Abstract
Viral fusion protein trimers can play a critical role in limiting lipids in membrane fusion. Because the trimeric oligomer of many viral fusion proteins is often stabilized by hydrophobic 4-3 heptad repeats, higher-order oligomers might be stabilized by similar sequences. There is a hydrophobic 4-3 heptad repeat contiguous to a putative oligomerization domain of Autographa californica multicapsid nucleopolyhedrovirus envelope glycoprotein GP64. We performed mutagenesis and peptide inhibition studies to determine if this sequence might play a role in catalysis of membrane fusion. First, leucine-to-alanine mutants within and flanking the amino terminus of the hydrophobic 4-3 heptad repeat motif that oligomerize into trimers and traffic to insect Sf9 cell surfaces were identified. These mutants retained their wild-type conformation at neutral pH and changed conformation in acidic conditions, as judged by the reactivity of a conformationally sensitive mAb. These mutants, however, were defective for membrane fusion. Second, a peptide encoding the portion flanking the GP64 hydrophobic 4-3 heptad repeat was synthesized. Adding peptide led to inhibition of membrane fusion, which occurred only when the peptide was present during low pH application. The presence of peptide during low pH application did not prevent low pH–induced conformational changes, as determined by the loss of a conformationally sensitive epitope. In control experiments, a peptide of identical composition but different sequence, or a peptide encoding a portion of the Ebola GP heptad motif, had no effect on GP64-mediated fusion. Furthermore, when the hemagglutinin (X31 strain) fusion protein of influenza was functionally expressed in Sf9 cells, no effect on hemagglutinin-mediated fusion was observed, suggesting that the peptide does not exert nonspecific effects on other fusion proteins or cell membranes. Collectively, these studies suggest that the specific peptide sequences of GP64 that are adjacent to and include portions of the hydrophobic 4-3 heptad repeat play a dynamic role in membrane fusion at a stage that is downstream of the initiation of protein conformational changes but upstream of lipid mixing.
INTRODUCTION
The mechanism of membrane fusion is currently a field of intense investigation, both for intracellular trafficking and enveloped viral infection. For viral fusion protein function, we have previously suggested that fusion protein oligomers form transient and cooperative higher-order structures or rings (Chernomordik et al., 1998). These transient ring structures are proposed to facilitate conversion of kinetic energy, released during protein conformational change, into work on the target and host membranes required for membrane fusion. Indeed, biochemical evidence for baculovirus GP64 aggregates during membrane fusion has recently been reported (Markovic et al. 1998). Whether and how viral fusion proteins might function in a coordinated and cooperative manner is a major question in the field of membrane fusion. Because hydrophobic 4-3 heptad repeats are observed in viral (Buckland and Wild, 1989) as well as cellular fusion proteins (Sutton et al., 1998) and might facilitate protein-protein interactions required for the cooperative ring fusion model, we investigated this region’s role in membrane fusion mediated by GP64.
GP64, a class I membrane glycoprotein, constitutes the major envelope protein of the Autographa californica multicapsid nucleopolyhedrovirus (AcMNPV) (Volkman and Goldsmith, 1984; Volkman et al., 1984). GP64 is temporally expressed during both the early and late phases of viral infection (Blissard and Rohrmann, 1989; Jarvis and Garcia, 1994; Kogan et al., 1994; Oomens et al., 1995; Garrity et al., 1997) and oligomerizes to form two electrophoretically distinct trimers (Oomens et al., 1995; Markovic et al., 1998). This protein is both necessary and sufficient for mediating pH-dependent membrane fusion (Blissard and Wenz, 1992), is essential for cell-to-cell transmission of the enveloped virion (Monsma et al., 1996), and has host cell receptor–binding activity (Hefferon et al., 1999). Mutagenesis and anti-peptide antibody studies identified a putative hydrophobic membrane fusion peptide between amino acids 220 and 230 of the highly homologous Orgyia pseudotsugata MNPV (OpMNPV) GP64 protein (Monsma and Blissard, 1995). A second hydrophobic region within OpMNPV has been identified between amino acids 327 and 335, which is important for oligomerization and cell surface expression. This second hydrophobic region lies within a highly conserved 4-3 heptad repeat from residues 299 to 346 that is predicted to form an amphipathic α helix (Monsma and Blissard, 1995). Disruption of the hydrophobic 4-3 heptad repeat’s putative α helical character by proline substitutions in one or two positions (leucines) in this region results in defective trimerization/oligomerization and cell surface expression (Monsma and Blissard, 1995). Studying AcMNPV GP64 protein, Kim and Yang (1996) found that mutation of two tandem leucines to alanine within the leucine zipper motif does not destroy the fusogenic properties of GP64.
Hydrophobic 4-3 heptad repeats, characteristic of coiled-coil sequences, are frequently observed within the extracellular domains of viral envelope proteins that mediate fusion of the envelope with cell surface or endosomal membranes during the infectious process (Buckland and Wild, 1989). Examples of this motif are encoded in the human immunodeficiency virus (HIV) GP41, paramyxovirus F, Ebola GP, and baculovirus GP64 membrane proteins (Blissard and Rohrmann, 1989; Whitford et al., 1989; Chambers et al., 1990; Sanchez et al., 1996; Weissenhorn et al., 1998). X-ray crystallographic studies on a number of viral fusion proteins have revealed a common theme of a central trimeric amino-terminal core sequence stabilized by coiled-coil packing, with an antiparallel packing of a second α helix against this central core. Specifically for HIV gp41, it has been shown that two separate heptad repeats form a six-helix bundle with the amino-terminal heptad repeats of gp41 to create a central trimeric core, with the carboxy-terminal heptad repeat packing in an antiparallel manner within the outside grooves of the core trimer (Weissenhorn et al., 1996, 1997; Chan et al., 1997). This general architecture is observed in other viral fusion proteins, such as other retroviruses (Blacklow et al., 1995; Fass and Kim, 1995; Lu et al., 1995; Fass et al., 1996; Malashkevich et al., 1998), Ebola GP (Wiessenhorn et al., 1998; Malashkevich et al., 1999), paramyxovirus F (Joshi et al., 1998; Baker et al., 1999; Dutch et al., 1999), and influenza hemagglutinin (HA) proteins (Carr and Kim, 1993; Bullough et al., 1994). Conversion of fusion proteins to a rod-like structure via formation of a long trimeric coiled coil upon low pH application has been observed and forms the basis of a proposed spring-loaded mechanism by which hydrophobic amino-terminal “fusion peptides” are projected into the target membrane, ultimately resulting in fusion of the viral envelope with the target cell membrane (Carr and Kim 1993; Chan et al., 1997; Weissenhorn et al., 1997, 1999).
For paramyxoviruses and HIV, evidence suggests that the hydrophobic 4-3 heptad repeats are directly involved in the viral fusion process. Single leucine-to-alanine mutations of the F proteins from paramyxoviruses do not block fusion, but multiple leucine-to-alanine mutations do abolish fusion activity of the protein but do not negatively affect F protein oligomerization or cell surface expression (Buckland et al., 1992; Sergel-Germano et al., 1994; Reitter et al., 1995). Nonconservative mutagenesis of the heptad repeat of HIV blocks cell-to-cell fusion and viral infectivity but does not affect oligomerization or cell surface transport of the GP120-GP41 protein (Dubay et al., 1992; Wild et al., 1994a; Chen et al., 1995).
Short peptides containing hydrophobic 4-3 heptad repeats (leucine zipper–like motifs) are potent inhibitors of syncytia formation and viral infection. Examples include peptides derived from envelope proteins of HIV (Wild et al., 1992, 1994b; Jiang et al., 1993a,b; Judice et al., 1997) and paramyxoviruses (Rapaport et al., 1995; Lambert et al., 1996; Yao and Compans, 1996; Ghosh et al., 1997; Wild and Buckland, 1997; Young et al., 1997; Ben-Efraim et al., 1999). These peptides are presumed to mimic functional domains of the paramyxovirus and HIV fusion proteins and therefore disrupt the activity of these proteins (Young et al., 1997). For HIV gp41, peptide (DP178) inhibition-escape mutants with altered sequences within the amino-terminal heptad repeat have recently been isolated (Rimsky et al., 1998). Furthermore, reduced binding of DP178 binding by these escape mutants indicates that the mechanism of peptide inhibition is via direct binding to the heptad repeat (Wild et al., 1995; Rimsky et al., 1998).
