communication of threatening images in worriers may provide a link that connects increased left-hemisphere activity with reduced autonomic responses, which are presumably controlled by the right hemisphere (e.g., Dalton et al., 2005; Wittling, 1995).
While prior studies of the neural basis of anxious apprehension have focused on asymmetries in frontal lobe regions, the present results involve interhemispheric exchange between posterior regions that are involved in perceptual processing. These two lines of research should be seen as complementary. It is plausible that a verbally-dominated strategy of avoidance could involve both increased left frontal activity (Engels et al., 2007) and an altered pattern of interhemispheric communication between posterior regions. Frontal lobe regions are known to exert top-down control over subcortical and cortical regions, including temporal cortex regions that represent visual images such as faces (e.g., Gazzaley, Cooney, McEvoy, Knight, & D’Esposito, 2005; Gazzaley et al., 2007). Such frontally-mediated control can establish a mental set that influences how information is processed in posterior regions (e.g., Miller & Cohen, 2001). Therefore, a cognitive strategy of threat avoidance in worry-prone people may involve activation of left-frontal systems that alter processing in the temporal regions that process visual image information.
Altered interhemispheric exchange is unlikely to be the only neural mechanism contributing to avoidance in anxiety. The transfer time effects were small in magnitude, implying that other mechanisms probably play a role in cognitive avoidance as well. For example, one study found that during an induced worry condition, cerebral blood flow increased in a left frontal lobe region and decreased in limbic structures such as the amygdala (Hoehn-Saric, Lee, McLeod, & Wong, 2005). These neuroimaging results suggest that worry may involve top-down regulation of subcortical regions by frontal regions, a possibility that is not mutually exclusive with the interhemispheric mechanism that we propose.
In addition to implications regarding cognitive avoidance in anxiety, the present results also underscore the importance of conceptualizing interhemispheric communication as a dynamic process, rather than a fixed information relay. Behavioral studies of interhemispheric interaction have long emphasized its dynamic nature. For example, researchers have conceived of the callosum as a selective filter that can adaptively control information flow between the hemispheres (e.g., Liederman, 1986; Mikels & Reuter-Lorenz, 2004; Weissman & Banich, 1999). Studies using behavioral methods have shown that interhemispheric communication is modulated by changing task demands (Weissman & Banich, 2000) and situational factors such as evaluation stress (Compton & Mintzer, 2001; Compton et al., 2004). In contrast, most ERP studies have tended to view interhemispheric transfer time as relatively fixed within a person, and individual differences in transfer time have often been viewed as reflecting anatomical differences between people (e.g., Barnett, Corballis, & Kirk, 2005; Moes, Brown, & Minnema, 2007; Patston, Kirk, Rolfe, Corballis, & Tippett, 2007; though see Nowicka, Grabowska, & Fersten, 1996). The present results suggest that individual differences in transfer time can be dependent on stimulus type, because the significant anxiety-related results were limited to angry faces. These data fit with a conception of interhemispheric communication as an active, selective process rather than a passive relay based on fixed anatomy.
While the pattern of contralateral versus ipsilateral latencies in the whole sample was robustly significant in the anatomically-predicted direction, a fraction of participants had anatomically impossible transfer times, and these participants had to be excluded in order for anxiety-related effects to be evident. This is one limitation of the current dataset. However, the percentage of participants with anatomically-impossible transfer times in our study was comparable to percentages reported in earlier research (Saron & Davidson, 1989). This most likely points to a general limitation of the ERP method of studying interhemispheric transfer, rather than a problem with how we implemented that method. Anatomically-incorrect transfer times may arise from a variety of sources, such as a waveform with a poorly defined peak, latency jitter, an atypically oriented dipole, or slight asymmetries in the correspondence between the electrode site on the scalp and the underlying cortex. It is possible that with larger samples of high and low worriers, participants with erroneous transfer times could still be included and have less statistical impact on the overall group comparison. However, in the present study, individual differences in anxiety were only evident when those with erroneous transfer times were excluded.
