Abstract
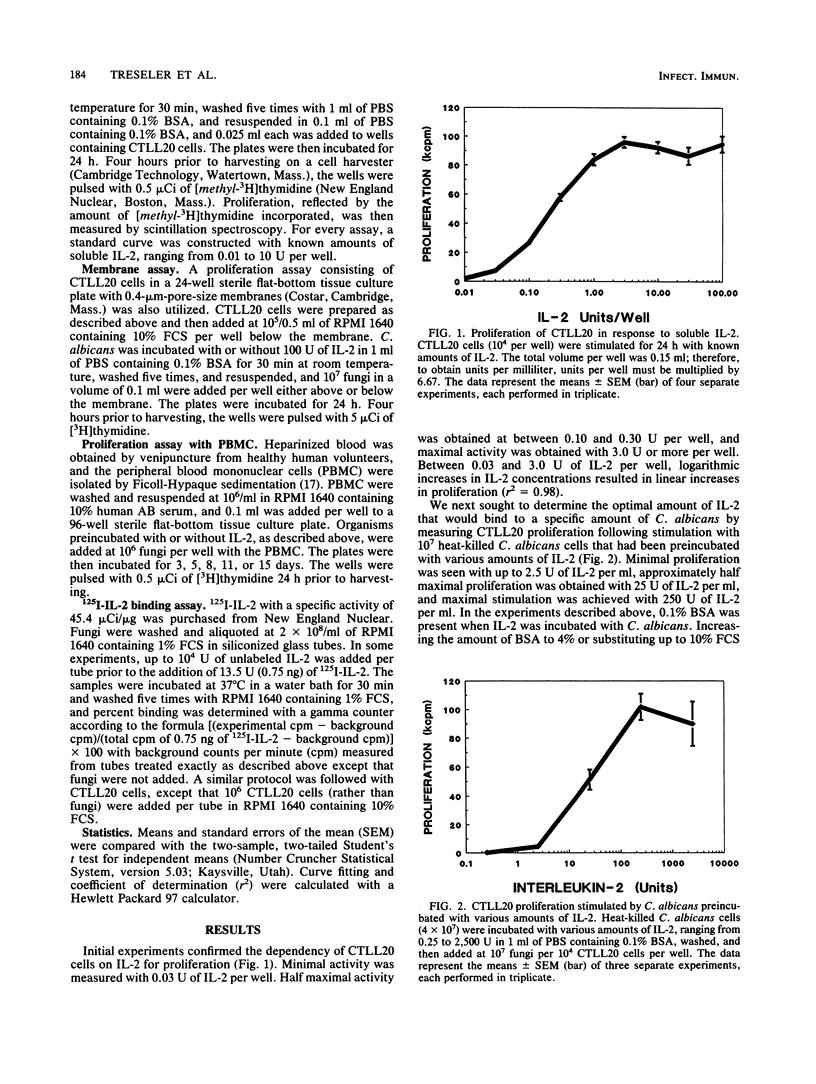
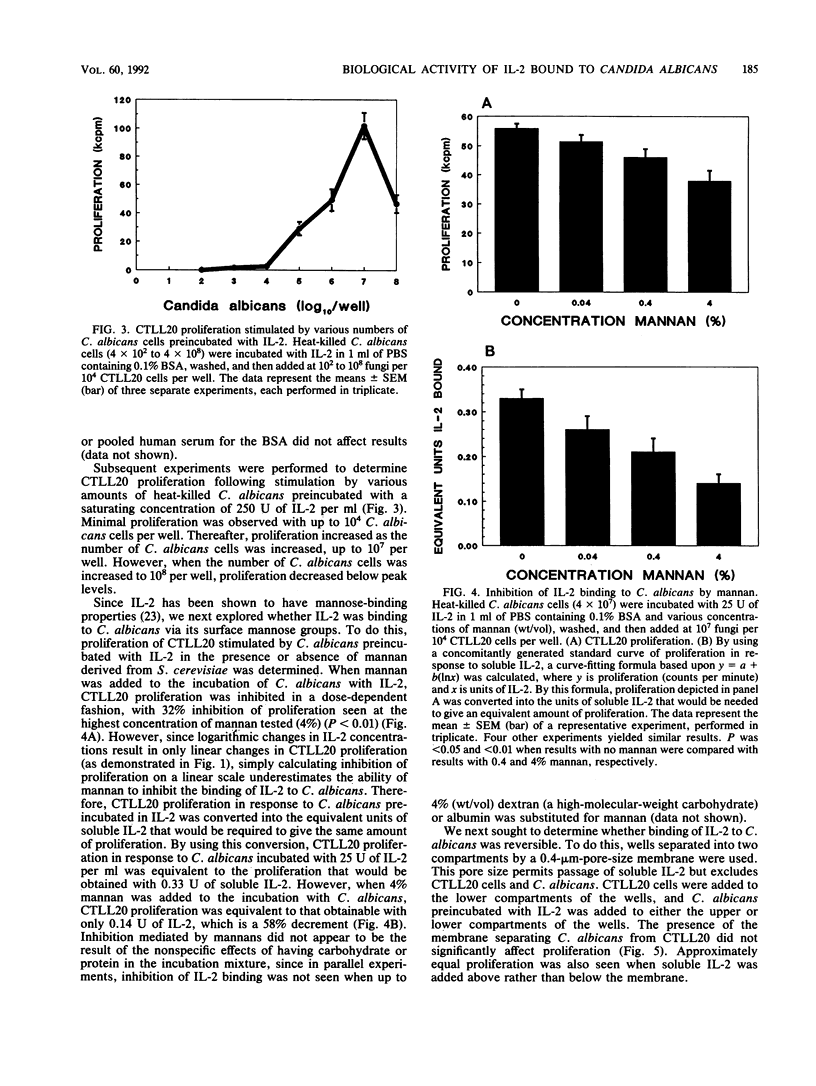
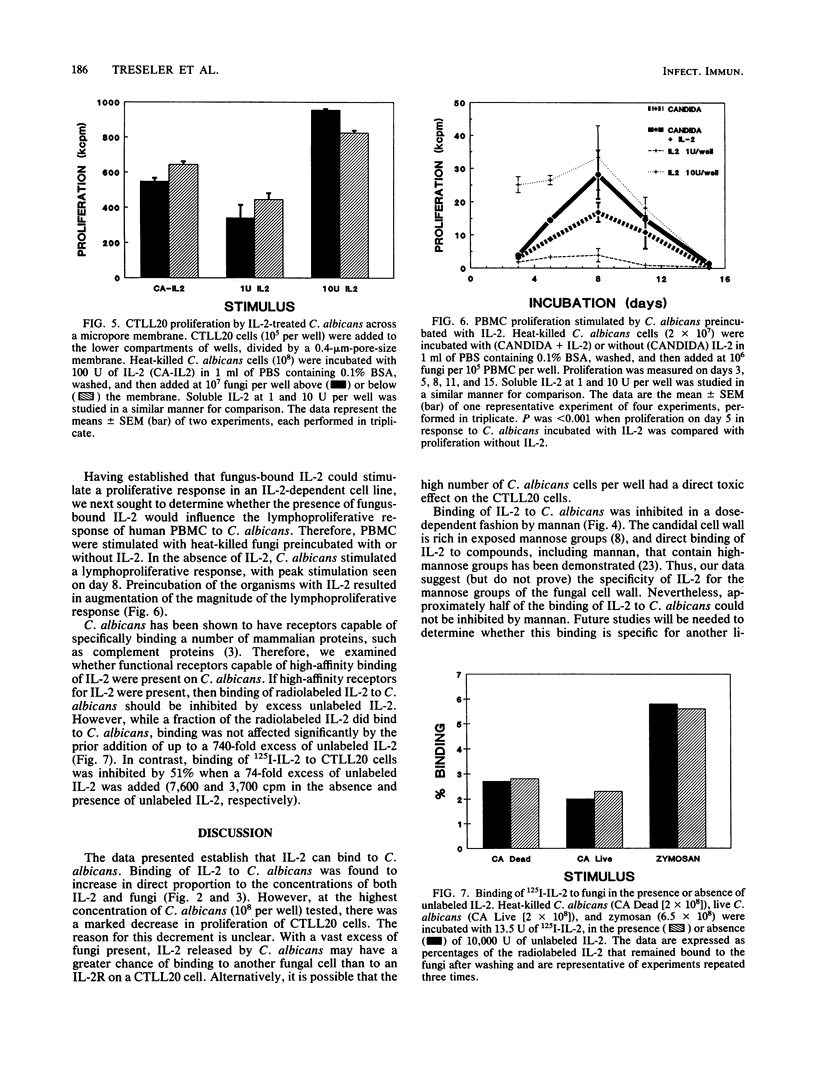
The lymphokine interleukin-2 (IL-2), which is necessary for the generation of an optimal cell-mediated immune response, has recently been shown to have lectinlike properties, with specificity for high-mannose groups. Therefore, the ability of IL-2 to bind to the mannose-rich fungus Candida albicans was examined. Heat-killed fungi preincubated with IL-2 stimulated, in a dose-dependent manner, proliferation of the IL-2-dependent cell line CTLL20. Soluble mannan, which is rich in exposed mannose groups, inhibited binding of IL-2 to C. albicans by approximately 60%, suggesting that the lectinlike properties of IL-2 are partially responsible for its fungal binding capacity. Binding of IL-2 to fungi appeared to be reversible, as C. albicans preincubated with IL-2 stimulated CTLL20 proliferation even when the fungi and cells were separated by an 0.4-microns-pore-size membrane. The lymphoproliferative response of normal human peripheral blood mononuclear cells to C. albicans was augmented when the fungus was preincubated with IL-2. Binding of 125I-IL-2 could not be inhibited by unlabeled IL-2, suggesting the absence of high-affinity receptors on C. albicans for IL-2. While the in vivo relevance remains to be determined, these data demonstrate that IL-2 can bind to C. albicans in vitro and thereby influence the host response to this medically important fungus.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burgaleta C., Territo M. C., Quan S. G., Golde D. W. Glucan-activated macrophages: functional characteristics and surface morphology. J Reticuloendothel Soc. 1978 Mar;23(3):195–204. [PubMed] [Google Scholar]
