Abstract
A high level of accuracy during protein synthesis is considered essential for life. Aminoacyl-tRNA synthetases (aaRSs) translate the genetic code by ensuring the correct pairing of amino acids with their cognate tRNAs. Because some aaRSs also produce misacylated aminoacyl-tRNA (aa-tRNA) in vivo, we addressed the question of protein quality within the context of missense suppression by Cys-tRNAPro, Ser-tRNAThr, Glu-tRNAGln, and Asp-tRNAAsn. Suppression of an active-site missense mutation leads to a mixture of inactive mutant protein (from translation with correctly acylated aa-tRNA) and active enzyme indistinguishable from the wild-type protein (from translation with misacylated aa-tRNA). Here, we provide genetic and biochemical evidence that under selective pressure, Escherichia coli not only tolerates the presence of misacylated aa-tRNA, but can even require it for growth. Furthermore, by using mass spectrometry of a reporter protein not subject to selection, we show that E. coli can survive the ambiguous genetic code imposed by misacylated aa-tRNA tolerating up to 10% of mismade protein. The editing function of aaRSs to hydrolyze misacylated aa-tRNA is not essential for survival, and the EF-Tu barrier against misacylated aa-tRNA is not absolute. Rather, E. coli copes with mistranslation by triggering the heat shock response that stimulates nonoptimized polypeptides to achieve a native conformation or to be degraded. In this way, E. coli ensures the presence of sufficient functional protein albeit at a considerable energetic cost.
Keywords: accuracy, aminoacyl-tRNA, fidelity, protein synthesis, missense suppression
Cells have adopted quite dissimilar levels of accuracy for various processes. Although mistakes in metabolism can be substantial (1), extreme precision in replication is needed to avoid mutations (2). Protein synthesis has reached an intermediate level of fidelity with an error rate of 1 in 10,000 (for reviews, see refs. 3 and 4). Several quality control mechanisms exist to safeguard translational fidelity: proofreading performed by the aminoacyl-tRNA synthetases (aaRSs) (5–8), deacylation of mismade tRNA (9), discrimination against misacylated aminoacyl-tRNA (aa-tRNA) by EF-Tu (10), and proper codon–anticodon alignment in the ribosomal A site (11, 12).
The fact that noncanonical amino acids get incorporated into proteins in vivo has been established long ago (for review, see refs. 13 and 14). Renewed more recent interest in the expansion of the standard genetic code via incorporation of a large repertoire of unnatural amino acids has given rise to new technologies (14, 15). The success of such protein-engineering efforts clearly indicates that misincorporation of unnatural amino acids into proteins can be tolerated by both prokaryotes (14) and eukaryotes (16).
The misincorporation of canonical amino acids via noncognate aa-tRNA (e.g., ref. 17) is also more widespread than previously appreciated. The in vivo presence of misacylated aa-tRNA introduces ambiguity into the genetic code and leads to the generation of “statistical” proteins (18), whereby the use of misacylated in addition to correctly charged aa-tRNA by the ribosome during translation results in a group of related proteins for any given messenger RNA. Such mistranslation may lead to observable cellular and disease pathologies such as cell degeneration and apoptosis in mammalian systems (19) and neurodegeneration and ataxia in mice (20). Moreover, since heritable mutations in human glycyl-tRNA synthetase and tyrosyl-tRNA synthetase genes have been directly associated with the peripheral neuropathy Charcot–Marie–Tooth (21, 22), the role of the aaRSs and their editing function in maintaining translational fidelity have become areas of intense study (23–28).
We were thus prompted to reconsider the quality control mechanisms in protein synthesis from cradle to grave. We use selection-based in vivo missense suppression assays with well-studied Escherichia coli model systems to evaluate the overall impact of misacylated aa-tRNA upon the cellular protein pool, and in the process, we determine an estimate of the level of mismade protein that the organism is willing to endure.
Results
In Vivo Misacylation of Four tRNAs Rescues Growth by Missense Suppression.
