Abstract
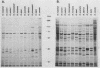
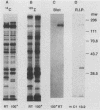
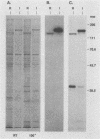
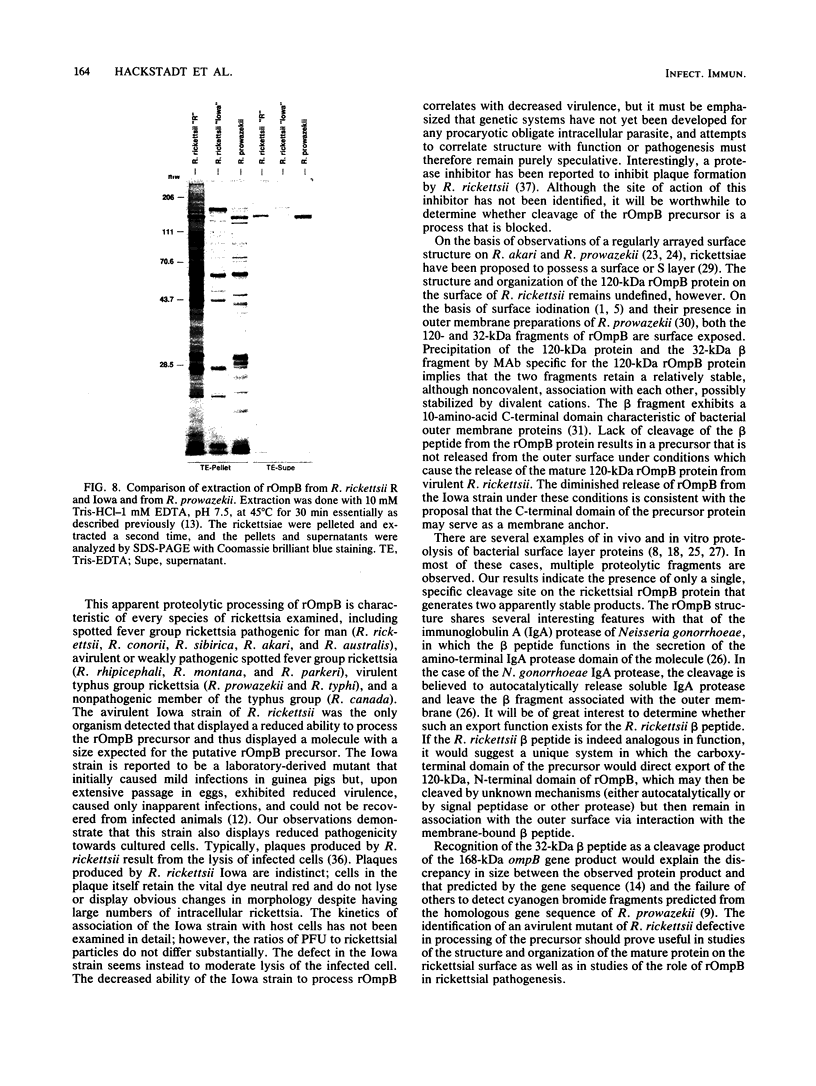
The 120-kDa rickettsial outer membrane protein (rOmpB) is encoded by a gene with the capacity to encode a protein of approximately 168 kDa. The carboxy-terminal end of the molecule is apparently cleaved to yield 120- and 32-kDa products. Both polypeptides are surface exposed and remain associated with the outer membrane of intact rickettsiae. All species of rickettsiae examined display similar cleavage of rOmpB. Comparison of diverse species of rickettsiae demonstrate a conserved N terminus of the 32-kDa fragment, with a predicted procaryotic secretory signal peptide immediately upstream of the proposed cleavage site. Coprecipitation of the 120-kDa rOmpB protein and the 32-kDa peptide by monoclonal antibodies specific for the 120-kDa portion of the molecule suggests that the two fragments remain noncovalently associated on the surface of rickettsiae. Analysis of an avirulent mutant of Rickettsia rickettsii revealed reduced amounts of the 120- and 32-kDa fragments, but with a correspondingly larger rOmpB protein that displayed properties expected of the putative precursor. This avirulent mutant grows intracellularly but fails to cause the lysis of infected cells that is typical of R. rickettsii. DNA sequence analysis of the region of the gene encoding the cleavage site of the avirulent strain revealed no difference from the sequence obtained from virulent R. rickettsii. The 168-kDa putative precursor of the avirulent strain of R. rickettsii was not extracted from the surface by dilute buffers, as is the 120-kDa protein of virulent R. rickettsii or R. prowazekii. These latter results suggest that the 32-kDa C-terminal region of the molecule may serve as a membrane anchor domain.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anacker R. L., List R. H., Mann R. E., Hayes S. F., Thomas L. A. Characterization of monoclonal antibodies protecting mice against Rickettsia rickettsii. J Infect Dis. 1985 Jun;151(6):1052–1060. doi: 10.1093/infdis/151.6.1052. [DOI] [PubMed] [Google Scholar]
- Anacker R. L., List R. H., Mann R. E., Wiedbrauk D. L. Antigenic heterogeneity in high- and low-virulence strains of Rickettsia rickettsii revealed by monoclonal antibodies. Infect Immun. 1986 Feb;51(2):653–660. doi: 10.1128/iai.51.2.653-660.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker R. L., Mann R. E., Gonzales C. Reactivity of monoclonal antibodies to Rickettsia rickettsii with spotted fever and typhus group rickettsiae. J Clin Microbiol. 1987 Jan;25(1):167–171. doi: 10.1128/jcm.25.1.167-171.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker R. L., McDonald G. A., List R. H., Mann R. E. Neutralizing activity of monoclonal antibodies to heat-sensitive and heat-resistant epitopes of Rickettsia rickettsii surface proteins. Infect Immun. 1987 Mar;55(3):825–827. doi: 10.1128/iai.55.3.825-827.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker R. L., Philip R. N., Williams J. C., List R. H., Mann R. E. Biochemical and immunochemical analysis of Rickettsia rickettsii strains of various degrees of virulence. Infect Immun. 1984 Jun;44(3):559–564. doi: 10.1128/iai.44.3.559-564.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson B. E., McDonald G. A., Jones D. C., Regnery R. L. A protective protein antigen of Rickettsia rickettsii has tandemly repeated, near-identical sequences. Infect Immun. 1990 Sep;58(9):2760–2769. doi: 10.1128/iai.58.9.2760-2769.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batteiger B., Newhall W. J., 5th, Jones R. B. The use of Tween 20 as a blocking agent in the immunological detection of proteins transferred to nitrocellulose membranes. J Immunol Methods. 1982 Dec 30;55(3):297–307. doi: 10.1016/0022-1759(82)90089-8. [DOI] [PubMed] [Google Scholar]