Several different functional roles for the hydrophobic 4-3 heptad repeat motifs in the pH-independent envelope fusion proteins of HIV and paramyxovirus are currently proposed. Because two discrete HIV GP41 hydrophobic 4-3 heptad repeats peptides (DP107 and DP178) interact in solution, these two regions may interact to form a “molecular clamp” that stabilizes the inactive conformation of the GP120-GP41 complex (Chen et al., 1995). Experiments with paramyxovirus heptad repeat peptides also appear to support the molecular clamp hypothesis because (a) these peptides demonstrate strain-specific inhibition of F protein function and (b) the two 4-3 heptad repeat regions interact in solution (Lambert et al., 1996; Young et al., 1997, 1999). It has also been suggested that the hydrophobic 4-3 heptad repeat sequences form a hydrophobic face that directly interacts with or disturbs the host membrane concomitant with disruption of the target membrane (Rabenstein and Shin, 1995; Reitter et al. 1995).
In the current study, we examined the single hydrophobic 4-3 heptad repeat and adjacent amino-terminal flanking sequences of the AcMNPV GP64 protein to determine if this region plays a functional role in the membrane fusion processes mediated by GP64. Although GP64 proteins are trimeric, there is currently no structural evidence that the baculovirus GP64 homologues form a six-helix bundle analogous to HIV gp41 or paramyxovirus F proteins. Furthermore, although the hydrophobic 4-3 heptad repeat predicts formation of an α helix (Monsma and Blissard, 1995), direct evidence for GP64 coiled-coil interactions is lacking. However, with the use of leucine-to-alanine scanning mutations that would be predicted not to disturb the α helical character of the hydrophobic 4-3 heptad repeat, we show that this region plays a direct role in membrane fusion. In addition, we demonstrate that a 29-amino acid peptide encoding a portion of the GP64 hydrophobic 4-3 heptad repeat motif is capable of inhibiting wild-type AcMNPV GP64-mediated membrane fusion.
MATERIALS AND METHODS
Sf9 cells were obtained from the American Type Culture Collection (Manassas, VA) and cultured at 28°C in Grace’s insect cell medium (Invitrogen, Carlsbad CA) supplemented with 10% FBS, 50 U/ml penicillin, and 50 mg/ml streptomycin. Cells were used only until their 25th passage. An AcMNPV GP64 expression plasmid, pATH-1, was generated by subcloning the AcMNPV gp64 ORF under the control of an optimized OpMNPV gp64 early promoter (p64CAT-166) (Blissard and Rohrmann, 1991) to generate plasmid p166B + 1-Ac Spe/Bgl. The AcMNPV gp64 ORF was inserted into the BamHI site at position +1 (relative to the original OpMNPV gp64 ATG) of plasmid p64–166B + 1 by ligating a 1732-base pair SpeI/BglII fragment containing the AcMNPV ORF and downstream poly(A) signals to BamHI-digested p64–166B + 1, followed by fill in and ligation. The pATH-1 plasmid was subsequently generated from plasmid p166B + 1-Ac Spe/Bgl by site-directed mutagenesis to engineer two silent point mutations (G to c and G to t) at nucleotides 627 and 1032 of the AcMNPV gp64 ORF, which created Tth111I and HincII sites, respectively.
Cell line Sf9Ac64–5 is a stably transformed cell line that constitutively expresses the AcMNPV GP64 protein. Sf9Ac64–5 was generated from Sf9 cells as described previously (Monsma et al., 1996; Plonsky et al., 1999) by transfection with plasmids p166B + 1-Ac Spe/Bgl and pAcie1-Neo, followed by selection in G418.
Mutagenesis was performed with the use of a site-directed mutagenesis kit (QuickChange, Stratagene, La Jolla, CA) according to the manufacturer’s recommendations. Supercoiled plasmids were prepared by double banding on CsCl gradients. Mutations were confirmed by automated DNA sequence analysis. The HA-Sf9 cell expression vector, pHArHD, was constructed by creating a HindIII site at −27 of the promoter in plasmid pATH-1 and excision of a HindIII/HpaI fragment, followed by ligation of a linker (HA1, 5′-agcttcaataaggaacacacaagcaaccatggtatcgtcgacccggg-3′; HA2, 5′-agttattccttgtgtgttcgttggtaccatagcagctgggccc-3′). A 1.7-kilobase BspHI-SalI fragment containing the HA ORF of the X31 strain of influenza virus was ligated to the NcoI site. To ensure that there were no functional differences between the pATH-1– and the HA-expressing plasmid promoters, the sequence within the promoter region was restored to its original sequence by mutagenesis. Transient transfection of Sf9 cells was performed by calcium phosphate precipitation, as described by O’Reily et al. (1994).
A cell surface ELISA (cELISA) was performed by calcium phosphate transfection of a confluent six-well tissue culture dish (4 × 106 cells) with 10 μg of pATH-1 (expressing wild-type gp64) or a leucine zipper mutant plasmid, as described by Monsma and Blissard (1995), with the following modifications. Cells were cultured for 24 h after transfection, followed by a 10-min fixation with 4% paraformaldehyde. The conformationally sensitive AcV1 mAb (which recognizes only the neutral pH form of GP64) was used as the primary antibody, followed by an alkaline phosphatase–conjugated goat anti-mouse immunoglobulin G (Pierce, Rockford, IL, and KPL Laboratories, Gaithersburg, MD). A dilute immunopure p-nitrophenyl phosphate substrate (Pierce) in Tris-buffered saline solution was used for a colorimetric absorbance assay at 405 nm. For studies of pH-sensitive GP64 and mutant conformational changes, cELISA was performed as described previously with the following modifications. A total of 1 × 106 cells were transfected, citrate buffer (50 mM NaCl, 10 mM KCl, 4 mM CaCl2, 5 mM MgCl2, 5 mM glucose, 50 mM citric acid, 20 mM sucrose, pH 4.8) was applied for 10 min before application of paraformaldehyde, and cells were cultured for 48 h after transfection.
For Western blots of nonreducing SDS-PAGE gels, ∼1 × 107 cells were transfected with 100 μg of plasmid DNA by calcium phosphate transfection. Cells were lysed at 48 h after transfection in 1.5% SDS, 10% sucrose, 50 mM Tris, pH 6.8, followed by heat treatment at 85°C for 10 min and sonication with a microtip for 30 s at setting 7 (Sonifer cell disrupter, Heat Systems-Ultrasonics, Plainview, NY). Fifty micrograms of protein was loaded on a 4–12% SDS-PAGE gel (Novex, San Diego, CA) and blotted on Immobilon transfer membranes (Millipore, Bedford, MA). The GP64 protein was visualized with the AcV5 mAb, which recognizes a denatured epitope within AcGP64, an alkaline phosphatase goat anti-mouse antibody (Kirkegaard and Perry Laboratories, Gaithersburg, MD), and ECF detection (Amersham, Arlington Heights, IL) with a scanner (Molecular Dynamics, Sunnyvale, CA).
Membrane fusion was assessed by measuring the transfer of a membrane-soluble dye from one cell to another and examining the extent of syncytia formation (Zimmerberg et al., 1994). Red blood cells (RBCs) from phenotypically normal donors were labeled with the lipid membrane dye PKH26 (Sigma Chemical, St. Louis, MO). Transfected Sf9 cells (5 μg of pATH-1 plasmid per 1.0–1.5 × 106 cells) at 48 h after transfection or stably transfected Sf9Ac64–5 cells (∼50% confluent) were mixed with labeled RBCs and allowed to settle. Membrane fusion was induced by treatment with PBS, pH 5.0, for 5 min, followed by PBS, pH 7.4. For experiments with HA-mediated fusion, 2 × 106 cells were transfected with 40 μg of HArHD plasmid for 48 h. Before fusion experiments, cells were treated with 100 μg/ml trypsin (Fluka Chemical, Milwaukee, WI) for 10 min at 37°C. HA incubations and fusion reactions were performed at 37°C. The extent of fusion was assessed with the use of a fluorescence microscope by visually counting the number of PKH26-labeled Sf9 cells and then dividing by the sum of that number and the number of nonlabeled Sf9 cells contacting RBCs.