Another limitation of the ERP method is that it focuses on interhemispheric communication within a certain time frame, that is the initial volleys of sensory-perceptual information exchange measured within the first 200 ms following stimulus presentation. Sharing of perceptual information is necessary to construct a unified perception of both sides of visual space and likely takes place over posterior sections of the callosum (Brown et al., 1999). However, information exchange across the callosum is not limited to this stage of processing or this anatomical sector, but rather involves many channels and many temporal stages (for reviews, see Banich, 2003; Clarke, 2003; Innocenti & Bressoud, 2003; Saron, Foxe, Simpson, & Vaughan, 2003). While the present study has demonstrated associations between anxiety and one aspect of callosal transfer, other aspects of callosal exchange are less amenable to measurement with the ERP transfer time method, and should be addressed with other methods in the future. In addition, the present method examines the brain’s initial response to externally presented stimuli, whereas worry is likely to influence the internal generation of threat-related imagery as well (e.g., Behar et al., 2005). Although perception and imagery are generally thought to rely upon similar neural systems (e.g., Behrmann, 2000; Ganis, Thompson, Mast, & Kosslyn, 2004), the relation between perceptual processing and imagery in the context of anxiety worry deserves future research attention.
Future studies can extend these results in a number of potentially fruitful directions. First, it would be beneficial to tie interhemispheric dynamics more directly to tonic levels of activity measured with EEG or hemodynamic measures. For example, worriers with higher levels of left frontal activity may also show more altered interhemispheric exchange, if the same verbalization strategy drives both the activity level and the interhemispheric dynamics. Researchers could also test whether autonomic responses to threatening images are lessened in those who display reduced left-to-right hemisphere exchange, tying interhemispheric processing even more directly to ongoing research on cognitive avoidance (Borkovec et al., 2004). In addition, a worry-induction paradigm could be used to determine whether individual differences in interhemispheric effects are due to trait differences, activation of a worry strategy, or some combination. In the present study, interhemispheric transfer effects were uncorrelated with state anxiety, suggesting a crucial role for trait differences in anxious apprehension. At the same time, the effect may be enhanced among worriers when they actively engage in worry, compared to baseline conditions. A study that manipulated state levels of worry could more directly address this issue.
Finally, while the present study focused on individual differences among a nonclinical undergraduate sample, future studies could examine individuals with generalized anxiety disorder (GAD). GAD is characterized by high levels of worry symptoms (Borkovec et al., 2004), along with the additional tendency towards “meta-worries”, or worries about worrying (Wells, 2004). Future research could help to determine whether GAD samples are qualitatively different than non-pathological high worriers or whether the two share common neural mechanisms of threat avoidance.
In sum, the present results add to our understanding of the neural basis of anxiety by demonstrating that information exchange between the hemispheres, particularly for threatening information, is altered in individuals with the propensity to worry. These results fit with an influential theory of cognitive avoidance in anxiety derived from the clinical literature (Borkovec et al., 2004). In addition, the results support a view of the corpus callosum as a selective filter that shapes information processing in ways that are influenced by emotional variables such as trait individual differences and stimulus content. Future research can examine the relationship between interhemispheric exchange and other physiological correlates of anxious apprehension to test further the possibility that altered interhemispheric communication may contribute to avoidance strategies in anxious individuals.