Because several aaRSs are known to misacylate tRNA in vivo (e.g., refs. 17, 23, and 29), missense suppression by misacylated aa-tRNA (30) provides a useful genetic tool to study misincorporation of canonical amino acids into proteins. Thus, we address the question of protein quality within the context of missense suppression by Cys-tRNAPro, Ser-tRNAThr, Glu-tRNAGln, and Asp-tRNAAsn. In each case, E. coli growth depends on missense suppression of an altered codon that specifies an active-site residue in an essential enzyme. Only incorporation of the amino acid carried by the misacylated aa-tRNA corrects the defect in the mutant allele to produce a functional enzyme. In contrast, faithful decoding with the cognate aa-tRNA species generates an inactive enzyme and prohibits growth under our selection conditions. For each misacylated aa-tRNA species tested, a different active-site mutation was used as a reporter. Thus, to study the in vivo effects of Cys-tRNAPro, Ser-tRNAThr, Glu-tRNAGln, and Asp-tRNAAsn, we relied on the following four E. coli missense suppression systems (Table 1).
Table 1.
Genetic systems used for missense suppression
| Missense reporter allele | Enzyme | Active-site residue | Mutated active-site codon | Faithful translation | Misacylating aaRS | Missense translation |
|---|---|---|---|---|---|---|
| thyA:146CCAC→P | Thymidylate synthase | Cys146 | 146CCA | Pro146 (Pro-tRNAPro) | ProRS | Cys146 (Cys-tRNAPro) |
| bla:68ACAS→T | β-Lactamase | Ser68 | 68ACA | Thr68 (Thr-tRNAThr) | ΔNThrRS | Ser68 (Ser-tRNAThr) |
| trpA:60AATD→N | Tryptophan synthase | Asp60 | 60AAT | Asn60 (Asn-tRNAAsn) | ND-AspRS | Asp60 (Asp-tRNAAsn) |
| TrpA:49CAAE→Q | Tryptophan synthase | Glu49 | 49CAA | Gln49 (Gln-tRNAGln) | ND-GluRS | Glu49 (Glu-tRNAGln) |
Cys-tRNAPro.
For Cys-tRNAPro incorporation, we constructed the thymidylate synthase (ThyA) missense mutant allele thyA:146CCAC→P. This notation indicates the Cys→Pro codon change at position 146, where the genetically encoded UGC codon for Cys has been mutated to the CCA codon specifying Pro. Thus, translation of the missense mutant allele thyA:146CCAC→P with the cognate Pro-tRNAPro gives rise to an inactive ThyA[146P] enzyme (31) as the active-site Cys at position 146 is replaced by Pro (Fig. 1, upper scheme). However, in the presence of Cys-tRNAPro [made by E. coli prolyl-tRNA synthetase, ProRS (32)] protein with a wild-type amino acid sequence, ThyA[146C], is produced from the mutant mRNA (Fig. 1, lower scheme). ThyA catalyzes the conversion of deoxyuridylate (dUMP) to thymidylate, and an E. coli strain that carries a deletion of the endogenous thyA gene is a thymine auxotroph (31). Only coexpression of the thyA:146CCAC→P missense mutation with a ProRS known to misacylate tRNAPro with Cys efficiently (32) rescues the thymine auxotrophy of the ΔthyA E. coli strain (Fig. 2Top). Therefore, missense suppression by Cys-tRNAPro is sufficient to generate active ThyA[146C] required for growth in the absence of thymine. In contrast, expression of the thyA:146CCAC→P allele alone in the ΔthyA background leads to production of the inactive ThyA[146P] protein and fails to rescue thymine auxotrophy.
Fig. 1.
Scheme of missense suppression. Misacylating ProRS forms Pro-tRNAPro and Cys-tRNAPro (32). When correctly acylated Pro-tRNAPro decodes the mRNA of thyA:146CCAC→P, the resulting inactive ThyA[146P] does not complement the ΔthyA strain. In contrast, decoding the mRNA of thyA:146CCAC→P with misacylated Cys-tRNAPro gives a wild-type ThyA[146C] and rescues thymine auxotrophy. In the absence of a misacylating aaRS, the Upper scheme prevails, and suppression is not observed.
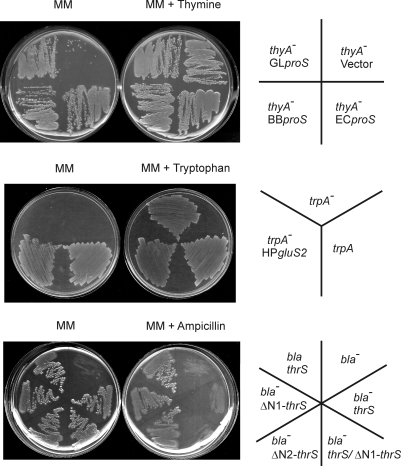
Fig. 2.