Syncytia formation (Sf9-Sf9 fusion) assays were performed by transfection of six-well culture dishes with 5 μg of plasmid DNA. At 48 h after transfection, cells were treated with 2 ml of PBS at pH 5.0. Syncytia formation was observed by microscopic inspection 1 h after treatment with acidic PBS. The extent of fusion was determined by counting the number of cells fused and dividing by the sum of the number of contacting cells and the number of fused cells and expressing that number as a percentage.
GP64 leucine zipper (DLMHIQEELMYENDLLKMNIELMHAHINK), a randomly scrambled GP64 leucine zipper (EMLQINLEMEDLHAHIMELNYKNMKILHD), and Ebola GP leucine zipper (GLMHNQDGLICGLRQLANETTQALQLFLRA) peptides were synthesized by Research Genetics (Huntsville, AL). Peptides were dissolved in 100% DMSO at 20 mg/ml and diluted to working concentrations in PBS of the appropriate pH. For studies of the inhibition of cell-to-cell membrane fusion by peptides, the appropriate peptide was diluted to the indicated concentration in PBS, pH 7.4, and added 10 min before the initiation of fusion. Fusion was induced with PBS, pH 5.0, for 5 min in the presence of peptide, followed by the addition of PBS, pH 7.4, in the presence of peptide. The extent of fusion was determined by membrane dye transfer and syncytia assay.
RESULTS
Mutagenesis of the Heptad Repeat
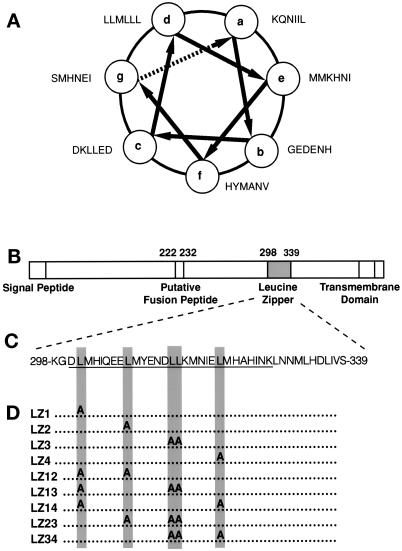
The hydrophobic 4-3 heptad repeat region of GP64 is displayed as a helical wheel (Figure 1A). The location and sequence of the 4-3 heptad repeat or leucine zipper–like motif within the GP64 polypeptide are also depicted (Figure 1, B and C). To evaluate the structural and functional roles of the heptad repeat motif, GP64 proteins containing various single and double leucine-to-alanine scanning mutations in the 4-3 heptad repeat region at positions 301, 308, 314, 315, and 321 were constructed (Figure 1D).
Figure 1.
(A) The AcMNPV GP64 leucine zipper (hydrophobic 4-3 heptad repeat) motif displayed as a helical wheel. (B) A representation of the AcMNPV gp64 protein. The locations of the signal peptide, the “putative fusion peptide,” the leucine zipper (hydrophobic 4-3 heptad repeat), and the transmembrane region are depicted. (C) The sequence of the AcMNPV leucine zipper motif is depicted. The GP64 peptide sequence used for inhibition studies is underlined. (D) Depiction of the leucine-to-alanine scanning mutations constructed.
Intracelluar Processing of Mutants
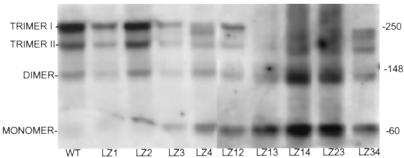
To characterize the function of mutant GP64 proteins containing heptad repeat mutations, expression plasmids encoding these mutant proteins (designated LZ) were transiently expressed in Sf9 cells as described in MATERIALS AND METHODS. At 48 h after transfection, oligomeric forms of heptad repeat mutants were resolved by nonreducing SDS-PAGE and evaluated by Western blotting (Figure 2) as described in MATERIALS AND METHODS. Wild-type GP64 extracts resulted in expression of two characteristic oligomeric forms (trimers I and II), with limited dimeric and monomeric forms observed. Cellular extracts expressing mutations LZ1, LZ2, and LZ3 (Figure 1D) resulted in oligomerization patterns similar to those in the wild type (Figure 2). Constructs LZ4 and LZ34 appeared to exhibit some processing or oligomerization defects, as indicated by an additional band that appeared between the trimer I and trimer II forms. Multiple mutants LZ13, LZ14, and LZ23 were grossly defective for oligomerization, as judged by the limited presence of trimers I and II and the proportionally large amounts of incompletely oligomerized/trimerized forms at 60 and 120 kDa. A portion of the LZ12 double mutant protein was oligomerized normally, as judged by the presence of trimers I and II.
Figure 2.
Analysis of oligomerization. Sf9 cells were transfected with expression plasmids encoding wild-type and GP64 mutant plasmids, and cells were lysed at 48 h after transfection under nonreducing conditions. GP64 proteins were resolved by SDS-PAGE and Western blotting. Apparent molecular weights as judged by molecular weight standards are shown on the right; GP64 oligomeric and monomeric species are identified on the left.
Cell Surface Expression and Conformation
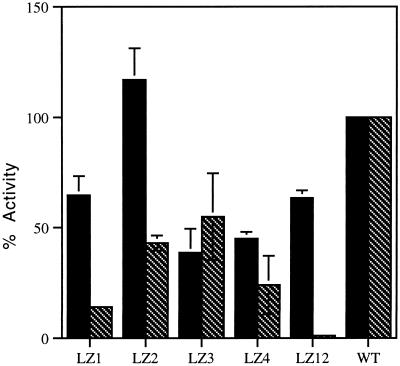
The relative cell surface expression of the LZ mutants was evaluated by cELISA (described in MATERIALS AND METHODS) with the use of the conformationally sensitive antibody AcV1. Variable surface expression was observed (Figure 3). Cell surface expression was generally lower for cell surface mutants than for wild-type GP64. Mutants LZ1, LZ2, LZ3, LZ4, and LZ12 all expressed at least one-third the level of wild-type GP64. Mutants expressing 25% or less of wild-type levels of surface GP64 were judged to be defective for cell surface localization. Mutants defective for surface localization included LZ34, LZ13, and LZ14. Because the AcV1 antibody recognizes a discontinuous epitope present in the native, neutral pH form of GP64 but not in conformations that result from exposure of GP64 to low pH, these results reflect the presence of surface protein in a conformation approximating or equivalent to the native, neutral pH conformation. Furthermore, treatment of mutants LZ1, LZ2, LZ3, LZ4, and LZ12 with citrate buffer, pH 4.8, before cELISA (with AcV1 antibody) resulted in a loss of immunoreactivity consistent with low pH induction of conformational change (see Figure 6).
Figure 3.
Relative cell surface expression and fusion activity of GP64 mutants. Cell surface expression (black bars) was determined by cELISA. Values represent the means from three separate experiments normalized to optical density signal from wild-type GP64 (100%). Relative cell-to-cell fusion activities (hatched bars) were determined by PKH26-membrane dye transfer from fusion target RBCs to fusion protein–expressing Sf9 cells. Values represent the means from three separate experiments normalized to wild-type GP64 (100%). Error bars represent SE. For mutants LZ13, LZ14, LZ23, and LZ34, all cell surface and relative fusion levels were <5% of wild-type values (data not shown).
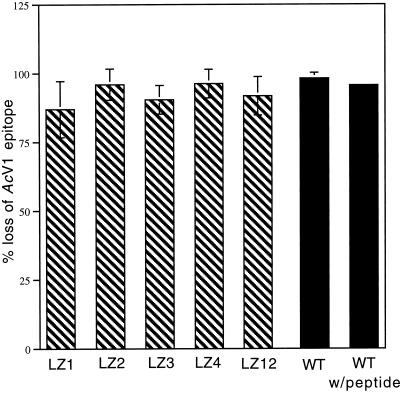
Figure 6.
