Abstract
In pancreatic β-cells, uncoupling protein 2 (UCP2) influences mitochondrial oxidative phosphorylation and insulin secretion. Here, we show that α-cells express significantly higher levels of UCP2 than do β-cells. Greater mitochondrial UCP2-related uncoupling was observed in α-cells compared with β-cells and was accompanied by a lower oxidative phosphorylation efficiency (ATP/O). Conversely, reducing UCP2 activity in α-cells was associated with higher mitochondrial membrane potential generated by glucose oxidation and with increased ATP synthesis, indicating more efficient metabolic coupling. In vitro, the suppression of UCP2 activity led to reduced glucagon secretion in response to low glucose; however, in vivo, fasting glucagon levels were normal in UCP2−/− mice. In addition to its effects on secretion, UCP2 played a cytoprotective role in islets, with UCP2−/− α-cells being more sensitive to specific death stimuli. In summary, we demonstrate a direct role for UCP2 in maintaining α-cell function at the level of glucose metabolism, glucagon secretion, and cytoprotection.
Keywords: ATP, glucagon, islet, mitochondria, diabetes
Blood-glucose levels are tightly regulated by the islet hormones insulin and glucagon. Insulin is secreted from β-cells when glucose levels are high to increase glucose utilization, whereas glucagon is secreted from α-cells when glucose levels are low to elevate blood glucose. It is well established that β-cell dysfunction, resulting in a lack of insulin secretion, is a key event in the development of hyperglycemia that is associated with both type 1 and 2 diabetes (1, 2). In type 2 diabetes, β-cell dysfunction can in part be explained by the loss of proper glucose sensing, leading to abnormal insulin secretion. However, in both forms of diabetes, glucagon secretion can be dysregulated during hyper- and hypoglycemia (3, 4), suggesting that glucose sensing by the α-cell is also impaired. For this reason, it is important to understand mechanistically how glucagon is regulated by glucose in normal and diseased states.
High plasma levels of glucose inhibit glucagon secretion; however, it is still unclear whether this in vivo response is mediated directly via glucose sensing or indirectly by neuronal modulation and/or paracrine/endocrine effects (5–8). Pancreatic α-cells, like β-cells, possess ATP-dependent K+ (KATP) channels; however, the metabolism/oxidation of glucose resulting in closure of the KATP channels causes inhibition of glucagon secretion (9, 10). It is suggested that N-type Ca2+ channels modulate this alternate excitability downstream of KATP-channel closure (10). Glucose metabolism in α-cells generates a proton-motive force (pmf) in the inner mitochondria that drives the synthesis of ATP via ATP synthase. Uncoupling proteins (UCPs) are mitochondrial carrier proteins that can dissipate the proton gradient to prevent the pmf from becoming excessive when there is nutrient overload, which can reduce reactive oxygen species (ROS) produced by electron transport (11). There are five mitochondrial UCP homologues in mammals (12). The closely related UCPs are UCP1–3. UCP1 is mainly expressed in brown adipose tissue and UCP3 in muscle and adipose tissue, whereas UCP2 has been found in liver, brain, pancreas, and adipose tissue and immune cells (13, 14). Specifically, UCP2 is expressed in pancreatic islets where its β-cell overexpression increases mitochondrial uncoupling, decreases mitochondrial membrane potential (ΔΨm), reduces mitochondrial ROS production and cytoplasmic ATP content, and therefore attenuates glucose stimulated insulin secretion (GSIS) by antagonizing the KATP-channel pathway (15–17). Uncoupling processes have not been studied in α-cells where they could regulate ATP production and glucagon secretion. UCP2 may be cytoprotective in some cell types, such as macrophages, cardiomyocytes, and neurons (18, 19), and thus expression of UCP2 in α-cells may modulate susceptibility to stress stimuli and influence cell survival (20). This study aims to identify whether UCP2 is expressed in α-cells, and if so, to characterize the role it plays in regulating glucagon secretion and cell survival.
Results
UCP2 Is Constitutively Expressed in α-Cells of Mouse Pancreatic Islets.
The expression and localization of native UCP2 in intact and dispersed murine islets was assessed using specific polyclonal antisera against either the N or C terminus of UCP2, and the results showed identical staining patterns. Cells were costained for glucagon or insulin to identify α- and β-cells, respectively. Confocal laser scanning microscopy (CLSM) images from both dispersed islet cells (Fig. 1) and intact islets [supporting information (SI) Fig. S1A], reveal that UCP2 was mainly expressed in non-β-cells (insulin-negative or glucagon-positive cells) (Fig. 1 A and B). UCP2 was not detected in islets from UCP2−/− mice (Fig. 1 C and D).
Fig. 1.
Expression of UCP2 in dispersed pancreatic islets. Dispersed islets from UCP2+/+ (A and B) and UCP2−/− (C and D) mice were immunostained for UCP2 (red), and either glucagon (A and C, green) or insulin (B and D, green). Light (Left) and merged images (Right) are shown in the lower rows.
The expression of UCP2 was also evaluated in the cultured pancreatic cell lines α-TC6 and MIN6, which are glucagon- and insulin-secreting cells, respectively. UCP2 protein was strongly detected in α-TC6 but not in MIN6 cells (Fig. 2Ai and Aii). In α-cells, UCP2 was mainly localized to the mitochondria (Fig. 2 Aiii and B and Fig. S1B). Additionally, RT-PCR analysis showed that UCP2 mRNA expression relative to β-actin mRNA was much higher in α-TC6 compared with MIN6 cells (Fig. 2C), whereas the levels of UCP1 and UCP3 mRNA were low in both cell types, suggesting that UCP2 is the predominant UCP expressed in islets. High levels of UCP2 expression were found in both α- and β-cells of human islets (Fig. S2).
Fig. 2.