Missense suppression by misacylated aa-tRNAs. (Top) Cys-tRNAPro corrects a nonfunctional thyA gene. The proS genes from G. lamblia (GL), Borrelia burgdorferi (BB), or E. coli (EC) and the thyA:146CCAC→P (thyA−) allele were expressed in the E. coli ΔthyA strain at 37 °C on minimal medium (MM) A agar plates with or without thymine (20 μg/ml). (Middle) Glu-tRNAGln corrects a nonfunctional tryptophan synthase gene. The H. pylori ND-GluRS (HPgluS2) and the mutant trpA:49CAAE→Q (trpA−) or wild-type (trpA) allele were expressed in the E. coli trpA− strain at 37 °C on minimal medium B agar plates with or without tryptophan (20 μg/ml). (Bottom) Ser-tRNAThr corrects a nonfunctional β-lactamase gene. The thrS derivatives and the wild-type (bla) or mutant bla:68ACAS→T (bla−) allele were expressed in the E. coli thrSts strain at 42 °C (nonpermissive temperature) on minimal medium C agar plates with or without ampicillin (20 μg/ml).
Glu-tRNAGln.
For Glu-tRNAGln, we used the mutant tryptophan synthase (TrpA) allele trpA:49CAAE→Q as the missense reporter. In this case, the genetically encoded GAG Glu codon is mutated to the CAA Gln codon resulting in a Glu→Gln change at position 49. Tryptophan synthase catalyzes the last step in Trp biosynthesis, and the E. coli strain KS463 harboring a loss-of-function mutation in trpA cannot grow in the absence of Trp (33). Expression of the trpA:49CAAE→Q allele in this strain leads to production of the inactive TrpA[49Q] protein and cannot grow without Trp (Fig. 2 Middle). However, coexpression of the trpA:49CAAE→Q allele with a nondiscriminating glutamyl-tRNA synthetase (ND-GluRS) that is known to misacylate tRNAGln with Glu (34, 35) rescued growth of the trpA− strain in the absence of exogenous Trp, thus implicating Glu-tRNAGln in missense suppression (Fig. 2 Middle).
Ser-tRNAThr.
For Ser-tRNAThr incorporation, the β-lactamase (Bla) allele bla:68ACAS→T was used as the missense reporter. Here, the Ser→Thr change involves mutating the Ser codon AGU at position 68 to ACA coding for Thr. β-Lactamase catalyzes the hydrolysis of the amide bond of the lactam rings of penicillin derivatives (e.g., ampicillin). In the absence of Ser-tRNAThr-mediated translation, expression of the bla:68ACAS→T allele leads to production of the inactive Bla[68T] enzyme that is unable to hydrolyze ampicillin (36). Because the endogenous E. coli threonyl-tRNA synthetase (ThrRS) possesses an N-terminal editing domain that hydrolyzes misacylated Ser-tRNAThr formed in vivo (6), it was necessary to use an E. coli strain that carries a temperature-sensitive mutant of wild-type ThrRS (37). Indeed, at the nonpermissive temperature (42 °C) no growth of the thrSts E. coli strain is observed (Fig. 2 Bottom). Complementation of the thrSts strain with two previously described thrS clones (6) that lack the ThrRS N-terminal editing domain (ΔN1-thrS and ΔN2-thrS) allows for translation of an active Bla[68S] enzyme from the bla:68ACAS→T mRNA, conferring ampicillin resistance (Fig. 2 Bottom). Coexpression of the wild-type E. coli ThrRS with the editing-defective ΔN1-ThrRS abolishes ampicillin resistance, indicating that formed Ser-tRNAThr is bound and transedited by the wild-type ThrRS before its use in protein synthesis (Fig. 2 Bottom).
Asp-tRNAAsn.
Finally, for Asp-tRNAAsn we used a different allele of tryptophan synthase, trpA:60AATD→N (Asp→Asn change at position 60 by mutating the wild-type GAU Asp codon to the AAT Asn codon). Expression of a nondiscriminating aspartyl-tRNA synthetase (ND-AspRS) capable of forming Asp-tRNAAsn in an E. coli strain that carries a chromosomal copy of trpA:60AATD→N (38) rescues Trp auxotrophy. The results for this system were published in ref. 17.
To support the phenotypic in vivo complementation results in a more quantitative manner, we determined the level of missense suppression present in cells under selection. The presence of functional reporter enzyme was monitored in vitro by measuring the specific activity present in cell extracts of the strains in Fig. 2. Total accumulation of full-length reporter protein (both active and inactive) was estimated from immunoblots (Tables 2–4).