Conformational changes of wild-type GP64 during peptide inhibition and fusion-defective mutants. The effect of the inhibitory peptide on the initiation of wild-type GP64 pH-induced conformational changes and the ability of leucine zipper mutants LZ1, LZ2, LZ3, LZ4, and LZ12 to undergo pH-induced conformational changes was assessed by cELISA. Black bars represent the percent change (loss) of wild-type (WT) GP64 immunoreactivity with the conformationally sensitive AcV1 antibody in the presence and absence of 100 μg/ml GP64 peptide compared with the wild-type untreated (neutral pH) value. Hatched bars represent the percent change (loss) of mutant immunoreactivity with the conformationally sensitive AcV1 antibody compared with the neutral pH value of each mutant. All results shown are from three separate trials. Error bars represent SE. Because citrate buffer, pH 4.8, rather than PBS, pH 5.0, was used for low pH application, peptide inhibition of membrane fusion with 100 μg/ml GP64 peptide and citrate buffer, pH 4.8, was measured. Results (48% of nonpeptide control) were comparable to those observed with PBS, pH 5.0.
Quantitation of GP64 Mutant Fusion Activity
Fusion activity was measured by assessing membrane lipid mixing between Sf9 cells expressing mutant constructs and RBCs labeled with a hydrophobic dye (Figure 3). All mutants displayed some reduced level of fusion activity. Mutants LZ13, LZ14, and LZ23, which were defective for oligomerization and cell surface localization, were also defective for fusion. Mutants LZ2 and LZ3 gave fusion results of 43 and 55% of wild-type levels, respectively. Results for the LZ3 mutation were consistent with results reported previously by Kim and Yang (1996). Interestingly, mutants LZ1 and LZ12, which did oligomerize and express mutant GP64 on the cell surface (65 and 63% of wild-type levels, respectively), were disproportionately more defective for fusion (13.8 and 3% of wild-type levels, respectively).
The Heptad Repeat Peptide Inhibits Wild-Type GP64 Activity
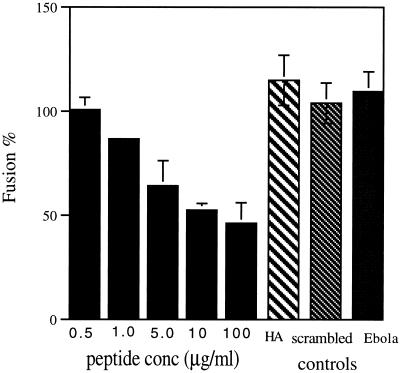
A 29-mer peptide (DLMHIQEELMYENDLLKMNIELMHAHINK), encoding a portion of the AcMNPV GP64 4-3 heptad repeat motif (amino acids 300–328; underlined in Figure 1C), was evaluated for inhibitory activity during GP64-mediated membrane fusion events. A concentration-dependent inhibition of GP64-mediated cell-to-cell fusion was observed (Figure 4). The presence of 10 μg/ml peptide throughout the process of membrane triggering resulted in ∼53% of fusion activity, as judged by fluorescent membrane dye transfer. Control experiments with the homologous peptide from the Ebola (Zaire strain) virus envelope GP (502-GLMHNQDGLICGLRQLANETTQALQLFLRA-532) and a randomly scrambled GP64 peptide (EMLQINLEMEDLHAHIMELNYKNMKILHD) of different sequence but having the same amino acid composition did not affect GP64-mediated membrane fusion. To determine whether the GP64 peptide was inhibiting fusion by a nonspecific interaction with the Sf9 cell membrane or by an interaction with the GP64 transmembrane protein, the effects of the GP64 peptide on influenza (X31 strain) HA-mediated Sf9-RBC membrane fusion were determined. Results indicate that the peptide had no effect on HA-mediated Sf9-RBC fusion (Figure 4).
Figure 4.
Fusion inhibition by the GP64 peptide. The effect of various concentrations of GP64 peptide present during the fusion of PKH26 membrane dye–labeled RBCs and stably transfected gp64-expressing Sf9 cells (Ac5 cell line) was determined by dye transfer assay. Controls to evaluate the specificity of the GP64 peptide’s effect were performed as follows. (HA) The GP64 peptide’s (10 μg/ml) effect on HA-mediated cell-to-cell fusion was evaluated with the use of transiently transfected Sf9 cells. (scrambled) A scrambled GP64 leucine zipper peptide (EMLQINLEMEDLHAHIMELNYKNMKILHD) of the same peptide composition but alternative sequence was tested for inhibition of GP64-mediated cell-to-cell fusion activity of transiently transfected Sf9 cells by membrane dye transfer at 10 μg/ml peptide. (Ebola) The GP leucine zipper peptide (GLMHNQDGLICGLRQLANETTQALQLFLRA) was tested for inhibition of GP64-mediated cell-to-cell fusion activity of transiently transfected Sf9 cells by membrane dye transfer at 10 μg/ml peptide. For all experiments, fusion was initiated by application of PBS, pH 5.0, and the extent of fusion was determined by counting fluorescently labeled Sf9 cells (fusion event) and dividing by the sum of PKH26-labeled RBCs in contact with Sf9 cells and fluorescently labeled Sf9 cells. Values represent the means from three separate experiments normalized to wild-type GP64-expressing (100%) or HA-expressing (100%) cells in the absence of peptide. All peptides were present before, during, and after induction of membrane fusion. Error bars represent SE.
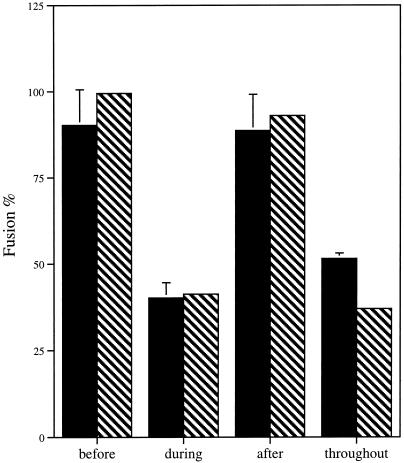
Because GP64-mediated fusion is a pH-triggered event, it is possible to determine at which temporal stage (before low pH triggering, during low pH triggering, or after low pH triggering) this peptide exerts its effects. The peptide (10 μg/ml) was effective only when present in the solution bathing the cells during the low pH application step (during low pH triggering), as judged by both syncytia formation and fluorescent membrane dye transfer (Figure 5). To determine if this stage-specific requirement for the peptide reflected an inhibition of the initial, low pH–dependent conformational changes of GP64, we measured cell surface GP64 conformation with the use of the mAb AcV1 that binds only to the native, neutral pH form of GP64. In the presence of 100 μg/ml peptide, cells were incubated at pH 4.8 and then cELISA was performed (Figure 6). The peptide had no significant effect on the loss of native conformation seen at low pH (compared with 100% mAb binding to neutral pH cells; after low pH treatment, 95.6 ± 1.3% loss of binding was detected in the presence of the peptide and 98.1 ± 2.1% loss of binding was observed without peptide). Because cELISA experiments used citrate buffer, pH 4.8, rather than PBS, pH 5.0, an independent experiment to confirm the inhibition of fusion by the peptide in citrate buffer was performed. Inhibition of membrane fusion (48% of nonpeptide control) was comparable to the inhibition observed with PBS, pH 5.0, as measured by fluorescent membrane dye transfer assay.
Figure 5.