- Calderone R. A., Braun P. C. Adherence and receptor relationships of Candida albicans. Microbiol Rev. 1991 Mar;55(1):1–20. doi: 10.1128/mr.55.1.1-20.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crislip M. A., Edwards J. E., Jr Candidiasis. Infect Dis Clin North Am. 1989 Mar;3(1):103–133. [PubMed] [Google Scholar]
- Crum E. D., Kaplan D. R. In vivo activity of solid phase interleukin 2. Cancer Res. 1991 Feb 1;51(3):875–879. [PubMed] [Google Scholar]
- Ezekowitz R. A., Kuhlman M., Groopman J. E., Byrn R. A. A human serum mannose-binding protein inhibits in vitro infection by the human immunodeficiency virus. J Exp Med. 1989 Jan 1;169(1):185–196. doi: 10.1084/jem.169.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukazawa Y., Kagaya K. Host defence mechanisms against fungal infection. Microbiol Sci. 1988 Apr;5(4):124–127. [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Herzberg V. L., Smith K. A. T cell growth without serum. J Immunol. 1987 Aug 15;139(4):998–1004. [PubMed] [Google Scholar]
- Hora M. S., Rana R. K., Nunberg J. H., Tice T. R., Gilley R. M., Hudson M. E. Controlled release of interleukin-2 from biodegradable microspheres. Biotechnology (N Y) 1990 Aug;8(8):755–758. doi: 10.1038/nbt0890-755. [DOI] [PubMed] [Google Scholar]