Table 2.
Analysis of missense-suppressed thymidylate synthase in ΔthyA background
| Reporter allele | Added aaRS | Active ThyA[146C], pmol/mg S100 | Total ThyA[146C] + ThyA[146P], pmol/mg S100 | Suppression, %* |
|---|---|---|---|---|
| thyA | 800 ± 80 | 800 | 100 | |
| ΔthyA | 0 | 0 | ||
| thyA:146CCAC→P | 3.8 ± 1 | 640 | 0.6 | |
| thyA:146CCAC→P | GLproS | 137 ± 13 | 685 | 20 |
| thyA:146CCAC→P | ECproS | 24.7 ± 1.6 | 561 | 4.4 |
| thyA:146CCAC→P | BBproS | 8.7 ± 1 | 483 | 1.8 |
*Suppression % represents the ratio between the active and total reporter proteins.
Table 3.
Analysis of missense-suppressed β-lactamase in thrSts background
| Reporter allele | Added aaRS | Ampicillin | Active Bla[68S], units/μL medium† | Total Bla[68S] + [68T], ng/μL medium | Suppression %* |
|---|---|---|---|---|---|
| bla | thrS | − | 39 ± 2 | 0.8 | |
| + | 40 ± 2 | 0.9 | |||
| bla:68ACAS→T | ΔN1-thrS | − | 0 | 0.4 | 0 |
| + | 22 ± 1 | 9.4 | 5.4 | ||
| bla:68ACAS→T | ΔN2-thrS | − | 0 | 0.3 | 0 |
| + | 16 ± 1 | 6.8 | 5.3 |
*Suppression % was determined by dividing the specific activity of each sample by that of the bla, thrS strain (47 units/ng).
†One unit of Bla enzyme is defined as hydrolyzing 1 nmol of nitrocefin per min using 1 μL of the test samples.
Table 4.
Analysis of missense-suppressed tryptophan synthase in trpA– background
| Reporter Allele | Added aaRS* | Active TrpA† | Total TrpA | Suppression % |
|---|---|---|---|---|
| TrpA[49E] | ||||
| TrpA | HPgluS2 | 1.6 ± 0.2 | NA‡ | |
| trpA:49CAAE→Q | HPgluS2 | 0.08 ± 0.01 | 4.6§ | |
| trpA:49CAAà[συπ]→Q | 0 | 0 | ||
| TrpA[60D] | TrpA[60D] + [60N]¶ | |||
| trpA (strain W3110) | 5.5 ± 0.2 | 2 ± 0.2 | NA | |
| trpA:60AATD→N | DRaspS2 | 1.2 ± 0.1 | 2.8 ± 0.3 | 16‖ |
| trpA:60AATDà[συπ]→]N | HSaspS2 | 2.1 ± 0.2 | 2.7 ± 0.3 | 28 |
| trpA:60AATD→N | 0 | 0.74 ± 0.1 | 0 |
*HP, H. pylori; DR, D. radiodurans; HS, H. salinarum.
†Units are units/mg S100. One unit of TrpA enzyme is defined as producing 0.1 μmol of tryptophan in 20 min at 37 °C using 1 mg of protein.
‡NA, not applicable.
§Suppression % was determined by dividing the activity seen in the second row (0.08 unit/mg) by the activity in the presence of the wild-type trpA allele (1.6 units/mg).
¶Units are ng/mg S100.
‖Suppression % was determined by dividing the specific activity of each sample by that calculated from the TrpA in the W3110 strain (2.75 units/ng TrpA[60D] in W3110).
In the Cys-tRNAPro missense suppression system, overexpression of E. coli or Giardia lamblia ProRS suppressed the thymine requirement of the thyA:146CCAC→P allele in the ΔthyA strain (Fig. 2 Top). As expected, only in the presence of the misacylating ProRSs did active ThyA[146C] protein represent a significant fraction of the expressed protein (Table 2, 1.8–20% versus 0.6% suppression in the absence of an added synthetase). No ThyA activity was detected in extracts of the ΔthyA strain (Table 2, second row). In all ΔthyA derivative strains, expression of ThyA from the thyA:146CCAC→P allele reached a level of up to 80% compared with its expression from the wild-type thyA allele (Table 2, 640 vs. 800 pmol/mg of total ThyA protein). Moreover, the differential ability to form Cys-tRNAPro by the E. coli and G. lamblia ProRS enzymes (32) is reflected in the disparate suppression levels of the thyA:146CCAC→P allele (4.4% vs. 20%, respectively).