Stage of peptide inhibition. The effect of the presence or absence of 10 μg/ml GP64 peptide was determined before, during, or after induction of membrane fusion on transiently GP64-transfected Sf9 cell lines. “Before” data represent GP64-expressing Sf9 cells exposed to the GP64 peptide 10 min before the addition of fusion-inducing PBS, pH 5.0. “During” data represent the presence of GP64 peptide in PBS, pH 5.0. “After” data represent the presence of GP64 peptide in PBS, pH 7.4, added immediately after the 5-min induction with acidic pH. “Throughout” data represent the presence of GP64 peptide 10 min before induction, during the 5-min induction period in PBS, pH 5.0, and after the induction period in PBS, pH 7.4. The extent of membrane fusion was determined by counting fluorescently labeled Sf9 cells (fusion event) and dividing by the sum of PKH26-labeled RBCs in contact with Sf9 cells and fluorescently labeled Sf9 cells. The extent of syncytia formation was determined by dividing fused Sf9 cells by the sum of fused Sf9 cells and nonfused cells in contact. Black bars represent mean values from three separate Sf9-RBC experiments normalized to wild-type GP64 (100%) in the absence of the GP64 peptide. Hatched bars represent average values from two separate Sf9-Sf9 syncytia experiments normalized to wild-type GP64 (100%) in the absence of the GP64 peptide. Error bars represent SE.
DISCUSSION
Targeting the hydrophobic 4-3 heptad repeat–like sequence, we examined whether the peptide sequence within and flanking this region plays a direct, dynamic role in GP64-mediated membrane fusion. Our results suggest that this sequence has an active role between those crucial stages of fusion during which the fusion protein leaves its prefusagenic conformation and mediates lipid rearrangement toward lipid continuity and fusion pore formation. We have identified mutations that were defective for fusion activity but (a) successfully oligomerized into trimers, (b) presented on the cell surface, (c) retained conformationally sensitive epitopes at neutral pH, and (d) lost conformationally sensitive epitopes upon exposure to low pH. Most importantly, we demonstrated that wild-type GP64-mediated membrane fusion is inhibited in a concentration-dependent manner by a peptide to this region, but only when the peptide is present during the low pH incubation that normally triggers fusion. This peptide inhibition is specific, because neither the homologous peptide from Ebola nor the scrambled GP64 peptide was inhibitory. Peptide inhibition was not caused by effects exerted on cell surface lipids because the GP64 peptide did not inhibit influenza HA-mediated fusion of Sf9-RBC membranes.
For peptide inhibition, there is the formal possibility that the pH-sensitive effect is due to the peptide’s conformation, i.e., that at neutral pH, the GP64 peptide assumes a tertiary structure that is unfavorable for interaction and inhibition. However, given that fusion-defective mutants do change conformation at low pH and that the effect of peptide, regardless of pH, is on conformationally changed GP64, sequences adjacent to and including the hydrophobic 4-3 heptad repeat must have an active function downstream of the initiation of GP64 protein conformational change. At present, it is unclear whether GP64 fusion activity can be reduced to negligible levels with the GP64 peptide. The limited decrease in GP64 fusion activity between 10 and 100 μg/ml (53–46%) suggests that the inhibitory activity of the peptide may be approaching a plateau value, perhaps reflecting competition between the identified sequence of GP64 and the inhibitory peptide-binding site within GP64. Alternatively, limiting amounts of free, putatively active monomeric GP64 peptide may result from peptide aggregation at higher peptide concentrations.
It is difficult to directly compare peptide inhibition values obtained for the pH-dependent, GP64-mediated, cell-to-cell fusion with values obtained for pH-independent processes of HIV and paramyxoviruses. However, at a concentration of 10 μg/ml or 2.8 μM, the GP64 peptide inhibits slightly <50% of Sf9-RBC fusion and Sf9 cell-to-cell fusion. This concentration is several orders of magnitude greater than the 50% syncytial inhibition value of 100 pM reported for DP178 inhibition of HIV-mediated cell-to-cell fusion (Wild et al., 1994b) but may be closer to values reported for paramyxovirus fusion, which range from 0.015 to 2.1 μM (Lambert et al., 1996; Yao and Compans, 1996).
Currently, there is no structural evidence that the conserved 4-3 heptad repeats found in baculovirus GP64 homologues form coiled-coil structures, because crystallographic studies have not yet been performed. Sequence-based criteria for predicted coiled-coil formation suggest that four or more heptad repeats are required for coiled-coil formation, with at least six of eight residues at the “a” and “d” positions being hydrophobic alanine, leucine, isoleucine, methionine, tyrosine, or valine (A, L, I, M, Y, or V), with the exclusion of tryptophan (W) (Lupas et al., 1991; Berger et al., 1995; Woolfson and Alber, 1995). Proline at any heptad position is predicted to be unfavorable for helix formation and consequently coiled-coil interaction. Based on these criteria, the first heptad repeat (positions 301–308) would not necessarily be within a coiled coil (see Figure 1). Glutamine (Q, residue 305) and asparagine (N, residue 312) at “a” positions, although not hydrophobic, are often found in naturally occurring coiled-coil sequences and may confer specificity by burying polar interactions within the hydrophobic face and destabilizing potential alternative interactions by requiring unfavorable polar/hydrophobic interactions (Lumb and Kim, 1995).
For initial mutagenesis experiments to determine if the 4-3 hydrophobic heptad region might be involved in membrane fusion, leucine-to-alanine scanning mutations were constructed to make conservative mutations that would not disturb the predicted α helical character of the hydrophobic 4-3 heptad repeat. Because structural information on GP64 is lacking, and noting that coiled-coil sequences sometimes contain phase shifts (so-called stammers and stutters [Brown et al., 1996]), all hydrophobic leucine residues within the first four heptad repeats were mutated to the less hydrophobic alanine residues. It should be noted that with the use of the general 4-3 heptad repeat pattern (a-b-c-d-e-f-g, where “a” and “d” form a hydrophobic face), mutants LZ1 and LZ2 would fall at “d” residues, LZ3, a tandem mutation, would fall at “c” and “d,” and LZ4 would actually fall at the “c” position. The predicted “d” (residue 322) in the fourth heptad is methionine (M), which was not evaluated. As noted previously, the LZ1 mutation likely would not fall within a predicted coiled coil. Because several proteins with leucine-to-alanine mutations were defective for oligomerization and surface expression, and mutants generally demonstrated reduced levels of cell surface expression, the mutagenesis results presented here do not conflict with the hypothesis that the sequences within the 4-3 heptad repeat are also important for correct folding and surface expression of the GP64 oligomer.
The Heptad Repeat–dependent Stage of Membrane Fusion
The stage at which the identified sequence functions during membrane fusion is after the onset of the low pH–triggered protein conformational changes associated with membrane fusion yet before lipidic contact between the two membranes. The heptad repeat acts downstream of initial low pH–induced conformational changes because (a) no peptide inhibition was observed before activation with low pH buffer and (b) in either mutants or peptide inhibition, the loss of a conformationally sensitive neutral pH epitope was observed upon low pH application. The heptad repeat acts upstream of unrestricted lipid continuity of target and host membranes, because either mutations in this region or the presence of GP64 peptide from this region prevented the redistribution of lipidic dye between cells.
Hydrophobic 4-3 heptad repeat motifs are clearly important for pH-independent viral protein–mediated fusion (Chen et al., 1995; Reitter et al., 1995; Lambert et al., 1996; Young et al., 1997). For HIV gp41 peptide inhibition, it has been predicted that peptides would exert inhibitory effects during the conformational change to the fusion-active conformation (Wiessenhorn et al., 1997). In fact, recent work on peptide inhibition of HIV gp41 and parainfluenza also suggests that peptide inhibition may function dynamically by preventing the hypothesized collapse of the extended coiled-coil structure back on itself as part of a proposed mechanism by which target and host membranes are merged (Joshi et al., 1998; Munoz-Barroso et al., 1998; Baker et al., 1999). Also, the HIV inhibitory peptide binds to gp41 only after gp120 interacts with its receptor(s) and gp41 is activated (Furata et al., 1998). By studying pH-dependent viral protein–mediated membrane fusion, we have been able to clearly stage its effect. The frequent observation of hydrophobic 4-3 heptad repeats (leucine zipper–like) motifs within viral fusion proteins, coupled with the evidence for functional roles for both pH-independent and now pH-dependent viral fusion processes, suggests that the hydrophobic 4-3 heptad motif performs a universal function critical to membrane fusion in general. It should be noted, however, that all hydrophobic 4-3 heptad repeat regions of pH-independent fusion proteins may not function at the same stage of membrane fusion, because Young et al. (1997) report peptide inhibition of paramyxovirus fusion before tryptic activation of the fusion protein. So whether all pH-independent 4-3 heptad repeats function directly during the fusion process remains an open question.