- Kuhlman M., Joiner K., Ezekowitz R. A. The human mannose-binding protein functions as an opsonin. J Exp Med. 1989 May 1;169(5):1733–1745. doi: 10.1084/jem.169.5.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitz S. M. Activation of human peripheral blood mononuclear cells by interleukin-2 and granulocyte-macrophage colony-stimulating factor to inhibit Cryptococcus neoformans. Infect Immun. 1991 Oct;59(10):3393–3397. doi: 10.1128/iai.59.10.3393-3397.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitz S. M., Diamond R. D. Killing of Aspergillus fumigatus spores and Candida albicans yeast phase by the iron-hydrogen peroxide-iodide cytotoxic system: comparison with the myeloperoxidase-hydrogen peroxide-halide system. Infect Immun. 1984 Mar;43(3):1100–1102. doi: 10.1128/iai.43.3.1100-1102.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchmore A. V., Decker J. M. Evidence that recombinant IL 1 alpha exhibits lectin-like specificity and binds to homogeneous uromodulin via N-linked oligosaccharides. J Immunol. 1987 Apr 15;138(8):2541–2546. [PubMed] [Google Scholar]
- Rosenberg S. A., Lotze M. T., Muul L. M., Chang A. E., Avis F. P., Leitman S., Linehan W. M., Robertson C. N., Lee R. E., Rubin J. T. A progress report on the treatment of 157 patients with advanced cancer using lymphokine-activated killer cells and interleukin-2 or high-dose interleukin-2 alone. N Engl J Med. 1987 Apr 9;316(15):889–897. doi: 10.1056/NEJM198704093161501. [DOI] [PubMed] [Google Scholar]
- Scaringi L., Marconi P., Boccanera M., Tissi L., Bistoni F., Cassone A. Cell wall components of Candida albicans as immunomodulators: induction of natural killer and macrophage-mediated peritoneal cell cytotoxicity in mice by mannoprotein and glucan fractions. J Gen Microbiol. 1988 May;134(5):1265–1274. doi: 10.1099/00221287-134-5-1265. [DOI] [PubMed] [Google Scholar]