Acknowledgments
This research was supported by NIH grant R15-MH63715.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at http://www.apa.org/journals/emo
References
- Asbjornsen A, Hugdahl K, Bryden MP. Manipulations of subjects’ level of arousal in dichotic listening. Brain and Cognition. 1992;19:183–194. doi: 10.1016/0278-2626(92)90044-m. [DOI] [PubMed] [Google Scholar]
- Banich MT. Interaction between the hemispheres and its implications for the processing capacity of the brain. In: Hugdahl K, Davidson RJ, editors. The Asymmetrical Brain. Cambridge, MA: MIT Press; 2003. pp. 261–302. [Google Scholar]
- Barnett KJ, Corballis MC, Kirk IJ. Symmetry of callosal information transfer in schizophrenia: A preliminary study. Schizophrenia Research. 2005;74:171–178. doi: 10.1016/j.schres.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Bayard S, Gosselin N, Robert M, Lassonde M. Inter- and intra-hemispheric processing of visual event-related potentials in the absence of the corpus callosum. Journal of Cognitive Neuroscience. 2004;16:401–414. doi: 10.1162/089892904322926746. [DOI] [PubMed] [Google Scholar]
- Behar E, Zuellig AR, Borkovec TD. Thought and imaginal activity during worry and trauma recall. Behavior Therapy. 2005;36:157–168. [Google Scholar]
- Behrmann M. The mind’s eye mapped onto the brain’s matter. Current Directions in Psychological Science. 2000;9:50–54. [Google Scholar]
- Bentin S, Allison T, Puce A, Perez E, McCarthy G. Electrophysiological studies of face perception in humans. Journal of Cognitive Neuroscience. 1996;8:551–565. doi: 10.1162/jocn.1996.8.6.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkovec TD, Alcaine OM, Behar E. Avoidance theory of worry and generalized anxiety disorder. In: Heimberg RG, Turk CL, Mennin DS, editors. Generalized Anxiety Disorder: Advances in Research and Practice. New York: Guilford Press; 2004. pp. 77–108. [Google Scholar]
- Borkovec TD, Hu S. The effect of worry on cardiovascular response to phobic imagery. Behaviour Research and Therapy. 1990;28:69–73. doi: 10.1016/0005-7967(90)90056-o. [DOI] [PubMed] [Google Scholar]
- Borkovec TD, Lyonfields JD, Wiser SL, Deihl L. The role of worrisome thinking in the suppression of cardiovascular response to phobic imagery. Behaviour Research and Therapy. 1993;31:321–324. doi: 10.1016/0005-7967(93)90031-o. [DOI] [PubMed] [Google Scholar]
- Borkovec TD, Ray WJ, Stöber J. Worry: A cognitive phenomenon intimately linked to affective, physiological, and interpersonal behavioral processes. Cognitive Therapy and Research. 1998;22:561–576. [Google Scholar]
- Borkovec TD, Roemer L. Perceived functions of worry among generalized anxiety disorder subjects: Distraction from more emotionally distressing topics? Journal of Behavior Therapy and Experimental Psychiatry. 1995;26:25–30. doi: 10.1016/0005-7916(94)00064-s. [DOI] [PubMed] [Google Scholar]
- Brown WS, Bjerke MD, Galbraith GC. Interhemispheric transfer in normals and acallosals: Latency adjusted evoked potential averaging. Cortex. 1998;34:677–692. doi: 10.1016/s0010-9452(08)70772-x. [DOI] [PubMed] [Google Scholar]
- Brown WS, Jeeves MA. Bilateral visual field processing and evoked potential interhemispheric transmission time. Neuropsychologia. 1993;31:1276–1281. doi: 10.1016/0028-3932(93)90097-j. [DOI] [PubMed] [Google Scholar]
- Brown WS, Jeeves MA, Dietrich R, Burnison DA. Bilateral field advantage and evoked potential interhemispheric transmission in commisurotomy and callosal agenesis. Neuropsychologia. 1999;37:1165–1180. doi: 10.1016/s0028-3932(99)00011-1. [DOI] [PubMed] [Google Scholar]