Exactly what specific functional role (or roles) the hydrophobic 4-3 heptad repeat plays during GP64-mediated membrane fusion also remains hypothetical. Indeed, it is not clear how many conformational intermediates ensue after the pH-driven triggering of GP64, nor which of the putative intermediate(s) is actually fusogenic. The inhibition of membrane fusion by the GP64 peptide reported here is presumably via direct interaction with activating or activated GP64 trimers rather than untriggered inactive trimers, because no inhibition is observed before activation with low pH buffer. We imagine that this inhibition is due to a stable interaction of the GP64 peptide with a newly exposed complementary sequence during low pH application, perhaps even a complementary sequence within the hydrophobic 4-3 heptad repeat of GP64, or with another domain of GP64. We are currently investigating methods to determine the binding site of the GP64 peptide. Regardless of the site of binding, these experiments suggest that this sequence mediates critical intramonomer/intratrimeric or intermonomer/intertrimeric interactions required during membrane fusion.
The Mechanism of Membrane Fusion
How do these results fit into our general model for virus-mediated membrane fusion (Chernomordik et al., 1998)? In that scheme, after activation of the fusion protein, conformational changes result in a ring of protein around the fusion site encasing a portion of the lipid bilayer. Subsequently, lipid continuity forms between the outer membrane leaflets of the host and target membranes, but lipid transmigration is prohibited by the ring of protein (restricted hemifusion). Finally, fusion pore formation gives aqueous continuity of cytoplasm, followed by fusion pore widening that breaks the ring to allow lipid redistribution. We view ring formation as essential because restricting membrane lipids would allow energy released during fusion protein conformational changes to translate into work on lipids required to overcome energy barriers to membrane fusion.
The concept of a ring-like GP64 fusion complex is indirectly supported by experimental evidence. Electrophysiological experiments on baculovirus-infected Sf9 cells led Plonsky and Zimmerberg (1996) to propose that a ring of five to seven GP64 trimers may form a fusion machine that facilitates opening of the fusion pore within this ring. Furthermore, using polypeptide cross-linking agents and sulfhydryl group reagents, Markovic et al. (1998) demonstrated that GP64 forms transient, higher-order complexes of trimers upon application of low pH buffer (only in the presence of target membrane) at the same time that fusion is catalyzed. The ring hypothesis is further supported by work with influenza HA, which suggests that HA trimers function cooperatively and inhibit lipid dye transmigration during the initial fusion phases (Ellens et al., 1990; Clague et al., 1991; Tse et al., 1993; Zimmerberg et al., 1994; Blumenthal et al., 1996; Danieli et al., 1996; Chernomordik et al., 1998).
Because the 4-3 heptad repeat–dependent stage of membrane fusion occurs after the initiation of individual trimer conformational change and before the functioning of the putative fusion complex, it is reasonable to think that GP64 facilitates interoligomeric assembly. Consequently, we propose that the hydrophobic 4-3 heptad repeat of GP64 mediates protein-protein interactions that result in the assembly of a ring of activated GP64 trimers into a fusion complex.
In conclusion, we have demonstrated that the GP64 hydrophobic 4-3 heptad repeat motif is a useful guide for investigation of critical sequences within GP64 for membrane fusion. This region functions after the initiation of conformational change and before any detectable mixing of target and host membranes. Further study and dissection of GP64 pH-triggered membrane fusion may provide more detailed and unique insights into the general biophysical mechanism of membrane fusion.
ACKNOWLEDGMENTS
We thank Dr. Peter Faulkner for providing mAbs AcV1 and AcV5, Dr. Scott Monsma for construction of plasmid pATH-1 and cell line Sf9 Ac64–5, Dr. Judy White for providing the X31 strain of HA-encoding plasmid, and Dr. Leonid Chernomordik for thoughtful discussions and critical reading of the manuscript.
REFERENCES
- Baker KA, Dutch RE, Lamb RA, Jardetzky T. Structural basis for paramyxovirus-mediated membrane fusion. Mol Cell. 1999;3:309–319. doi: 10.1016/s1097-2765(00)80458-x. [DOI] [PubMed] [Google Scholar]
- Ben-Efraim I, Kliger Y, Hermesh C, Shai Y. Membrane-induced step in the activation of Sendai virus fusion protein. J Mol Biol. 1999;285:609–625. doi: 10.1006/jmbi.1998.2370. [DOI] [PubMed] [Google Scholar]
- Berger B, Wilson DB, Wolf E, Tonchev T, Milla M, Kim PS. Predicting coiled coils by use of pairwise residue correlations. Proc Natl Acad Sci USA. 1995;92:8259–8263. doi: 10.1073/pnas.92.18.8259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blacklow SC, Lu M, Kim PS. A trimeric subdomain of the simian immunodeficiency virus envelope glycoprotein. Biochemistry. 1995;34:14955–14962. doi: 10.1021/bi00046a001. [DOI] [PubMed] [Google Scholar]
- Blissard GW, Rohrmann GF. Location sequence transcriptional mapping, and temporal expression of the gp64 envelope glycoprotein gene of Orgyia pseudotsugata multicapsid nuclearpolyhedrosis virus. Virology. 1989;170:537–555. doi: 10.1016/0042-6822(89)90445-5. [DOI] [PubMed] [Google Scholar]
- Blissard GW, Rohrmann GF. Baculovirus GP64 gene expression: analysis of sequences modulating early transcription and transactivation by IE1. J Virol. 1991;65:5820–5827. doi: 10.1128/jvi.65.11.5820-5827.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blissard GW, Wenz JR. Baculovirus GP64 envelope glycoprotein is sufficient to mediate pH-dependent membrane fusion. J Virol. 1992;66:6829–6835. doi: 10.1128/jvi.66.11.6829-6835.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal R, Sarkar DP, Durell S, Howard DE, Morris SJ. Dilation of the influenza hemagglutinin fusion pore revealed by the kinetics of individual cell-cell fusion events. J Cell Biol. 1996;135:63–71. doi: 10.1083/jcb.135.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JH, Cohen C, Parry DAD. Heptad breaks in alpha-helical coiled-coils: stutters and stammers. Proteins. 1996;26:134–145. doi: 10.1002/(SICI)1097-0134(199610)26:2<134::AID-PROT3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Buckland R, Malvoisin E, Beauverger P, Wild F. A leucine zipper structure present in the measles virus fusion protein is not required for its tetramerization but is essential for fusion. J Gen Virol. 1992;73:1703–1707. doi: 10.1099/0022-1317-73-7-1703. [DOI] [PubMed] [Google Scholar]
- Buckland R, Wild F. Leucine zipper motif extends. Nature. 1989;338:547. doi: 10.1038/338547a0. (Letter). [DOI] [PubMed] [Google Scholar]
- Bullough PA, Hughson FM, Skehel JJ, Wiley DC. Structure of influenza hemagglutinin at the pH of membrane fusion. Nature. 1994;371:37–43. doi: 10.1038/371037a0. [DOI] [PubMed] [Google Scholar]
- Carr CM, Kim PS. A spring-loaded mechanism for the conformational change of influenza hemagglutinin. Cell. 1993;73:823–832. doi: 10.1016/0092-8674(93)90260-w. [DOI] [PubMed] [Google Scholar]
- Chambers P, Pringle CR, Easton AJ. Heptad repeat sequences are located adjacent to hydrophobic regions in several types of virus fusion glycoproteins. J Gen Virol. 1990;71:3075–3080. doi: 10.1099/0022-1317-71-12-3075. [DOI] [PubMed] [Google Scholar]
- Chan DC, Fass D, Berger JM, Kim PS. Core structure of gp41 from the HIV envelope glycoprotein. Cell. 1997;89:263–273. doi: 10.1016/s0092-8674(00)80205-6. [DOI] [PubMed] [Google Scholar]