- Sherblom A. P., Decker J. M., Muchmore A. V. The lectin-like interaction between recombinant tumor necrosis factor and uromodulin. J Biol Chem. 1988 Apr 15;263(11):5418–5424. [PubMed] [Google Scholar]
- Sherblom A. P., Sathyamoorthy N., Decker J. M., Muchmore A. V. IL-2, a lectin with specificity for high mannose glycopeptides. J Immunol. 1989 Aug 1;143(3):939–944. [PubMed] [Google Scholar]
- Sherwood E. R., Williams D. L., Di Luzio N. R. Glucan stimulates production of antitumor cytolytic/cytostatic factor(s) by macrophages. J Biol Response Mod. 1986 Dec;5(6):504–526. [PubMed] [Google Scholar]
- Smith K. A. Interleukin-2: inception, impact, and implications. Science. 1988 May 27;240(4856):1169–1176. doi: 10.1126/science.3131876. [DOI] [PubMed] [Google Scholar]
- Waldmann T. A. The multi-subunit interleukin-2 receptor. Annu Rev Biochem. 1989;58:875–911. doi: 10.1146/annurev.bi.58.070189.004303. [DOI] [PubMed] [Google Scholar]
- West W. H., Tauer K. W., Yannelli J. R., Marshall G. D., Orr D. W., Thurman G. B., Oldham R. K. Constant-infusion recombinant interleukin-2 in adoptive immunotherapy of advanced cancer. N Engl J Med. 1987 Apr 9;316(15):898–905. doi: 10.1056/NEJM198704093161502. [DOI] [PubMed] [Google Scholar]
- Winkelhake J. L., Gauny S. S. Human recombinant interleukin-2 as an experimental therapeutic. Pharmacol Rev. 1990 Mar;42(1):1–28. [PubMed] [Google Scholar]