- Baumeister W., Karrenberg F., Rachel R., Engel A., ten Heggeler B., Saxton W. O. The major cell envelope protein of Micrococcus radiodurans (R1). Structural and chemical characterization. Eur J Biochem. 1982 Jul;125(3):535–544. doi: 10.1111/j.1432-1033.1982.tb06715.x. [DOI] [PubMed] [Google Scholar]
- Carl M., Dobson M. E., Ching W. M., Dasch G. A. Characterization of the gene encoding the protective paracrystalline-surface-layer protein of Rickettsia prowazekii: presence of a truncated identical homolog in Rickettsia typhi. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8237–8241. doi: 10.1073/pnas.87.21.8237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching W. M., Dasch G. A., Carl M., Dobson M. E. Structural analyses of the 120-kDa serotype protein antigens of typhus group rickettsiae. Comparison with other S-layer proteins. Ann N Y Acad Sci. 1990;590:334–351. doi: 10.1111/j.1749-6632.1990.tb42241.x. [DOI] [PubMed] [Google Scholar]
- Cory J., Yunker C. E., Ormsbee R. A., Peacock M., Meibos H., Tallent G. Plaque assay of rickettsiae in a mammalian cell line. Appl Microbiol. 1974 Jun;27(6):1157–1161. doi: 10.1128/am.27.6.1157-1161.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox H. R. CULTIVATION OF RICKETTSIAE OF THE ROCKY MOUNTAIN SPOTTED FEVER, TYPHUS AND Q FEVER GROUPS IN THE EMBRYONIC TISSUES OF DEVELOPING CHICKS. Science. 1941 Oct 31;94(2444):399–403. doi: 10.1126/science.94.2444.399. [DOI] [PubMed] [Google Scholar]
- Dasch G. A. Isolation of species-specific protein antigens of Rickettsia typhi and Rickettsia prowazekii for immunodiagnosis and immunoprophylaxis. J Clin Microbiol. 1981 Sep;14(3):333–341. doi: 10.1128/jcm.14.3.333-341.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore R. D., Jr, Cieplak W., Jr, Policastro P. F., Hackstadt T. The 120 kilodalton outer membrane protein (rOmp B) of Rickettsia rickettsii is encoded by an unusually long open reading frame: evidence for protein processing from a large precursor. Mol Microbiol. 1991 Oct;5(10):2361–2370. doi: 10.1111/j.1365-2958.1991.tb02082.x. [DOI] [PubMed] [Google Scholar]
- Gilmore R. D., Jr, Joste N., McDonald G. A. Cloning, expression and sequence analysis of the gene encoding the 120 kD surface-exposed protein of Rickettsia rickettsii. Mol Microbiol. 1989 Nov;3(11):1579–1586. doi: 10.1111/j.1365-2958.1989.tb00143.x. [DOI] [PubMed] [Google Scholar]
- Klein P., Kanehisa M., DeLisi C. The detection and classification of membrane-spanning proteins. Biochim Biophys Acta. 1985 May 28;815(3):468–476. doi: 10.1016/0005-2736(85)90375-x. [DOI] [PubMed] [Google Scholar]
- Klose M., Schwarz H., MacIntyre S., Freudl R., Eschbach M. L., Henning U. Internal deletions in the gene for an Escherichia coli outer membrane protein define an area possibly important for recognition of the outer membrane by this polypeptide. J Biol Chem. 1988 Sep 15;263(26):13291–13296. [PubMed] [Google Scholar]
- Koval S. F., Murray R. G. The isolation of surface array proteins from bacteria. Can J Biochem Cell Biol. 1984 Nov;62(11):1181–1189. doi: 10.1139/o84-152. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Mohana Rao J. K., Argos P. A conformational preference parameter to predict helices in integral membrane proteins. Biochim Biophys Acta. 1986 Jan 30;869(2):197–214. doi: 10.1016/0167-4838(86)90295-5. [DOI] [PubMed] [Google Scholar]