Expression of UCP2 in pancreatic cell lines. (Ai) MIN6 cells were immunostained for UCP2 (red) and insulin (green). (Aii) α-TC6 cells were stained for UCP2 (red) and glucagon (green). (Aiii) α-TC6 cells were stained for UCP2 (green) and mitochondria (red). Light (Left) and merged (Right) images are shown in the lower rows. (B) Western blot analysis of cytosolic, membrane, and mitochondrial fractions of MIN6 and α-TC6 cells with equal protein loading. Q, fractions prepared by using Qproteome Cell Compartment Kit; IM, fractions prepared by using Mitochondria Extraction Kit. (C) RT-PCR analysis of UCP1, 2, and 3 mRNA levels in MIN6 and α-TC6 cells (n = 6).
UCP2 Regulates ΔΨm and ATP Synthesis in Pancreatic α- and β-Cells.
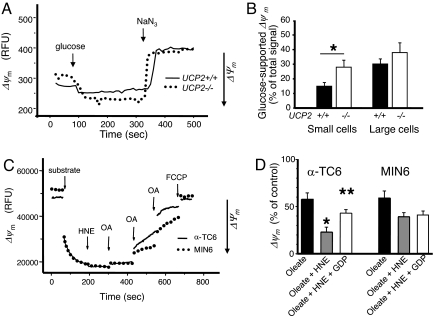
To determine the functional role of UCP2 in pancreatic cells, glucose-induced formation of ΔΨm and ATP synthesis were measured. In primary cells, ΔΨm was estimated by imaging the fluorescent probe rhodamine 123 in quenching mode so that a decrease in fluorescence corresponded to an increase in ΔΨm (Fig. 3 A and B and Fig. S3A). Non-β- [∼65% of which are α-cells (21)] and β-cells were distinguished by cell size [large cells were counted as β-cells (21)] (Fig. 3 A and B) or selective expression of GFP in β-cells (Fig. S3A). The change in glucose-induced ΔΨm was significantly lower in UCP2+/+ vs. UCP2−/− α-cells (P < 0.05, n = 3) (Fig. 3B and Fig. S3A). In contrast, there was no significant difference in glucose-driven ΔΨm in β-cells (large or GFP-positive cells) isolated from UCP2−/− and UCP2+/+ mice (Fig. 3B and Fig. S3A).
Fig. 3.
Glucose-stimulated alterations in the mitochondrial membrane potential in islets and pancreatic cell lines. (A and B) Islets dispersed from UCP2+/+ and UCP2−/− mice were cultured for 1–3 days. The ΔΨm-dependent Rh123 fluorescent signal (in relative fluorescence units, RFU) in selected regions of interest was obtained after the addition of 20 mM glucose and maximal mitochondrial polarization. The ΔΨm was then dissipated by the addition of the respiratory inhibitor NaN3 (1 mM). (A) Representative kinetic traces of the fluorescent signal in small (non-β) cells are shown. (B) Quantitation of these traces is shown (n = 21–54 cells per condition; the experiment was repeated in islets taken from three animals per group on different days). (C) A representative trace showing the effects of the UCP2 activators OA and HNE on ΔΨm as determined by safranin fluorescence. (D) Quantitation of these traces and the effect of the UCP2 inactivator GDP. Values are mean ± SEM (n = 5; ∼30 cells measured per cell type in each experiment). *, P < 0.05; **, P < 0.01.
Permeabilized clonal α-cells (α-TC6) and β-cells (MIN6) were used to test the effect of UCP2 activators 4-hydroxynonenal (HNE) and oleic (OA) or palmitic acid (PA) on ΔΨm. HNE in the presence of ≤ 75 μM OA (or PA, data not shown) reduced ΔΨm more efficiently in α-TC6 than in MIN6 cells (Fig. 3C). Quantitatively, HNE significantly enhanced OA-mediated uncoupling in α-TC6 cells (P < 0.05, n = 5), and this could be partially reversed by the prior addition of the UCP2 inhibitor, guanosine diphosphate (GDP) (Fig. 3D). In contrast, β-cell (MIN6) mitochondria exhibited a much weaker response to GDP (Fig. 3D).
To confirm the role of UCP2 in uncoupling in α-TC6 cells, ΔΨm was measured in cells transfected with a specific siRNA against UCP2 (UCP2-siRNA) or a control-siRNA (Fig. 4 A and B). In α-TC6 cells transfected with control-siRNA, OA significantly reduced ΔΨm, which was further dissipated by HNE (P < 0.05, n = 5) (Fig. 4C and Fig. S3Bi). Prior addition of GDP significantly recovered the amount of fluorescence (P < 0.05, n = 5). In contrast, cells transfected with UCP2-siRNA showed a weaker nonsignificant response to uncoupling modulators (Fig. 4C and Fig. S3Bii).
Fig. 4.
Effect of UCP2 knockdown on mitochondrial uncoupling in α-TC6 cells. (A) UCP2 protein levels were evaluated by costaining cells with specific antibodies against UCP2 (red) and glucagon (green). Merged images are shown, and yellow represents colocalization. (B) UCP2 mRNA levels were evaluated by RT- PCR in UCP2- vs. control-siRNA cells (n = 3). (C) Effect of UCP2 modulators OA, HNE, and GDP on ΔΨm were evaluated (n = 5). *, P < 0.01. Representative kinetic traces are shown in Fig. S3B.
