Abstract
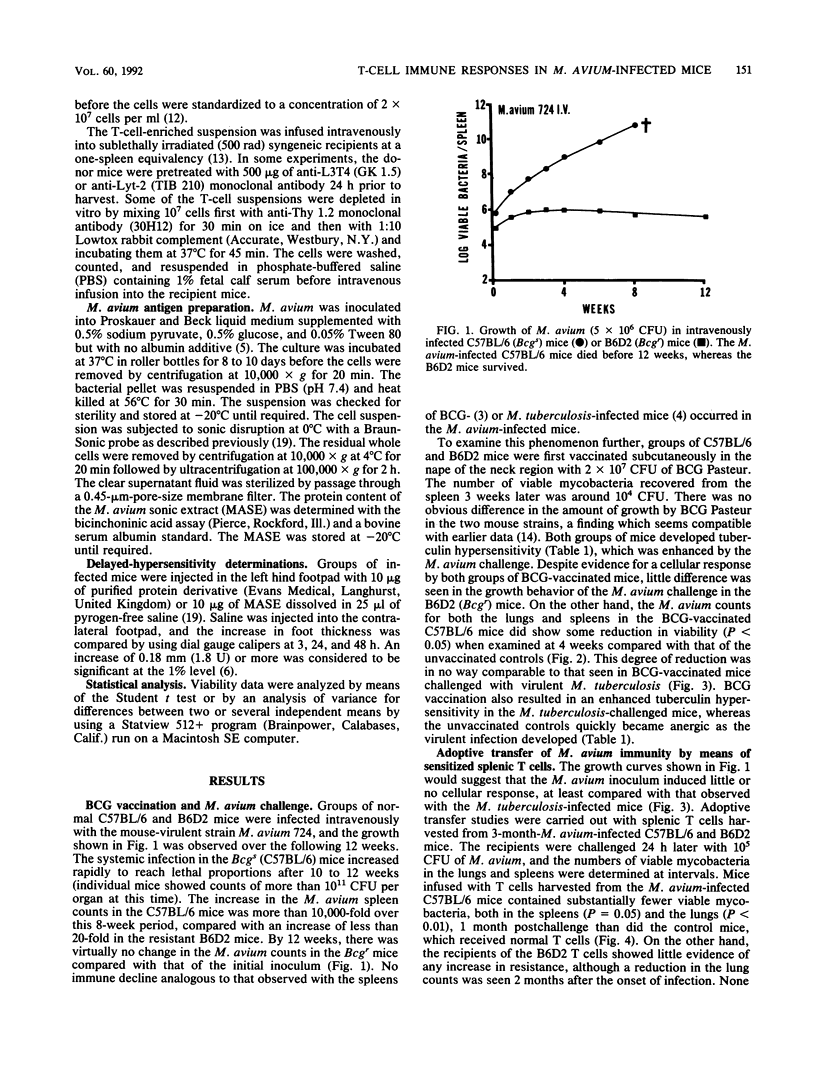
Mycobacterium avium infection was substantially more severe in C57BL/6 (Bcgs) than in (C57BL/6 x DBA/2)F1 hybrid (Bcgr) mice both in terms of bacterial growth in the spleens and lungs and in host survival. Prior Mycobacterium bovis BCG vaccination resulted in increased resistance as well as enhanced tuberculin hypersensitivity to both PPD-S (Mycobacterium tuberculosis) and PPD-A (M. avium). Mice heavily infected with M. avium were used as T-cell donors in an adoptive transfer system. Substantial resistance was observed for both recipient hosts regardless of the genotype of the donor strain. Transfer of resistance was ablated by treatment of the immune spleen cells with anti-Thy 1.2 monoclonal antibody and complement or by cyclophosphamide treatment. Spleen cells which were monodepleted of L3T4+ or Lyt-2+ T cells did not lose their ability to transfer resistance against a subsequent challenge. However, when these cells were doubly deleted, all resistance was ablated in both the BCG-susceptible and -resistant mice. The recipient host expressed a detectable adoptive immune response although the donor had been unable to reduce the growth of the primary M. avium infection in vivo.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chaisson R. E., Hopewell P. C. Mycobacteria and AIDS mortality. Am Rev Respir Dis. 1989 Jan;139(1):1–3. doi: 10.1164/ajrccm/139.1.1. [DOI] [PubMed] [Google Scholar]
- Collins F. M. Antituberculous immunity: new solutions to an old problem. Rev Infect Dis. 1991 Sep-Oct;13(5):940–950. doi: 10.1093/clinids/13.5.940. [DOI] [PubMed] [Google Scholar]
- Collins F. M. In vivo vs. in vitro killing of virulent Mycobacterium tuberculosis. Res Microbiol. 1990 Feb;141(2):212–266. doi: 10.1016/0923-2508(90)90033-m. [DOI] [PubMed] [Google Scholar]
- Collins F. M., Lamb J. R., Young D. B. Biological activity of protein antigens isolated from Mycobacterium tuberculosis culture filtrate. Infect Immun. 1988 May;56(5):1260–1266. doi: 10.1128/iai.56.5.1260-1266.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M., Mackaness G. B. The relationship of delayed hypersensitivity to acquired antituberculous immunity. I. Tuberculin sensitivity and resistance to reinfection in BCG-vaccinated mice. Cell Immunol. 1970 Sep;1(3):253–265. doi: 10.1016/0008-8749(70)90047-x. [DOI] [PubMed] [Google Scholar]
- Collins F. M., Morrison N. E., Montalbine V. Immune response to persistent mycobacterial infection in mice. Infect Immun. 1978 May;20(2):430–438. doi: 10.1128/iai.20.2.430-438.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M., Stokes R. W. Mycobacterium avium-complex infections in normal and immunodeficient mice. Tubercle. 1987 Jun;68(2):127–136. doi: 10.1016/0041-3879(87)90028-6. [DOI] [PubMed] [Google Scholar]