- Chen CH, Matthews TJ, McDanal CB, Bolognesi P, Greenberg ML. A molecular clamp in the human immunodeficiency virus (HIV) type 1 TM protein determines the anti-HIV activity of gp41 derivatives: implications for viral function. J Virol. 1995;69:3771–3777. doi: 10.1128/jvi.69.6.3771-3777.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernomordik LV, Frolov VA, Leikina E, Bronk P, Zimmerberg J. The pathway of membrane fusion catalyzed by influenza hemagglutinin: restriction of lipids, hemifusion, and lipidic fusion pore formation. J Cell Biol. 1998;140:1369–1382. doi: 10.1083/jcb.140.6.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clague MJ, Schoch C, Blumenthal R. Delay time for influenza hemagglutinin-induced membrane fusion depends on hemagglutinin surface density. J Virol. 1991;65:2402–2407. doi: 10.1128/jvi.65.5.2402-2407.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danieli T, Pelletier SL, Henis YI, White JM. Membrane fusion mediated by the influenza virus hemagglutinin requires the concerted action of at least three hemagglutinin trimers. J Cell Biol. 1996;133:559–569. doi: 10.1083/jcb.133.3.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubay JW, Roberts SJ, Brody B, Hunter E. Mutations in the leucine zipper of the human immunodeficiency virus type 1 transmembrane glycoprotein affect fusion and infectivity. J Virol. 1992;66:4748–4756. doi: 10.1128/jvi.66.8.4748-4756.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutch RE, Leser GP, Lamb RA. Paramyxovirus fusion protein: characterization of the core trimer, a rod-shaped complex with helices in antiparallel orientation. Virology. 1999;254:147–159. doi: 10.1006/viro.1998.9532. [DOI] [PubMed] [Google Scholar]
- Ellens H, Bentz J, Mason D, Zhang F, White JM. Fusion of influenza hemagglutinin-expressing fibroblasts with glycophorin-bearing liposomes: role of hemagglutinin surface density. Biochemistry. 1990;29:9697–9707. doi: 10.1021/bi00493a027. [DOI] [PubMed] [Google Scholar]
- Fass D, Harrison SC, Kim PS. Structure of Moloney murine virus envelope domain at 1.7-A resolution. Nat Struct Biol. 1996;3:465–469. doi: 10.1038/nsb0596-465. [DOI] [PubMed] [Google Scholar]
- Fass D, Kim PS. Dissection of a retrovirus envelope protein reveals structural similarity to influenza hemagglutinin. Curr Biol. 1995;5:1377–1383. doi: 10.1016/s0960-9822(95)00275-2. [DOI] [PubMed] [Google Scholar]
- Furata RA, Wild CT, Weng Y, Weiss CA. Capture of an early fusion-active conformation of HIV gp41. Nat Struct Biol. 1998;5:276–279. doi: 10.1038/nsb0498-276. [DOI] [PubMed] [Google Scholar]
- Garrity DB, Chang MJ, Blissard GW. Late promoter selection in the baculovirus gp64 envelope fusion gene. Virology. 1997;231:167–181. doi: 10.1006/viro.1997.8540. [DOI] [PubMed] [Google Scholar]
- Ghosh JK, Ovadia M, Shai Y. A leucine zipper motif in the ectodomain of Sendai virus fusion protein assembles in solution and in the membranes and specifically binds biologically active peptides and the virus. Biochemistry. 1997;36:15451–15462. doi: 10.1021/bi971152i. [DOI] [PubMed] [Google Scholar]
- Hefferon KL, Oomens AGP, Monsma SA, Finnerty CM, Blissard GW. Host cell receptor binding by baculovirus GP64 and kinetics of viral entry. Virology. 1999;258:455–468. doi: 10.1006/viro.1999.9758. [DOI] [PubMed] [Google Scholar]
- Jarvis DL, Garcia AJ. Biosynthesis and processing of the Autographa californica nuclear polyhedrosis virus gp64 protein. Virology. 1994;205:300–313. doi: 10.1006/viro.1994.1646. [DOI] [PubMed] [Google Scholar]
- Jiang S, Lin K, Strick N, Neurath AR. HIV inhibition by a peptide. Nature. 1993a;365:113. doi: 10.1038/365113a0. (Letter). [DOI] [PubMed] [Google Scholar]
- Jiang S, Lin K, Strick N, Neurath AR. Inhibition of HIV-1 infection by a fusion domain binding peptide from the HIV-1 envelope glycoprotein gp41. Biochem Biophys Res Commun. 1993b;195:533–538. doi: 10.1006/bbrc.1993.2078. [DOI] [PubMed] [Google Scholar]
- Joshi SB, Dutch RE, Lamb RA. A core trimer of paramyxovirus fusion protein: parallels to influenza virus hemagglutinin and HIVgp41. Virology. 1998;248:20–34. doi: 10.1006/viro.1998.9242. [DOI] [PubMed] [Google Scholar]
- Judice JK, Tom JYK, Huang W, Wrin T, Vennari J, Petropoulos CJ, McDowell RS. Inhibition of HIV type 1 infectivity by constrained alpha-helical peptides: implications for the fusion mechanism. Proc Natl Acad Sci USA. 1997;94:13426–13430. doi: 10.1073/pnas.94.25.13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Yang JM. Analysis of the fusogenic activity of Autographa californica nuclear polyhedrosis virus (AcMNPV) gp64 envelope glycoprotein. J Microbiol. 1996;34:7–14. [Google Scholar]
- Kogan PH, Chen XY, Blissard GW. A baculovirus gp64 early promoter is activated by host transcription factor-binding to CACGTG and GATA elements. J Virol. 1994;68:813–822. doi: 10.1128/jvi.68.2.813-822.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert DM, Barney S, Lambert A, Guthrie K, Medinas R, Davis DE, Bucy T, Erickson J, Merutka G, Petteway SR. Peptides from conserved regions of paramyxovirus fusion (F) proteins are potent inhibitors of viral fusion. Proc Natl Acad Sci USA. 1996;93:2186–2191. doi: 10.1073/pnas.93.5.2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M, Blacklow SC, Kim PS. A trimeric structural domain of the HIV-1 transmembrane. Nat Struct Biol. 1995;2:1075–1082. doi: 10.1038/nsb1295-1075. [DOI] [PubMed] [Google Scholar]
- Lumb KJ, Kim PS. A buried polar interaction imparts structural uniqueness in a designed heterodimeric coiled coil. Biochemistry. 1995;34:8642–8648. doi: 10.1021/bi00027a013. [DOI] [PubMed] [Google Scholar]
- Lupas A, Van Dyke M, Stock J. Predicting coiled coils from protein sequences. Science. 1991;252:1162–1164. doi: 10.1126/science.252.5009.1162. [DOI] [PubMed] [Google Scholar]
- Malashkevich VN, Chan DC, Chutkowski CT, Kim PS. Crystal structure of the simian immunodeficiency virus (SIV) gp41 core: conserved helical interactions underlie broad inhibitory activity of gp41 peptides. Proc Natl Acad Sci USA. 1998;95:9134–9139. doi: 10.1073/pnas.95.16.9134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malashkevich VN, Schneider BJ, McNally ML, Millhollen MA, Pang JX, Kim PS. Core structure of the envelope glycoprotein GP2 from Ebola virus at 1.9-A resolution. Proc Natl Acad Sci USA. 1999;96:2662–2667. doi: 10.1073/pnas.96.6.2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markovic I, Pulyaeva H, Sokoloff A, Chernomordik LV. Membrane fusion mediated by baculovirus gp64 involves assembly of stable gp64 trimers into multiprotein aggregates. J Cell Biol. 1998;143:1155–1166. doi: 10.1083/jcb.143.5.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsma SA, Blissard GW. Identification of a membrane fusion domain and an oligomerization domain in the baculovirus GP64 envelope fusion protein. J Virol. 1995;69:2583–2595. doi: 10.1128/jvi.69.4.2583-2595.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsma SA, Oomens AGP, Blissard GW. The GP64 envelope fusion protein is an essential baculovirus protein required for cell-to-cell transmission of infection. J Virol. 1996;70:4607–4616. doi: 10.1128/jvi.70.7.4607-4616.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Barroso I, Durell S, Sakaguchi K, Appella E, Blumenthal R. The dilation of the human immunodeficiency virus-1 envelope glycoprotein fusion pore revealed by the inhibitory action of a synthetic peptide from gp41. J Cell Biol. 1998;140:315–323. doi: 10.1083/jcb.140.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oomens AGP, Monsma SA, Blissard GW. The baculovirus gp64 envelope fusion protein: synthesis, oligomerization, and processing. Virology. 1995;209:592–603. doi: 10.1006/viro.1995.1291. [DOI] [PubMed] [Google Scholar]