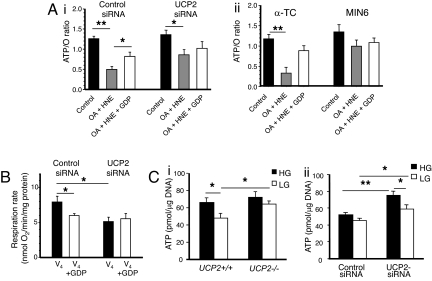
In line with the ΔΨm results, the ATP/O ratio in control-siRNA-transfected α-TC6 cells was significantly decreased by OA and HNE (P < 0.001, n = 5) (Fig. 5Ai). In contrast, activation of UCP-mediated uncoupling did not affect the ATP/O ratio as much in UCP2-siRNA-transfected α-TC6 cells (Fig. 5Ai) or MIN6 cells (Fig. 5Aii), and the addition of GDP had no effect. State IV respiration, which is proton-leak dependent, was reduced by 25% in control-siRNA-transfected α-TC6 cells after the addition of GDP (P < 0.05, n = 5) (Fig. 5B). Conversely, in UCP2-siRNA-transfected cells, state IV respiration was basally lower (P < 0.05, n = 5) and was not altered by the addition of GDP (Fig. 5B). State III respiration, which occurs independent of UCP activity, was not different (13.5 ± 1.93 and 13.7 ± 1.83 nmol of O2 per minute per milligram of protein in control-siRNA vs. UCP2-siRNA-transfected cells, n = 5) (data not shown).
Fig. 5.
Effects of UCP2 on ATP synthesis, respiration, and cellular ATP levels in pancreatic islets and cell lines. (A) Effect of UCP2 modulators OA, HNE, and GDP on ATP synthesis (ATP/O) in α-TC6 transfected with control- or UCP2-siRNA (i) or α-TC6 compared with MIN6 cells (ii). Values are mean ± SEM (n = 5). (B) State IV (V4) respiration rate was measured in control- or UCP2-siRNA-transfected α-TC6 cells. Values are mean ± SEM (n = 5). (C) ATP levels were evaluated in islets (20 islets per sample) isolated from UCP2+/+ and UCP2−/− mice (n = 4–6 mice per group) (i) and α-TC6 cells transfected with control- and UCP2-siRNA (n = 5) (ii). Both islets and α-TC6 cells were pretreated with high glucose (HG, 25 mM) for 15 min followed by incubation with either HG or low glucose (LG, 0 mM) for 1 h. *, P < 0.05; **, P < 0.01.
UCP2−/− islets exhibited significantly higher basal ATP levels compared with UCP2+/+ islets when incubated in 2.8 mM glucose (Fig. 5Ci). UCP2+/+ islets significantly reduced their cellular ATP levels (P < 0.05, n = 4) after glucose withdrawal for 1h, indicating a metabolic response to reduced glucose oxidation. In contrast, ATP levels remained elevated in UCP2−/− islets when glucose was switched from a high to a low concentration (n = 6). α-TC6 cells transfected with control-siRNA did not show significant changes in their cellular ATP levels in response to changes in glucose concentration (Fig. 5Cii). However, α-TC6 cells transfected with UCP2-siRNA showed significantly higher ATP levels compared with the control-siRNA-transfected cells under both high- and low-glucose conditions (P < 0.05, n = 3, in triplicate) (Fig. 5Cii), suggesting that UCP2 can reduce ATP generation in α-cells.
UCP2 Protein Expression Modulates Glucose-Regulated Glucagon Secretion from Isolated Islets and α-TC6 Cells.
Islets isolated from UCP2−/− mice had elevated basal glucagon secretion in the presence of 25 mM glucose (HG) (Fig. 6A). In addition, when compared with UCP2+/+ islets, UCP2−/− islets displayed reduced glucagon secretion in response to 2.8 mM glucose (LG). Therefore, the ability of UCP2−/− islets to secrete glucagon in response to changes in glucose concentration was diminished (1.05 ± 0.14-fold vs. 3.75 ± 0.15-fold in UCP2+/+ islets, P < 0.0001). Both UCP2+/+ and UCP2−/− islets incubated with high (11 mM) or low (1 mM) glucose and the glucagon secretagogue l-arginine (10 mM) displayed significantly enhanced glucagon secretion compared with HG or LG alone, respectively (P < 0.01, n = 6–7 animals). The stimulatory effect of arginine was modulated by glucose concentration; however, the increase was similar in UCP2+/+ islets and UCP2−/− islets (Fig. 6A). In keeping with a previous report (17), β-cells from UCP2−/− islets maintained sensitivity to glucose and secreted significantly more insulin in response to 25 mM glucose when compared with UCP2+/+ islets (Fig. 6B). Similar results were obtained by using islets isolated from MIP-GFP-UCP2−/− mice and confirmed that the lack of glucagon secretion was mouse-strain independent (data not shown).
Fig. 6.
Effect of UCP2 on glucagon secretion and pancreatic α-cell mass. Glucagon (A) and insulin (B) secretion from isolated islets in response to changes in glucose concentration (n = 8 for UCP2−/− mice; n = 6 for UCP2+/+ mice). Islets were preincubated in 25 mM glucose for 15 min and then switched to 25 mM glucose (HG) or 2.8 mM glucose (LG) for 1h. For secretion in the presence of arginine (10 mM), islets were incubated with HG (11 mM) or LG (1 mM) for 1h (n = 7 for UCP2−/− mice; n = 6 for UCP2+/+ mice). (C) Glucagon secretion was assessed in α-TC6 cells transfected with UCP2- or control-siRNA in the presence of 25 mM (HG) or 2.8 mM (LG) glucose (n = 3). (D and E) Fasting plasma glucagon (D) (n = 11 mice per group) and intracellular glucagon levels (E) (n = 6 mice per group) of isolated islets are shown. (F) α-cell mass was determined in pancreatic sections from UCP2+/+ (n = 4) and UCP2−/− (n = 5) mice. The glucagon-positive area was calculated and normalized to the islet area. *, P < 0.05; ***, P < 0.001.
The role of UCP2 in controlling glucagon secretion was evaluated by using α-TC6 cells transfected with UCP2- or control-siRNA. Cells transfected with UCP2-siRNA had lower glucagon secretion when compared with control-siRNA cells at 2.8 mM glucose (Fig. 6C), but secretion was similar at 25 mM glucose.