- Goto Y., Nakamura R. M., Takahashi H., Tokunaga T. Genetic control of resistance to Mycobacterium intracellulare infection in mice. Infect Immun. 1984 Oct;46(1):135–140. doi: 10.1128/iai.46.1.135-140.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Källenius G., Hoffner S. E., Svenson S. B. Does vaccination with bacille Calmette-Guérin protect against AIDS? Rev Infect Dis. 1989 Mar-Apr;11(2):349–351. doi: 10.1093/clinids/11.2.349. [DOI] [PubMed] [Google Scholar]
- Orme I. M., Collins F. M. Crossprotection against nontuberculous mycobacterial infections by Mycobacterium tuberculosis memory immune T lymphocytes. J Exp Med. 1986 Jan 1;163(1):203–208. doi: 10.1084/jem.163.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme I. M., Collins F. M. Protection against Mycobacterium tuberculosis infection by adoptive immunotherapy. Requirement for T cell-deficient recipients. J Exp Med. 1983 Jul 1;158(1):74–83. doi: 10.1084/jem.158.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme I. M., Stokes R. W., Collins F. M. Genetic control of natural resistance to nontuberculous mycobacterial infections in mice. Infect Immun. 1986 Oct;54(1):56–62. doi: 10.1128/iai.54.1.56-62.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanus V. Childhood tuberculosis in Sweden. An epidemiological study made six years after the cessation of general BCG vaccination of the newborn. Tubercle. 1983 Jun;64(2):101–110. doi: 10.1016/0041-3879(83)90034-x. [DOI] [PubMed] [Google Scholar]
- Stokes R. W., Collins F. M. Passive transfer of immunity of Mycobacterium avium in susceptible and resistant strains of mice. Clin Exp Immunol. 1990 Jul;81(1):109–115. doi: 10.1111/j.1365-2249.1990.tb05299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes R. W., Orme I. M., Collins F. M. Role of mononuclear phagocytes in expression of resistance and susceptibility to Mycobacterium avium infections in mice. Infect Immun. 1986 Dec;54(3):811–819. doi: 10.1128/iai.54.3.811-819.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson S. R., Morrison N. E., Collins F. M. Delayed hypersensitivity responses in mice and guinea pigs to Mycobacterium leprae, Mycobacterium vaccae, and Mycobacterium nonchromogenicum cytoplasmic proteins. Infect Immun. 1979 Jul;25(1):229–236. doi: 10.1128/iai.25.1.229-236.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolinsky E. Nontuberculous mycobacteria and associated diseases. Am Rev Respir Dis. 1979 Jan;119(1):107–159. doi: 10.1164/arrd.1979.119.1.107. [DOI] [PubMed] [Google Scholar]