- Carter WR, Johnson MC, Borkovec TD. Worry: An electrocortical analysis. Advances in Behavioral Research and Therapy. 1986;8:193–204. [Google Scholar]
- Clarke S. The role of homotopic and heterotopic callosal connections in humans. In: Zaidel E, Iacoboni M, editors. The Parallel Brain: The Cognitive Neuroscience of the Corpus Callosum. Cambridge, MA: MIT Press; 2003. pp. 461–472. [Google Scholar]
- Coan JA, Allen JJB. The state and trait nature of frontal EEG asymmetry in emotion. In: Hugdahl K, Davidson RJ, editors. The Asymmetrical Brain. Cambridge, MA: MIT Press; 2003. pp. 565–615. [Google Scholar]
- Collins KA, Westra HA, Dozois DJA, Stewart SH. The validity of the brief version of the Fear of Negative Evaluation Scale. Anxiety Disorders. 2005;19:345–359. doi: 10.1016/j.janxdis.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Compton RJ, Mintzer DA. Effects of worry and evaluation stress on interhemispheric interaction. Neuropsychology. 2001;15:427–433. [PubMed] [Google Scholar]
- Compton RJ, Wilson K, Wolf K. Mind the gap: Interhemispheric communication about emotional faces. Emotion. 2004;4:219–232. doi: 10.1037/1528-3542.4.3.219. [DOI] [PubMed] [Google Scholar]
- Craig AD. Forebrain emotional asymmetry: A neuroanatomical basis? Trends in Cognitive Sciences. 2005;9:566–571. doi: 10.1016/j.tics.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Dalton KM, Kalin NH, Grist TM, Davidson RJ. Neural-cardiac coupling in threat-evoked anxiety. Journal of Cognitive Neuroscience. 2005;17:969–980. doi: 10.1162/0898929054021094. [DOI] [PubMed] [Google Scholar]
- Ekman P, Friesen WV. Pictures of Facial Affect. Palo Alto, CA: Consulting Psychologists Press; 1976. [Google Scholar]
- Engels AS, Heller W, Mohanty A, Herrington JD, Banich MT, Webb AG, Miller GA. Specificity of regional brain activity in anxiety types during emotion processing. Psychophysiology. 2007;44:352–363. doi: 10.1111/j.1469-8986.2007.00518.x. [DOI] [PubMed] [Google Scholar]
- Ganis G, Thompson WL, Mast F, Kosslyn SM. The brain’s mind’s images: The cognitive neuroscience of mental imagery. In: Gazzaniga MS, editor. The cognitive neurosciences III. Cambridge, MA: MIT Press; 2004. pp. 931–941. [Google Scholar]
- Gazzaley A, Cooney JW, McEvoy K, Knight RT, D’Esposito M. Top-down enhancement and suppression of the magnitude and speed of neural activity. Journal of Cognitive Neuroscience. 2005;17:507–517. doi: 10.1162/0898929053279522. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Rissman J, Cooney J, Rutman A, Seibert T, Clapp W, D’Esposito M. Functional interactions between prefrontal and visual association cortex contribute to top-down modulation of visual processing. Cerebral Cortex. 2007;17:i125–i135. doi: 10.1093/cercor/bhm113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller W, Koven NS, Miller GA. Regional brain activity in anxiety and depression, cognition/emotion interaction, and emotion regulation. In: Hugdahl K, Davidson RJ, editors. The asymmetrical brain. Cambridge, MA: MIT Press; 2003. pp. 533–564. [Google Scholar]
- Heller W, Nitschke JB. The puzzle of regional brain activity in depression and anxiety: The importance of subtypes and comorbidity. Cognition and Emotion. 1998;12:421–446. [Google Scholar]
- Heller W, Nitschke JB, Etienne MA, Miller GA. Patterns of regional brain activity differentiate types of anxiety. Journal of Abnormal Psychology. 1997;106:376–385. doi: 10.1037//0021-843x.106.3.376. [DOI] [PubMed] [Google Scholar]
- Hellige JB. Hemispheric asymmetry: What’s right and what’s left. Cambridge, MA: Harvard University Press; 1993. [Google Scholar]