There was no difference in the fasting plasma glucagon concentrations between UCP2+/+ and UCP2−/− mice or total islet glucagon content (Fig. 6 D and E). Arginine stimulation of glucagon secretion in vivo was not significantly different in UCP2+/+ or UCP2−/− mice (Fig. S4). The effect of UCP2 expression on pancreatic islet α-cell mass was also evaluated in UCP2+/+ and UCP2−/− mice, and there was no difference in α-cell area per islet area (Fig. 6F).
UCP2 Expression Affects Islet Cell Viability in Vitro.
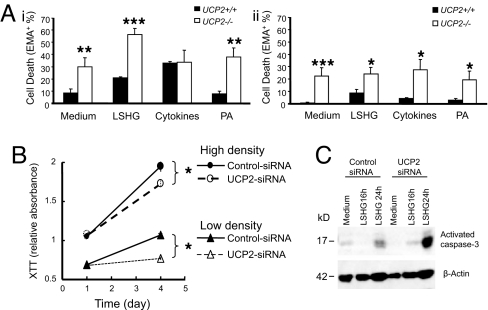
Dispersed islets were incubated in medium containing 10% FBS (medium), no serum (LSHG), proinflammatory cytokines, or 0.5 mM palmitate (PA). Cell viability was determined using a DNA-intercalating dye, ethidium monoazide (EMA) (22), and was assessed in β- and non-β-cells based on insulin-positive or insulin-negative staining, respectively. Under all treatment conditions, except in the presence of cytokines, the percentage of death in non-β-cells from control UCP2+/+ islets was significantly lower than in the non-β-cells from UCP2−/− islets (Fig. 7Ai). The maximal rate of death in β-cells was lower than in the non-β-cells and only reached 8.6 ± 3.1% in UCP2+/+ islets incubated in LSHG medium and 27.4 ± 8.6% in UCP2−/− cells incubated with cytokines. UCP2+/+ β-cells were more resistant to all death stimuli tested (Fig. 7Aii).
Fig. 7.
Effect of UCP2 on islet cell viability under different treatments. (A) Islets from UCP2−/− and UCP2+/+ mice were dispersed (experiment n = 3–6; animal n = 3–8 per group; 623.6 ± 115.8 cells counted per treatment). Insulin negative (i) or positive (ii) cells were treated with medium (10% FBS and 11.5 mM glucose), serum-starved (LSHG) (0.01% FBS and 11.5 mM glucose), cytokines (a combination of IL-1, TNF-α, and IFN-γ at 100 ng of each per milliliter) in culture medium, or PA (0.5 mM) in medium. (B) α-TC6 cells transfected with UCP2- or control-siRNA were seeded at a high or low density and cultured in medium containing 0.05% FBS for three days. Cell viability was determined by using an XTT assay (n = 4). (C) Cell apoptosis was evaluated in α-TC6 cells transfected with UCP2- or control-siRNA by Western blot analysis for activated caspase-3 after treatment as indicated. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
UCP2-siRNA-transfected α-TC6 cells were more sensitive to serum starvation than control-siRNA cells. UCP2-siRNA cells showed a significantly slower growth rate over 3 days, whether they were seeded at a high or low density (11–28% reduction in cell viability, respectively, when compared with control-siRNA cells, P < 0.005) (Fig. 7B). Western blot analysis indicated that serum starvation induced higher activation of caspase 3 in the UCP2-siRNA-transfected cells compared with control cells (Fig. 7C).
Discussion
UCP2 mediates mitochondrial uncoupling, but its physiological function is unresolved in most cell types where it is expressed (23). This study reveals that UCP2 was abundantly expressed in α-cells and that it was at a higher level than in β-cells. We then demonstrate that UCP2 functions as a mitochondrial uncoupler, regulating glucose-mediated changes in Δψm and ATP synthesis. Lack of UCP2 not only impaired glucose-coupled glucagon secretion, but also islet α-cell survival under normal physiological conditions and during metabolic stress.
In resting thymocytes, UCP2 accounts for ≥50% of all proton leaks observed (24), but its effects have not been fully characterized in other cell types. Glucose-dependent mitochondrial hyperpolarization in primary α-cells showed a greater dependence on UCP2 when compared with β-cells. The uncoupling effect of UCP2 was further confirmed in cultured α-TC6 cells when UCP2 expression was reduced by a specific siRNA. Similarly, α-TC6 cells were more responsive than MIN6 β-cells to UCP2 activators (OA and HNE) and displayed partial repolarization of ΔΨm in the presence of the UCP2 inhibitor GDP. In addition, α-TC6 cells responded to these UCP2 activators by reducing ATP synthesis relative to oxygen consumption more significantly than cultured β-cells or cells transfected with UCP2-siRNA. Glucose metabolism triggers a lower rate of ATP generation and leads to a much smaller change in the ATP/ADP ratio in α- compared with β-cells, partially because of the prevalence of anaerobic glycolysis (25). The current data suggest that this difference may also be because of a higher basal expression of UCP2 in α-cells, which functions to uncouple respiration and reduce ATP production. This idea was further confirmed in islets isolated from UCP2−/− mice and α-TC6 cells with UCP2 knockdown, which had significantly higher intracellular ATP levels when compared with UCP2+/+ islets and control α-TC6 cells.