- O’Reily DR, Miller LK, Luckow VA. Baculovirus Expression Vectors: A Laboratory Manual. New York: Oxford University Press; 1994. [Google Scholar]
- Plonsky I, Cho MS, Oomens AGP, Blissard G, Zimmerberg J. An analysis of the role of the target membrane on the gp64-induced fusion pore. Virology. 1999;253:65–76. doi: 10.1006/viro.1998.9493. [DOI] [PubMed] [Google Scholar]
- Plonsky I, Zimmerberg J. The initial fusion pore induced by baculovirus gp64 is large and forms quickly. J Cell Biol. 1996;135:1831–1839. doi: 10.1083/jcb.135.6.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinstein M, Shin YK. A peptide from the heptad repeat of human immunodeficiency virus gp41 shows both membrane binding and coiled-coil formation. Biochemistry. 1995;34:13390–13397. doi: 10.1021/bi00041a016. [DOI] [PubMed] [Google Scholar]
- Rapaport D, Ovadia M, Shai Y. A synthetic peptide corresponding to a conserved heptad repeat domain is a potent inhibitor of Sendai virus-cell fusion: an emerging similarity with functional domains of other viruses. EMBO J. 1995;14:5524–5531. doi: 10.1002/j.1460-2075.1995.tb00239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitter JN, Sergel T, Morrison TG. Mutational analysis of the leucine zipper motif in the Newcastle disease virus fusion protein. J Virol. 1995;69:5995–6004. doi: 10.1128/jvi.69.10.5995-6004.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimsky LT, Shugars DC, Matthews TJ. Determinants of human immunodeficiency virus type 1 resistance to gp41-derived inhibitory peptides. J Virol. 1998;72:986–993. doi: 10.1128/jvi.72.2.986-993.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez A, Trappier SG, Mahy BWJ, Peters CJ, Nichol ST. The virion glycoproteins of Ebola viruses are encoded in two reading frames and are expressed through transcriptional editing. Proc Natl Acad Sci USA. 1996;93:3602–3607. doi: 10.1073/pnas.93.8.3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergel-Germano T, McQuain C, Morrison T. Mutations in the fusion peptide and heptad repeat regions of Newcastle disease virus fusion protein block fusion. J Virol. 1994;68:7654–7658. doi: 10.1128/jvi.68.11.7654-7658.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton RB, Fasshauer D, Jahn R, Brunger AT. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 angstrom resolution. Nature. 1998;395:347–353. doi: 10.1038/26412. [DOI] [PubMed] [Google Scholar]
- Tse FW, Iwata A, Almers W. Membrane flux through the pore formed by a fusogenic viral envelope protein during cell fusion. J Cell Biol. 1993;121:543–552. doi: 10.1083/jcb.121.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissenhorn W, Calder LJ, Wharton SA, Skehel JJ, Wiley DC. The central structural feature of the membrane fusion protein subunit from the Ebola virus glycoprotein is a long triple-stranded coiled coil. Proc Natl Acad Sci USA. 1998;95:6032–6036. doi: 10.1073/pnas.95.11.6032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissenhorn W, Dessen A, Calder LJ, Harrison SC, Skehel JJ, Wiley DC. Structural basis for membrane fusion by enveloped viruses. Mol Membr Biol. 1999;16:3–9. doi: 10.1080/096876899294706. [DOI] [PubMed] [Google Scholar]
- Weissenhorn W, Dessen A, Harrison SC, Skehel JJ, Wiley DC. Atomic structure of the ectodomain from HIV gp41. Nature. 1997;387:426–430. doi: 10.1038/387426a0. [DOI] [PubMed] [Google Scholar]
- Weissenhorn W, Wharton SA, Calderp LJ, Earl L, Moss B, Aliprandis E, Skehel JJ, Wiley DC. The ectodomain of HIV env subunit gp41 forms a soluble, alpha-helical, rod-like oligomer in the absence of gp120 and the N-terminal fusion peptide. EMBO J. 1996;15:1507–1514. [PMC free article] [PubMed] [Google Scholar]
- Whitford M, Stewart S, Kuzio J, Faulkner P. Identification and sequence analysis of a gene encoding gp67, an abundant envelope glycoprotein of the baculovirus Autographa californica nuclear polyhedrosis virus. J Virol. 1989;63:1393–1399. doi: 10.1128/jvi.63.3.1393-1399.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild C, Greenwell T, Shugars D, Rimsky-Clarke L, Matthews T. The inhibitory activity of an HIV type 1 peptide correlates with its ability to interact with a leucine zipper structure. AIDS Res Hum Retroviruses. 1995;11:323–325. doi: 10.1089/aid.1995.11.323. [DOI] [PubMed] [Google Scholar]
- Wild CT, Dubay W, Greenwell T, Baird T, Oas TG, McDanal C, Hunter E, Matthews T. Propensity for a leucine zipper-like domain of human immunodeficiency virus type 1 gp41 to form oligomers correlates with a role in virus-induced fusion rather than assembly of the glycoprotein complex. Proc Natl Acad Sci USA. 1994a;91:12676–12680. doi: 10.1073/pnas.91.26.12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild CT, Oas TG, McDanal C, Bolognesi D, Matthews T. A synthetic peptide inhibitor of human immunodeficiency virus replication: correlation between solution structure and viral inhibition. Proc Natl Acad Sci USA. 1992;89:10537–10541. doi: 10.1073/pnas.89.21.10537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild CT, Shugars DC, Greenwell TK, McDanal CB, Matthews TJ. Peptides corresponding to a predictive alpha helical domain of human immunodeficiency virus type 1 gp41 are potent inhibitors of virus infection. Proc Natl Acad Sci USA. 1994b;91:9770–9774. doi: 10.1073/pnas.91.21.9770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild FT, Buckland R. Inhibition of measles virus infection and fusion with peptides corresponding to the leucine zipper region of the fusion protein. J Gen Virol. 1997;78:107–111. doi: 10.1099/0022-1317-78-1-107. [DOI] [PubMed] [Google Scholar]
- Volkman LE, Goldsmith PA. Budded Autographa californica NPV 64k protein: further biochemical analysis and effects of postimmunoprecipitation sample preparation. Virology. 1984;139:295–302. doi: 10.1016/0042-6822(84)90375-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkman LE, Goldsmith PA, Hess RJ, Faulkner P. Neutralization of budded Autographa californica NPV by a monoclonal antibody: identification of the target antigen. Virology. 1984;133:354–362. doi: 10.1016/0042-6822(84)90401-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolfson DN, Alber T. Predicting oligomerization states of coiled coils. Protein Sci. 1995;4:1596–1607. doi: 10.1002/pro.5560040818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Q, Compans RW. Peptides corresponding to the heptad repeat region of human parainfluenza virus fusion protein are potent inhibitors of virus infection. Virology. 1996;223:103–112. doi: 10.1006/viro.1996.0459. [DOI] [PubMed] [Google Scholar]
- Young JK, Hicks RP, Wright GE, Morrison TG. Analysis of a peptide inhibitor of paramyxovirus (NDV) fusion using biological assays, NMR, and molecular modeling. Virology. 1997;238:291–304. doi: 10.1006/viro.1997.8834. [DOI] [PubMed] [Google Scholar]
- Young JK, Li D, Abramowitz MC, Morrison TG. Interaction of peptides with sequences from the Newcastle disease virus fusion protein heptad repeat regions. J Virol. 1999;73:5945–5956. doi: 10.1128/jvi.73.7.5945-5956.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerberg J, Blumenthal R, Sarkar DP, Curran M, Morris SJ. Restricted movement of lipid and aqueous dyes through pores formed by influenza hemagglutinin during cell fusion. J Cell Biol. 1994;127:1885–1894. doi: 10.1083/jcb.127.6.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]