- Morrison M. The determination of the exposed proteins on membranes by the use of lactoperoxidase. Methods Enzymol. 1974;32:103–109. doi: 10.1016/0076-6879(74)32013-7. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Mizushima S. Effects of heating in dodecyl sulfate solution on the conformation and electrophoretic mobility of isolated major outer membrane proteins from Escherichia coli K-12. J Biochem. 1976 Dec;80(6):1411–1422. doi: 10.1093/oxfordjournals.jbchem.a131414. [DOI] [PubMed] [Google Scholar]
- Palmer E. L., Mallavia L. P., Tzianabos T., Obijeski J. F. Electron microscopy of the cell wall of Rickettsia prowazeki. J Bacteriol. 1974 Jun;118(3):1158–1166. doi: 10.1128/jb.118.3.1158-1166.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer E. L., Martin M. L., Mallavia L. Ultrastucture of the surface of Rickettsia prowazeki and Rickettsia akari. Appl Microbiol. 1974 Oct;28(4):713–716. doi: 10.1128/am.28.4.713-716.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei Z., Ellison R. T., 3rd, Lewis R. V., Blaser M. J. Purification and characterization of a family of high molecular weight surface-array proteins from Campylobacter fetus. J Biol Chem. 1988 May 5;263(13):6416–6420. [PubMed] [Google Scholar]
- Pohlner J., Halter R., Beyreuther K., Meyer T. F. Gene structure and extracellular secretion of Neisseria gonorrhoeae IgA protease. 1987 Jan 29-Feb 4Nature. 325(6103):458–462. doi: 10.1038/325458a0. [DOI] [PubMed] [Google Scholar]
- Sleytr U. B., Messner P. Crystalline surface layers on bacteria. Annu Rev Microbiol. 1983;37:311–339. doi: 10.1146/annurev.mi.37.100183.001523. [DOI] [PubMed] [Google Scholar]
- Smith D. K., Winkler H. H. Separation of inner and outer membranes of Rickettsia prowazeki and characterization of their polypeptide compositions. J Bacteriol. 1979 Feb;137(2):963–971. doi: 10.1128/jb.137.2.963-971.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struyvé M., Moons M., Tommassen J. Carboxy-terminal phenylalanine is essential for the correct assembly of a bacterial outer membrane protein. J Mol Biol. 1991 Mar 5;218(1):141–148. doi: 10.1016/0022-2836(91)90880-f. [DOI] [PubMed] [Google Scholar]
- Swanson J., Barrera O. Immunological characteristics of gonococcal outer membrane protein II assessed by immunoprecipitation, immunoblotting, and coagglutination. J Exp Med. 1983 May 1;157(5):1405–1420. doi: 10.1084/jem.157.5.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Colony opacity and protein II compositions of gonococci. Infect Immun. 1982 Jul;37(1):359–368. doi: 10.1128/iai.37.1.359-368.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D. H., Cain B. G. The rickettsial plaque. Evidence for direct cytopathic effect of Rickettsia rickettsii. Lab Invest. 1980 Oct;43(4):388–396. [PubMed] [Google Scholar]
- Walker D. H., Tidwell R. R., Rector T. M., Geratz J. D. Effect of synthetic protease inhibitors of the amidine type on cell injury by Rickettsia rickettsii. Antimicrob Agents Chemother. 1984 May;25(5):582–585. doi: 10.1128/aac.25.5.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. C., Walker D. H., Peacock M. G., Stewart S. T. Humoral immune response to Rocky Mountain spotted fever in experimentally infected guinea pigs: immunoprecipitation of lactoperoxidase 125I-labeled proteins and detection of soluble antigens of Rickettsia rickettsii. Infect Immun. 1986 Apr;52(1):120–127. doi: 10.1128/iai.52.1.120-127.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. Signal sequences. The limits of variation. J Mol Biol. 1985 Jul 5;184(1):99–105. doi: 10.1016/0022-2836(85)90046-4. [DOI] [PubMed] [Google Scholar]