The expression of UCP2 is modulated cell specifically. Fatty acids such as OA and PA up-regulate UCP2 expression in pancreatic β-cells (26), whereas cytokines regulate its expression in macrophages (27), and hypoxia is a modulator in cardiomyocytes (28). Interestingly, high levels of UCP2 expression were found in both α- and β-cells of human islets (Fig. S2), possibly induced by stresses during isolation and transport. Although basally there is lower UCP2 expression in β-cells isolated from murine islets or MIN6 cells, its expression is enhanced by stressors such as PA, inflammatory cytokines, or serum starvation (Fig. S5). The increased UCP2 expression correlates with previous data showing that UCP2 expression is up-regulated in β-cells isolated from the obesity-related diabetes model ob/ob when compared with wild-type mice (17). There is strong evidence that the induction of UCP2 reduces insulin secretion in islet β-cells, which is linked to its ability to adjust ΔΨm and the cytoplasmic ATP/ADP ratio and is related to the regulation of the plasma membrane KATP channel (17, 29). Importantly, fatty acid oxidation in β-cells is critical for the activation of UCP2-mediated mitochondrial uncoupling and is associated with impaired GSIS and ROS production (15, 24, 30). Recently, transgenic overexpression of UCP2 specifically in β-cells of mice fed a chow diet had no effect on plasma glucose, insulin, or in vivo GSIS (31). Therefore, UCP2 likely plays a more prominent uncoupling role in β-cells when its expression is induced under pathological or stress conditions.
The principal role of plasma glucose and cytosolic ATP concentrations in the regulation of glucagon secretion is still a matter of debate (32). α-cells possess KATP channels that are identical to those in β-cells, but reports vary on the ability of glucose to inhibit α-cell KATP channels (9, 33). Recent data show that depolarization-induced inhibition of rodent and human α-cell-glucagon release was associated with KATP inhibition and a voltage-dependent inactivation of N-type voltage-dependent Ca2+ channels when compared with L-type channel activation in β-cells (10). This difference may explain the opposite secretory response to glucose in these two islet cell types. In the current study, null or reduced UCP2 expression resulted in significantly higher basal glucagon secretion and blunted secretion in response to a switch from high to low glucose. Possibly, the chronically elevated ATP levels observed in UCP2-deficient α-cells, even in the absence of glucose, could account for the lack of a response to low glucose. These data are in line with the model of glucagon secretion proposed by Macdonald et al. (10), whereby elevated ATP levels would inhibit the KATP channel and decrease glucagon secretion. The UCP2-deficient islets maintained their ability to secrete glucagon in response to l-arginine, which is suggested to electrogenically enter the cell, directly depolarize the plasma membrane, and cause an influx of Ca2+ to stimulate secretion (32). Therefore, l-arginine potentially bypasses the glucose-sensing defect in UCP2−/− islets. These data suggest that UCP2 regulates glucagon secretion from α-cells by altering glucose metabolism coupling and ATP levels. However, in addition to ATP synthesis, UCP2 may modulate the transport of other substrates within mitochondria, such as pyruvate, that could also regulate glucagon secretion (34). Additionally, inhibition of islet glucagon secretion from UCP2−/− islets could be an indirect effect because of UCP2 deletion in β-cells causing enhanced intraislet insulin, γ-aminobutyric acid, and/or Zn2+ secretion (5). Collectively, the data suggest that UCP2 plays a role in regulating glucagon secretion from α-cells and that glucose sensing directly modulates glucagon secretion.
In certain neurons, UCP2-mediated uncoupling activity is associated with decreased ΔΨm, ROS production, and oxidative stress, maintaining neuron survival and mitochondrial biogenesis (18, 19). However, a discrepancy exists regarding the cytoprotective role of UCP2 in different cell types and in response to different stimuli. For example, UCP2 does not protect macrophages from lipopolysaccharide/nitric oxide induced apoptosis (35). Overexpression of UCP2 inhibits oxidative damage and related apoptosis in cultured hepatocytes (36) and β-cell lines (6, unpublished data). In the current study, deletion of UCP2 made dispersed α-cells more susceptible to death stimuli. Although it is uncertain which protective pathways are used, the data indicate that UCP2 may act through a growth-factor-related and/or fatty-acid-mediated pathway because UCP2 provided significant protection for islets from serum deprivation and PA. ROS may also play a role, but regulation of ROS production by UCP2 in α-cells is yet to be determined. Investigation of a few key molecules in related signaling pathways (e.g., PKB, mammalian target of rapamycin, and AMP-activated protein kinase) did not reveal any linkage between UCP2 and cytoprotection (unpublished data). Previous studies have suggested that nuclear factor-κB is constitutively activated in UCP2-null cells including β-cells, although this observation varies according to cell type and was not investigated here in α-cells (27).
Deletion of UCP2 attenuated glucagon secretion from isolated islets, whereas total islet glucagon, fasting plasma glucagon concentrations, and the ability of α-cells to secrete glucagon in response to arginine in vivo were not significantly altered. Collectively, this data suggests that the lack of a net change in blood glucagon levels in UCP2−/− mice may reflect chronic compensation to prevent hypoglucagonemia and hypoglycemia (30). Glucagon secretion is under neuronal control via glucose-sensing neurons in the hypothalamic-pituitary axis (37). A fall in blood-glucose levels is rapidly detected, and hypoglycemia is prevented via stimulation of epinephrine and glucagon secretion. Catecholamine secretion may be enhanced in UCP2−/− mice, and therefore, when glucagon secretion from isolated islets is assessed, it is independent of this control and is attenuated. Similarly, i.v. injection of l-arginine might bypass any chronic adaption as arginine causes the rapid release (within 2 min) of glucagon, probably via enhanced intracellular Ca2+ levels in the α-cell. Normoglucagonemia could also be achieved in vivo through an increase in α-cell mass, yet α-cell mass per pancreatic islet in UCP2−/− mice was not significantly changed under the conditions tested. The increased susceptibility of UCP2−/−-derived α- and β-cells to death stimuli ex vivo suggests that islet mass might have been reduced if the mice had been exposed to a stressor to stimulate cell death, such as starvation or a high fat diet. Collectively, UCP2 may play an important role in regulating α-cell mass in vivo under certain stress conditions, whereas under basal conditions, UCP2−/− mice are able to compensate to maintain normal plasma levels of glucagon.