- Hoehn-Saric R, Lee JS, McLeod DR, Wong DF. Effect of worry on regional cerebral blood flow in nonanxious subjects. Psychiatry Research-Neuroimaging. 2005;140:259–269. doi: 10.1016/j.pscychresns.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Hofmann SG, Moscovitch DA, Litz BT, Kim HJ, Davis LL, Pizzagalli DA. The worried mind: Autonomic and prefrontal activation during worrying. Emotion. 2005;5:464–475. doi: 10.1037/1528-3542.5.4.464. [DOI] [PubMed] [Google Scholar]
- Innocenti G, Bressoud R. Callosal axons and their development. In: Zaidel E, Iacoboni M, editors. The Parallel Brain: The Cognitive Neuroscience of the Corpus Callosum. Cambridge, MA: MIT Press; 2003. pp. 11–26. [Google Scholar]
- Kolassa IT, Miltner WHR. Psychophysiological correlates of face processing in social phobia. Brain Research. 2006;1118:130–141. doi: 10.1016/j.brainres.2006.08.019. [DOI] [PubMed] [Google Scholar]
- Larson EB, Brown WS. Bilateral field interactions, hemispheric specialization and evoked potential interhemispheric transmission time. Neuropsychologia. 1997;35:573–581. doi: 10.1016/s0028-3932(96)00099-1. [DOI] [PubMed] [Google Scholar]
- Leary MR. A brief version of the Fear of Negative Evaluation Scale. Personality and Social Psychology Bulletin. 1983;9:371–375. [Google Scholar]
- Liederman J. Subtraction in addition to addition: Dual task performance improves when tasks are presented to separate hemispheres. Journal of Clinical and Experimental Neuropsychology. 1986;8:486–502. doi: 10.1080/01688638608405172. [DOI] [PubMed] [Google Scholar]
- MacLeod C, Rutherford E. Information-processing approaches: Assessing the selective functioning of attention, interpretation, and retrieval. In: Heimberg RG, Turk CL, Mennin DS, editors. Generalized Anxiety Disorder: Advances in Research and Practice. New York: Guilford Press; 2004. pp. 109–142. [Google Scholar]
- Meyer TJ, Miller ML, Metzger RL, Borkovec TD. Development and validation of the Penn State Worry Questionnaire. Behaviour Research and Therapy. 1990;28:487–495. doi: 10.1016/0005-7967(90)90135-6. [DOI] [PubMed] [Google Scholar]
- Mikels JA, Reuter-Lorenz PA. Neural gate keeping: The role of interhemispheric interactions in resource allocation and selective filtering. Neuropsychology. 2004;18:328–339. doi: 10.1037/0894-4105.18.2.328. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Mineka S, Rafaeli E, Yovel I. Cognitive biases in emotional disorders: Information processing and social-cognitive perspectives. In: Davidson RJ, Scherer KR, Goldsmith HH, editors. Handbook of Affective Sciences. Oxford: Oxford University Press; 2003. pp. 976–1009. [Google Scholar]
- Moes PE, Brown WS, Minnema MT. Individual differences in interhemispheric transfer time (IHTT) as measured by event related potentials. Neuropsychologia. 2007;45:2626–2630. doi: 10.1016/j.neuropsychologia.2007.03.017. [DOI] [PubMed] [Google Scholar]
- Mogg K, Philippot P, Bradley BP. Selective attention to angry faces in clinical social phobia. Journal of Abnormal Psychology. 2004;113:160–165. doi: 10.1037/0021-843X.113.1.160. [DOI] [PubMed] [Google Scholar]
- Nitschke JB, Heller W, Miller GA. The neuropsychology of anxiety. In: Borod JC, editor. The Neuropsychology of Emotion. New York: Oxford University Press; 2000. pp. 298–319. [Google Scholar]