In the Ser-tRNAThr missense suppression system, complementation of the E. coli thrSts strain by plasmid-borne editing-defective thrS alleles led to suppression of the bla:68ACAS→T mutant allele (Fig. 2 Bottom). This suppression correlated with β-lactamase activity measured in Table 3. In the absence of ampicillin, only the strain containing the wild-type bla gene produced active β-lactamase (Table 3, compare first row with third and fifth rows). Under selection, coexpression of the bla:68ACAS→T allele and the ΔN-thrS genes was required to produce substantial amounts of β-lactamase (Table 3, compare second row with fourth and sixth rows). Western blotting showed a 10-fold increase in total β-lactamase protein when these strains were grown under ampicillin selection (9.4 and 6.8 ng/μL vs. 0.9 ng/μL).
In vivo missense suppression of the trpA:49CAAE→Q and the trpA:60AATD→N alleles was brought about by Glu-tRNAGln and Asp-tRNAAsn, respectively. The nondiscriminating GluRS enzyme from Helicobacter pylori allowed an E. coli Trp auxotroph cotransformed with the missense reporter trpA:49CAAE→Q to grow in the absence of Trp (Fig. 2 Middle). In this case, 4.6% of the total TrpA protein was active compared with wild-type (Table 4 Upper). The presence of the nondiscriminating AspRS enzymes from Deinococcus radiodurans (DR) and Halobacterium salinarum (HS) allowed the E. coli strain harboring the trpA:60AATD→N allele to grow in the absence of Trp (17); suppression levels of up to 28% (compared with wild-type) were measured in the presence of the H. salinarum ND-AspRS (Table 4 Lower).
Effective Missense Suppression Relies on Induction of the Heat Shock Response.
To assess the cellular response to misfolded proteins made in the presence of misacylated aa-tRNA, we monitored the mRNA levels of the two major E. coli chaperones MopA and DnaK by quantitative real-time PCR. Transcription of heat shock genes that aid in protein folding (e.g., mopA and dnaK) and recycling (e.g., lon, clpA, clpP, and clpX) are coupled to the degree of cellular protein misfolding present (39). Expression of the nondiscriminating D. radiodurans ND-AspRS in the E. coli strain MG1655 increased the abundance of dnaK and mopA transcripts by 49- and 8.7-fold, respectively. The magnitude of this induction was lower (2.5 and 1.3 for the dnaK and mopA mRNAs, respectively) when the nondiscriminating AspRS was expressed in strain KY2350 that lacks the major heat shock proteases lon, clpP, clpX, hslV, and hslU (40) and therefore cannot recycle mismade or misfolded proteins. This lower induction is misleading because the levels of dnaK and mopA mRNAs in the protease-deficient KY2350 strain are 370- and 69-fold higher relative to the protease-replete MG1655 strain, i.e., the heat shock machinery in KY2350 is already highly expressed even in the absence of misacylated aa-tRNA. In the presence of misacylated aa-tRNA, the protease-deficient E. coli cells are unable to cope with the additional burden of misfolded proteins caused by expression of the nondiscriminating AspRS. In fact, misincorporation caused by expression of any one of the misacylating aaRSs used in our in vivo missense suppression systems has a severe impact on cell growth in the protease-deficient background (Fig. 3Right) whereas it is easily tolerated in the wild-type strain (Fig. 3 Left).
Fig. 3.
Proteases are essential for coping with mismade proteins. (Left) Growth curves of protease-replete derivatives. The E. coli strain MG1655 (○) was transformed with the nondiscriminating HPgluS2 (●), the GLproS (□), or the nondiscriminating DRaspS2 (■) and grown at 37 °C in minimal medium. (Right) Growth curves for the protease-deficient strain derivatives. The isogenic strain KY2350 was transformed with the same set of misacylating aaRSs and grown under the same conditions as described above.
Surviving Mistranslation.