In summary, the present study reveals a fundamental role for UCP2 in α-cell function. In islets, expression of UCP2 is essential for preserving appropriate glucagon secretion in response to glucose and cellular responses to stress stimuli. Of particular importance is the finding that mitochondrial function and hormone secretion from α-cells is more responsive to UCP2 modulation when compared with β-cells. Abundant evidence suggests that hyperglucagonemia may play a key role in the development of hyperglycemia in type 2 diabetes (32). Therefore, determining the upstream and downstream signaling events linking UCP2 to mitochondrial metabolism, glucagon secretion, and α-cell survival will be important.
Materials and Methods
Antibodies and Reagents.
Polyclonal antisera directed against UCP2 (N-19 and C-20) were purchased from Santa Cruz Biotechnology. Mouse monoclonal anti-glucagon (Sigma), rabbit polyclonal anti-human glucagon (Dako), guinea pig anti-swine insulin (Dako), fluorescein (FITC)- and cyanine (Cy5)-labeled secondary antibodies (Jackson ImmunoResearch), and anti-caspase 3 (Cell Signaling) were also used. Ethidium monoazide bromide (EMA) was purchased from Invitrogen. Cytokines (IL-1, TNF-α and IFN-γ) were from Chemicon. l-arginine and arginine monohydrochloride were from Sigma. Sodium oleate or palmitate (75 μM) (Sigma) were complexed to fatty-acid-free BSA (Sigma) solution as described previously (38). The approximate concentration of free fatty acid was 5 nM (39).
Animals and Islet Cell Lines.
UCP2−/− mice on a mixed C57BL/6/129 background were generated previously (17). In the present study, UCP2−/− mice were also bred with MIP-GFP transgenic mice in a CD1 background (kindly provided by M. Hara, University of Chicago, Chicago) (40). Multiplex PCR was used to genotype mice (17). The Animal Care Committee at the University of Toronto approved all animal protocols, and animals were handled according to the guidelines of the Canadian Council on Animal Care. Pancreatic cell lines MIN6 and α-TC6 cells were cultured in standard media.
UCP2 siRNA and Transfection.
Sequence-specific siRNA against the mouse UCP2 gene (NM_011671, Cat: AM16704, ID: 69962) and control siRNA (Cat: 4611) were from Ambion. The efficiency of these siRNAs has been reported previously (41). Transfection of α-TC6 cells with siRNA duplexes was performed by using Lipofectamine 2000 (Invitrogen). Cells with ≥50% knockdown efficiency of mRNA were obtained 72h after transfection and were used for functional analysis. All transfection experiments were n = 5 with triplicates for each condition.
RT-PCR.
RT-PCR analysis was performed by using specific primer sequences that were reported previously (26, 41) and are described in detail in SI Materials and Methods.
Pancreatic Islet Isolation and Dispersion.
Pancreatic islets were isolated as described previously (5, 26) and are described in detail in SI Materials and Methods.
Immunostaining and Immunofluorescence Confocal Microscopy.
Cells were fixed with 4% paraformaldehyde in PBS for 15 min and washed once with PBS followed by permeabilization with 0.2% Triton X-100 in PBS at room temperature for 10 min. Cells were then incubated with blocking solution containing 5% BSA and 0.1% Triton X-100 in PBS at 4°C overnight. Subsequently, cells were incubated with primary antibodies (1:100, 1:4,000, and 1:200 for goat anti-UCP2, mouse anti-glucagon, and rabbit anti-insulin, respectively) for 16 h at 4°C. After washing, cells were stained with the secondary antibodies and mounted on slides with ProLong Gold antifade reagent (Invitrogen) and CLSM image analysis was performed.
Mitochondrial and Membrane Fractionation.
Isolation of mitochondria from α-TC6 and MIN6 cells was performed based on differential centrifugation according to the procedure in the Mitochondrial Extraction Kit (Imgenex). The mitochondrial fraction was confirmed by staining with a mitochondrial staining solution. The subcellular membrane fractionation of α-TC6 and MIN6 cells was performed according to the protocol of the Qproteome cell compartment kit (Qiagen).
Parameters of Mitochondrial Metabolism.
Mitochondrial metabolism was monitored by using conventional methods (fluorescent probes rhodamine 123 and safranin O for ΔΨm, polarography for respiration, and luciferase assay for ATP synthesis) and are described in detail in SI Materials and Methods.
In Vitro Secretion Assays.
Experiments were designed to optimize glucagon secretion by using isolated islets or cultured α-TC6 cells as described previously (5). Insulin and glucagon content was measured by using RIA (5).
Pancreatic Islet Morphology.
Pancreata were extracted from mice and fixed in 10% neutral buffered formalin for ≥48 h before being processed for islet morphology as previously described (42).
Cell Viability Assays.
To measure the viability of specific islet cells, a DNA- photocross-linking reagent EMA, was used. A mixed population of live and dead islets was incubated with 0.5 μg/ml EMA for 10 min at room temperature, followed by illumination with a visible light source for 10 min. Cells were washed, fixed, and stained with insulin antibodies as described in the “Immunostaining” section. Wavelengths of 633 nm and 488 nm were used to detect the red and green signal from EMA and FITC, respectively, by CSLM. Cell proliferation and viability in cell lines were measured by using an XTT-based colorimetric assay, as described previously (5).
Statistical Analysis.
Data are expressed as mean ± SEM. Significance was determined using the Student's t test or one-way ANOVA with Tukey–Kramer or Dunn's multiple comparisons post test. P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments.
We thank Daphne Yau, Fuzheng Xia, and Robert Tsushima for their insightful discussions and technical assistance. This work was supported by Canadian Institutes of Health Research Grant MOP 12898 (to M.B.W. and C.B.C.) and partially by MOP 69018 (to A.G.). M.B.W. was supported by an investigator award from Canadian Institutes of Health Research, and J.D. and E.M.A. were supported by fellowship awards from the Canadian Diabetes Association.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0710434105/DCSupplemental.