- Nowicka A, Grabowska A, Fersten E. Interhemispheric transmission of information and functional asymmetry of the human brain. Neuropsychologia. 1996;34:147–151. doi: 10.1016/0028-3932(95)00064-x. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh Inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Patston LLM, Kirk IJ, Rolfe MHS, Corballis MC, Tippett LJ. The unusual symmetry of musicians: Musicians have equilateral interhemispheric transfer for visual information. Neuropsychologia. 2007;45:2059–2065. doi: 10.1016/j.neuropsychologia.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Peasley-Miklus C, Vrana SR. Effect of worrisome and relaxing thinking on fearful emotional processing. Behaviour Research and Therapy. 2000;38:129–144. doi: 10.1016/s0005-7967(99)00025-x. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Raichle ME, Butler FK, Herscovitch P, Robins E. A focal brain abnormality in panic disorder, a severe form of anxiety. Nature. 1984;310:683–685. doi: 10.1038/310683a0. [DOI] [PubMed] [Google Scholar]
- Rodebaugh TL, Woods CM, Thissen DM, Heimberg RG, Chambless DL, Rapee RM. More information from fewer questions: The factor structure and item properties of the original and brief Fear of Negative Evaluation scale. Psychological Assessment. 2004;16:169–181. doi: 10.1037/1040-3590.16.2.169. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Lines CR, Milner AD. Visual evoked potentials to lateralized visual stimuli and the measurement of interhemispheric transmission time. Neuropsychologia. 1984;22:215–225. doi: 10.1016/0028-3932(84)90064-2. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Milner AD, Lines C. Visual evoked potentials to lateralised stimuli in two cases of callosal agenesis. Journal of Neurology, Neurosurgery, and Psychiatry. 1985;48:367–373. doi: 10.1136/jnnp.48.4.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saron CD, Davidson RJ. Visual evoked potential measures of interhemispheric transfer time in humans. Behavioral Neuroscience. 1989;103:1115–1138. doi: 10.1037//0735-7044.103.5.1115. [DOI] [PubMed] [Google Scholar]
- Saron CD, Foxe JJ, Simpson GV, Vaughan HG. Interhemispheric visuomotor activation: Spatiotemporal electrophysiology related to reaction time. In: Zaidel E, Iacoboni M, editors. The Parallel Brain: The Cognitive Neuroscience of the Corpus Callosum. Cambridge, MA: MIT Press; 2003. pp. 171–219. [Google Scholar]
- Spielberger CD. Self-evaluation questionnaire. STAI Form X-2. Palo Alto, CA: Consulting Psychologists Press; 1968. [Google Scholar]
- Terasaki O, Okazaki M. Transcallosal conduction time measured by visual hemifield stimulation with face images. NeuroReport. 2002;13:97–99. doi: 10.1097/00001756-200201210-00023. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Banich MT. Global-local interference modulated by communication between the hemispheres. Journal of Experimental Psychology: General. 1999;128:283–308. doi: 10.1037//0096-3445.128.3.283. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Banich MT. The cerebral hemispheres cooperate to perform complex but not simple tasks. Neuropsychology. 2000;14:41–59. doi: 10.1037//0894-4105.14.1.41. [DOI] [PubMed] [Google Scholar]
- Wells A. A cognitive model of GAD: Metacognitions and pathological worry. In: Heimberg RG, Turk CL, Mennin DS, editors. Generalized Anxiety Disorder: Advances in Research and Practice. New York: Guilford Press; 2004. pp. 164–186. [Google Scholar]
- Wittling W. Brain asymmetry in the control of autonomic-physiologic activity. In: Davidson RJ, Hugdahl K, editors. Brain Asymmetry. Cambridge, MA: MIT Press; 1995. pp. 305–357. [Google Scholar]
- Wittling W, Block A, Schweiger E, Genzel S. Hemisphere asymmetry in sympathetic control of the human myocardium. Brain and Cognition. 1998;38:17–35. doi: 10.1006/brcg.1998.1000. [DOI] [PubMed] [Google Scholar]
- Zaidel E, Iacoboni M, editors. The parallel brain: The cognitive neuroscience of the corpus callosum. Cambridge, MA: MIT Press; 2003. [Google Scholar]