We next sought to quantify more rigorously the degree of misincorporation caused by the presence of misacylated aa-tRNA in a polypeptide that is not subject to selection. We chose the E. coli dihydrofolate reductase (DHFR) protein as a nonselected, missense indicator. The third codon, specifying Ser in the wild-type folA gene was changed to AAT (denoted folA:3AATS→N) that is decoded by tRNAAsn. This is a permissive site for mutations in this protein since the presence of amino acid residues other than the wild-type Ser at position 3 does not affect the structure and function of the mature DHFR protein (41). Thus, in the absence of misacylated aa-tRNA, expression of the folA:3AATS→N allele will produce DHFR with a Ser→Asn substitution at position 3. The folA:3AATS→N allele was coexpressed with the D. radiodurans ND-AspRS in the E. coli strain harboring a chromosomal trpA:60AATD→N allele in minimal media in the absence of exogenous Trp (selecting for TrpA[60D] function as in Fig. 2) or in minimal media supplemented with Trp. An analysis of the aminoacyl-tRNA pool by acid gel electrophoresis followed with tRNAAsn-specific probing (data not shown) indicated that both Asp-tRNAAsn (made by D. radiodurans ND-AspRS) and the cognate Asn-tRNAAsn were present. In the presence of selection (i.e., without Trp), equal amounts of Asp-tRNA and Asn-tRNA were observed, whereas there was less Asp-tRNA in the presence of Trp. This presence of Asp-tRNAAsn in addition to the cognate Asn-tRNAAsn should lead to a mixture of Asp and Asn at position 3 of the DHFR protein. After growth to late log phase, the two DHFR protein samples were extensively purified and subjected to MALDI-MS analysis. The activity of ND-AspRS caused similar levels of misincorporation in the indicator DHFR protein both in the presence and absence of Trp selection (Fig. 4), that is 12 ± 1% vs. 9 ± 1%, respectively. Thus, based on this experiment with one indicator protein, one may conclude that ≈10% of the accumulated proteins can differ from the ORF sequence in the presence or absence of nutritional selection in E. coli.
Fig. 4.
Asp-tRNAAsn caused misincorporation in the reporter protein DHFR. The folA:3AATS→N missense mutant allele was expressed in the E. coli trpA:60AATD→N strain containing the nondiscriminating DRaspS2 with (Upper) or without (Lower) tryptophan. Tryptic digests of both DHFR protein preparations were methylated before MALDI-TOF MS analysis. Two N-terminal fragments of interest are shown: MINLIAALAVD*R (monoisotopic mass, MH+ = 1,327.78) and MI D*LIAALAV D*R (monoisotopic mass MH+ = 1,342.78). The ratio of absolute intensities of peaks at m/z 1,327.78 and 1,342.78 corresponds to the Asp/Asn ratio of the amino acid at position 3.
Discussion
Using four established missense suppression systems, we show that the misacylating aaRSs, ProRS, ΔN-ThrRS, ND-GluRS, and ND-AspRS generate both misacylated and cognate aa-tRNAs in vivo in E. coli. The misacylated aa-tRNA may be rebound and reedited by a second cognate aaRS, as evidenced by the lack of misincorporation of Ser-tRNAThr when the editing defective ΔN-ThrRS and wild-type ThrRS are both present in the thrSts strain (Fig. 2). This in vivo finding contributes to the discussion of the posttransfer editing mechanism by class II aaRSs and the binding of misacylated aa-tRNA to EF-Tu (42). In the absence of complete tRNA editing, and driven by our missense selection system, the misacylated aa-tRNA is then presented to the ribosome by EF-Tu. This leakage of the EF-Tu barrier (10, 43) allows mistranslation, although the level of misincorporation depends on the concentration and the nature of the misacylated aa-tRNAs in vivo.
Our genetic and biochemical evidence demonstrated that E. coli tolerates a significant amount of mistranslation. However, this work does not identify a single editing function (e.g., by the aaRSs, EF-Tu, the ribosome, or the chaperones and proteases) as the most important step in ensuring the production and maintenance of a functional cellular proteome. Our current understanding does not permit us to define the relative contributions of each of the above protein quality control mechanisms to diverse bacterial or eukaryotic growth conditions.
It is intuitively assumed that mistranslation causes reduced growth rate and fitness because it results in altered proteins that may have less overall activity. Indeed, the presence of misacylated aa-tRNA in a protease-deficient strain caused a significant reduction of the growth rate (Fig. 3B). Here, however, we also show that E. coli survives the errors of an ambiguous genetic code and does not require a perfectly accurate proteome. An ambiguous code may be beneficial under certain situations (27). The tolerance of mistranslation may be attributed partly to protein plasticity; it is known that many positions in a protein molecule, except the catalytic active-site residues, permit multiple substitutions (44, 45).