References
- 1.Ahren B. Type 2 diabetes, insulin secretion and beta-cell mass. Curr Mol Med. 2005;5:275–286. doi: 10.2174/1566524053766004. [DOI] [PubMed] [Google Scholar]
- 2.Greenbaum CJ, Prigeon RL, D'Alessio DA. Impaired beta-cell function, incretin effect, and glucagon suppression in patients with type 1 diabetes who have normal fasting glucose. Diabetes. 2002;51:951–957. doi: 10.2337/diabetes.51.4.951. [DOI] [PubMed] [Google Scholar]
- 3.Shah P, Basu A, Basu R, Rizza R. Impact of lack of suppression of glucagon on glucose tolerance in humans. Am J Physiol. 1999;277:E283–290. doi: 10.1152/ajpendo.1999.277.2.E283. [DOI] [PubMed] [Google Scholar]
- 4.Cryer PE. Diverse causes of hypoglycemia-associated autonomic failure in diabetes. N Engl J Med. 2004;350:2272–2279. doi: 10.1056/NEJMra031354. [DOI] [PubMed] [Google Scholar]
- 5.Diao J, Asghar Z, Chan CB, Wheeler MB. Glucose-regulated glucagon secretion requires insulin receptor expression in pancreatic alpha-cells. J Biol Chem. 2005;280:33487–33496. doi: 10.1074/jbc.M506276200. [DOI] [PubMed] [Google Scholar]
- 6.Gyulkhandanyan AV, et al. Investigation of Transport mechanisms and regulation of intracellular Zn2+ in pancreatic α-cells. J Biol Chem. 2008;283:10184–10197. doi: 10.1074/jbc.M707005200. [DOI] [PubMed] [Google Scholar]
- 7.Ishihara H, et al. Islet beta-cell secretion determines glucagon release from neighbouring alpha-cells. Nat Cell Biol. 2003;5:330–335. doi: 10.1038/ncb951. [DOI] [PubMed] [Google Scholar]
- 8.Rorsman P, et al. Glucose-inhibition of glucagon secretion involves activation of GABAA-receptor chloride channels. Nature. 1989;341:233–236. doi: 10.1038/341233a0. [DOI] [PubMed] [Google Scholar]
- 9.Gromada J, et al. ATP-sensitive K+ channel-dependent regulation of glucagon release and electrical activity by glucose in wild-type and SUR1−/− mouse α-cells. Diabetes 53 Suppl. 2004;3:S181–189. doi: 10.2337/diabetes.53.suppl_3.s181. [DOI] [PubMed] [Google Scholar]
- 10.Macdonald PE, et al. A KATP channel-dependent pathway within α-cells regulates glucagon release from both rodent and human islets of Langerhans. PLoS Biol. 2007;5:e143. doi: 10.1371/journal.pbio.0050143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garlid KD, Jaburek M, Jezek P, Varecha M. How do uncoupling proteins uncouple? Biochim Biophys Acta. 2000;1459:383–389. doi: 10.1016/s0005-2728(00)00175-4. [DOI] [PubMed] [Google Scholar]
- 12.Jezek P, Urbankova E. Specific sequence of motifs of mitochondrial uncoupling proteins. IUBMB Life. 2000;49:63–70. doi: 10.1080/713803586. [DOI] [PubMed] [Google Scholar]
- 13.Ledesma A, de Lacoba MG, Rial E. The mitochondrial uncoupling proteins. Genome Biol. 2002;3:REVIEWS3015. doi: 10.1186/gb-2002-3-12-reviews3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arsenijevic D, et al. Disruption of the uncoupling protein-2 gene in mice reveals a role in immunity and reactive oxygen species production. Nat Genet. 2000;26:435–439. doi: 10.1038/82565. [DOI] [PubMed] [Google Scholar]
- 15.Chan CB, et al. Increased uncoupling protein-2 levels in β-cells are associated with impaired glucose-stimulated insulin secretion: Mechanism of action. Diabetes. 2001;50:1302–1310. doi: 10.2337/diabetes.50.6.1302. [DOI] [PubMed] [Google Scholar]
- 16.Li LX, et al. Uncoupling protein-2 participates in cellular defense against oxidative stress in clonal beta-cells. Biochem Biophys Res Commun. 2001;282:273–277. doi: 10.1006/bbrc.2001.4577. [DOI] [PubMed] [Google Scholar]
- 17.Zhang CY, et al. Uncoupling protein-2 negatively regulates insulin secretion and is a major link between obesity, beta cell dysfunction, and type 2 diabetes. Cell. 2001;105:745–755. doi: 10.1016/s0092-8674(01)00378-6. [DOI] [PubMed] [Google Scholar]
- 18.Fridell YW, Sanchez-Blanco A, Silvia BA, Helfand SL. Targeted expression of the human uncoupling protein 2 (hUCP2) to adult neurons extends life span in the fly. Cell Metab. 2005;1:145–152. doi: 10.1016/j.cmet.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 19.Andrews ZB, Diano S, Horvath TL. Mitochondrial uncoupling proteins in the CNS: In support of function and survival. Nat Rev Neurosci. 2005;6:829–840. doi: 10.1038/nrn1767. [DOI] [PubMed] [Google Scholar]
- 20.Dunning BE, Foley JE, Ahren B. α-cell function in health and disease: Influence of glucagon-like peptide-1. Diabetologia. 2005;48:1700–1713. doi: 10.1007/s00125-005-1878-0. [DOI] [PubMed] [Google Scholar]
- 21.Gopel S, et al. Voltage-gated and resting membrane currents recorded from β-cells in intact mouse pancreatic islets. J Physiol. 1999;521:717–728. doi: 10.1111/j.1469-7793.1999.00717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riedy MC, Muirhead KA, Jensen CP, Stewart CC. Use of a photolabeling technique to identify nonviable cells in fixed homologous or heterologous cell populations. Cytometry. 1991;12:133–139. doi: 10.1002/cyto.990120206. [DOI] [PubMed] [Google Scholar]