Our results illustrate that the presence of ≈10% mismade protein is not detrimental to growing cells. The energetic cost of error correction requires a considerable investment of the cell's resources, and the extra energetic cost of proofreading and protein turnover will tend to reduce the growth rate (46). Thus, the level of mistranslation tolerated is a balance between achieving a functional proteome and an optimal growth rate. The inherent plasticity of the error-minimizing network can buffer against the serious effects of debilitating mutations because it allows for chaperones and proteases to take over when the editing functions of aaRSs are insufficient for error correction. In fact, this is the case for mitochondrial protein synthesis, where three aaRSs inherently lack the editing function (47), and quality control is achieved downstream by degradation of mismade proteins, leaving only properly folded proteins for functional insertion into the membrane.
Yet, the ways of dealing with mistranslation probably vary greatly in different organisms and distinct outcomes are to be expected. For example, an editing-defective alanyl-tRNA synthetase (AlaRS) is linked to ataxia and neurodegeneration in the mouse. The inability to clear Ser-tRNAAla and Gly-tRNAAla causes global misfolding of mistranslated proteins in neurons that lead to the disease phenotype (20). Apart from neurons, other tissues do not exhibit any dramatic malfunctions because of the presence of misacylated aa-tRNA. Thus, the extent that mistranslation can be tolerated and dealt with by the unfolded protein response varies even within an organism. This allows for very exciting future research on diseases caused by the devastating effects of single amino acid substitutions in a particular protein (e.g., in cystic fibrosis, Alzheimer's disease, cancer). To the degree that mistranslation can be tolerated, expression of editing-defective aaRSs in the affected tissues may correct for the genetic mutation and significantly improve the disease progression.
Materials and Methods
For additional procedures, see supporting information (SI) Text.
Strains, Plasmids, and Culture Media.
The E. coli strain W3110, the ΔthyA strain χ2913 (31), the thrSts strain EJJ320, the trpA− strain KS463 (33) and the trpA34 strain (38) were obtained from the E. coli Genetic Stock Center (Yale University). E. coli strains MG1655 and its protease-deficient derivative KY2350 [ΔhslVU, Δ(clpPX-lon)] (40) were obtained from M. Kanemori (HSP Research Institute, Kyoto, Japan).
Minimal medium supplemented with 0.4% glucose, 1 μg/mL thiamine, 20 μg/mL amino acids, and, if needed, 0.02% arabinose, 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG), 20 μg/mL ampicillin (Amp), 20 μg/mL chloramphenicol (Cm), 20 μg/mL kanamycin (Kan) were used for the in vivo tests. The following media were derived accordingly. Medium A contains 19 aa (10 μg/ml), in addition to 0.5 mM Cys, 0.008% uracil, 0.02% arabinose, and IPTG. Medium B has only 19 aa (lacking Trp). Medium C contains 19 aa, as well as 1 mM Ser, 0.02% arabinose, and IPTG. Medium D contains 20 aa.
The vectors pCYB1 and pACYC177 were from New England Biolabs; pBad18-Cm and pBad18-Kan were from American Type Culture Collection. pCBS and pTech were described in ref. 48. All ORF sequences were amplified by PCR: trpA, thyA, thrS, and folA from E. coli W3110 genomic DNA, bla from pUC19 (GenBank accession no. L09137; the active-site residue is Ser68), gluS2 from H. pylori DNA (49); the proS genes (32) and D. radiodurans aspS2 (17) were described earlier. Mutant genes thyA:146CCAC→P (Cys→Pro codon change at position 146), trpA:49CAAE→Q (Glu→Gln codon change at position 49), bla:68ACAS→T (Ser→Thr codon change at position 68) (36), folA:3AATS→N (Ser→Asn codon change at position 3), ΔN1-thrS (removal of amino acids 2–224 of ThrRS), and ΔN2-thrS (removal of amino acids 2–241 of ThrRS) (6) were made by PCR mutagenesis with primers containing the corresponding mutations. PCR products were cloned into the pCR 2.1-TOPO vector and then subcloned into desired vectors. The pACYC-Kan vector was constructed by blunt-end ligation of 2 fragments: the replicating fragment of pACYC177 (BstEII-filled, AhdI-filled) and the multiple-cloning sites of pBad18-Cm (ClaI-filled, PvuI-filled). The thrS, ΔN1-, and ΔN2-thrS genes were cloned into pBAD-Kan under the control of an arabinose promoter. The aspS2 and gluS2 genes were cloned into pCBS and pCYB1 vectors under the control of the E. coli trpS promoter and Tac promoter, respectively. The proS genes were cloned into pACYC-Kan and pCYB1 vectors under the control of the arabinose and Tac promoter, respectively. The thyA:146CCAC→P and C-terminally His6-tagged folA:3AATS→N were cloned into pCYB1. The bla:68ACAS→T and trpA:49CAAE→Q genes were cloned into pTech.