- 23.Cannon B, et al. Uncoupling proteins: A role in protection against reactive oxygen species–or not? Biochim Biophys Acta. 2006;1757:449–458. doi: 10.1016/j.bbabio.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 24.Krauss S, Zhang CY, Lowell BB. A significant portion of mitochondrial proton leak in intact thymocytes depends on expression of UCP2. Proc Natl Acad Sci USA. 2002;99:118–122. doi: 10.1073/pnas.012410699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schuit F, et al. Metabolic fate of glucose in purified islet cells. Glucose-regulated anaplerosis in beta cells. J Biol Chem. 1997;272:18572–18579. doi: 10.1074/jbc.272.30.18572. [DOI] [PubMed] [Google Scholar]
- 26.Joseph JW, et al. Free fatty acid-induced β-cell defects are dependent on uncoupling protein 2 expression. J Biol Chem. 2004;279:51049–51056. doi: 10.1074/jbc.M409189200. [DOI] [PubMed] [Google Scholar]
- 27.Bai Y, et al. Persistent nuclear factor-kappa B activation in Ucp2−/− mice leads to enhanced nitric oxide and inflammatory cytokine production. J Biol Chem. 2005;280:19062–19069. doi: 10.1074/jbc.M500566200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bodyak N, et al. Uncoupling Protein-2 Modulates Cell Viability in Adult Rat Cardiomyocytes. Am J Physiol Heart Circ Physiol. 2007;293:H829–H835. doi: 10.1152/ajpheart.01409.2006. [DOI] [PubMed] [Google Scholar]
- 29.Chan CB, et al. Overexpression of uncoupling protein 2 inhibits glucose-stimulated insulin secretion from rat islets. Diabetes. 1999;48:1482–1486. doi: 10.2337/diabetes.48.7.1482. [DOI] [PubMed] [Google Scholar]
- 30.Joseph JW, et al. Uncoupling protein 2 knockout mice have enhanced insulin secretory capacity after a high-fat diet. Diabetes. 2002;51:3211–3219. doi: 10.2337/diabetes.51.11.3211. [DOI] [PubMed] [Google Scholar]
- 31.Produit-Zengaffinen N, et al. Increasing uncoupling protein-2 in pancreatic beta cells does not alter glucose-induced insulin secretion but decreases production of reactive oxygen species. Diabetologia. 2007;50:84–93. doi: 10.1007/s00125-006-0499-6. [DOI] [PubMed] [Google Scholar]
- 32.Gromada J, Franklin I, Wollheim CB. α-cells of the endocrine pancreas: 35 years of research but the enigma remains. Endocr Rev. 2007;28:84–116. doi: 10.1210/er.2006-0007. [DOI] [PubMed] [Google Scholar]
- 33.Olsen HL, et al. Glucose stimulates glucagon release in single rat α-cells by mechanisms that mirror the stimulus-secretion coupling in β-cells. Endocrinology. 2005;146:4861–4870. doi: 10.1210/en.2005-0800. [DOI] [PubMed] [Google Scholar]
- 34.Pecqueur C, et al. Uncoupling protein-2 controls proliferation by promoting fatty acid oxidation and limiting glycolysis-derived pyruvate utilization. FASEB J. 2008;22:9–18. doi: 10.1096/fj.07-8945com. [DOI] [PubMed] [Google Scholar]
- 35.Emre Y, et al. Mitochondria contribute to LPS-induced MAPK activation via uncoupling protein UCP2 in macrophages. Biochem J. 2007;402:271–278. doi: 10.1042/BJ20061430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Collins P, et al. Increased expression of uncoupling protein 2 in HepG2 cells attenuates oxidative damage and apoptosis. Liver Int. 2005;25:880–887. doi: 10.1111/j.1478-3231.2005.01104.x. [DOI] [PubMed] [Google Scholar]
- 37.Evans ML, et al. Hypothalamic ATP-sensitive K+ channels play a key role in sensing hypoglycemia and triggering counterregulatory epinephrine and glucagon responses. Diabetes. 2004;53:2542–2551. doi: 10.2337/diabetes.53.10.2542. [DOI] [PubMed] [Google Scholar]
- 38.Wang X, et al. Gene and protein kinase expression profiling of reactive oxygen species-associated lipotoxicity in the pancreatic β-cell line MIN6. Diabetes. 2004;53:129–140. doi: 10.2337/diabetes.53.1.129. [DOI] [PubMed] [Google Scholar]
- 39.Cnop M, et al. Inverse relationship between cytotoxicity of free fatty acids in pancreatic islet cells and cellular triglyceride accumulation. Diabetes. 2001;50:1771–1777. doi: 10.2337/diabetes.50.8.1771. [DOI] [PubMed] [Google Scholar]
- 40.Hara M, et al. Transgenic mice with green fluorescent protein-labeled pancreatic β-cells. Am J Physiol Endocrinol Metab. 2003;284:E177–183. doi: 10.1152/ajpendo.00321.2002. [DOI] [PubMed] [Google Scholar]
- 41.Saleh MC, Wheeler MB, Chan CB. Endogenous islet uncoupling protein-2 expression and loss of glucose homeostasis in ob/ob mice. J Endocrinol. 2006;190:659–667. doi: 10.1677/joe.1.06715. [DOI] [PubMed] [Google Scholar]
- 42.Asghar Z, et al. Insulin resistance causes increased β-cell mass but defective glucose-stimulated insulin secretion in a murine model of type 2 diabetes. Diabetologia. 2006;49:90–99. doi: 10.1007/s00125-005-0045-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.