Genetic Systems for Missense Suppression.
Four E. coli systems were used.
Suppression of the thyA:146CCAC→P mutation by Cys-tRNAPro.
This system was tested in derivatives of the E. coli thymine auxotroph χ2913 (31). The strain harboring pCYB-thyA or thyA:146CCAC→P was cotransformed with pACYC-Kan-proS. Transformants were grown in medium A plus thymine (1 μg/mL) to A600 = 0.4. Cells were harvested, washed with minimal medium, and streaked on medium A agar plates in the presence or absence of thymine and grown at 37 °C.
Suppression of the trpA:49CAAE→Q mutation by Glu-tRNAGln.
This system was tested in recombinants of the E. coli Trp auxotroph KS463 (33). The strain harboring pTech-trpA or trpA:49CAAE→Q was cotransformed with pCBS-gluS2. Single colonies, streaked on medium B agar plates in the presence or absence of Trp, were grown at 37 °C.
Suppression of the bla:68ACAS→T mutation by Ser-tRNAThr.
This system was tested in descendents of the E. coli thrSts strain EJJ320 (37) at 42 °C. Progeny harboring pTech-bla or bla:68ACAS→T were cotransformed with pBAD-Kan-thrS, ΔN1-thrS, and ΔN2-thrS. Cultures in medium C grown to A600 = 0.4 were harvested, washed with minimal medium, streaked on medium C agar plates in the presence or absence of Amp (20 μg/mL), and incubated at 42 °C.
Suppression of the trpA:60AATD→N mutation by Asp-tRNAAsn.
This system was tested in the E. coli trpA34 background (17, 38). After transformation with pCBS-aspS2, single colonies, streaked on medium B agar plates in the presence or absence of Trp, were incubated at 37 °C.
Quantifying the Heat Shock Response.
The transcript levels of mopA, dnaK, and gapA were measured using primers and methods described in ref. 50. The strains (KY2350 or MG1655 alone or containing the aspS2 plasmid) were grown for 1 h at 37 °C. Then, aspS2 expression was induced with 1 mM IPTG, whereas cultures of the strains lacking the aspS2 plasmid were left uninduced. Cells were harvested at A600 = 0.4–0.7. Total RNA was extracted, reverse transcribed, and quantified by RT-PCR. Expression ratios were generated by the 2−ΔΔCt method (51). The relative abundance of each message in a sample was normalized to gapA transcript levels because gapA remains constant for strains growing in medium containing glucose.
Growth of Cells Containing Misacylated aa-tRNA.
The E. coli strain MG1655 and its protease-deficient derivative KY2350 were transformed with pCYB-GLproS, pCYB-DRaspS2, or pCYB-HPgluS2. Overnight cultures of the transformants were used to inoculate medium D. After growth for 1 h at 37 °C, 1 mM IPTG was added to induce expression of the nondiscriminating aaRS genes. Cell growth was monitored (A600) as a function of time.
Supplementary Material
Acknowledgments.
We thank R. A. Bonono (Louis Stokes Veterans Affairs Medical Center, Cleveland, OH), G. Chin, L. Fay, L. Feng, M. Ibba, M. Kanemori (HSP Research Institute, Kyoto, Japan), S. G. Kreft, J. Ling, K. B. Low, B. Min, S. Namgoong, A. Pfeifer, J. Pober, J. Salazar, D. Steege, M. Springer (Institut de Biologie Physico-Chimique, Paris, France), P. van Bladeren, and C. Yanofsky (Stanford University, Stanford, CA) for gifts of materials, advice, and encouragement. This work was supported by grants from the National Institute of General Medical Sciences and the National Science Foundation (to D.S.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0809179105/DCSupplemental.
References
- 1.LaRossa RA, Van Dyk TK. Metabolic mayhem caused by 2-ketoacid imbalances. Bioessays. 1987;7:125–130. doi: 10.1002/bies.950070308. [DOI] [PubMed] [Google Scholar]
- 2.Marinus KJ. In: Escherichia coli and Salmonella: Cellular and Molecular Biology. Neidhardt FC, editor. Washington, DC: Am Soc Microbiol; 1996. pp. 749–763. [Google Scholar]
- 3.Kurland CG. Translational accuracy and the fitness of bacteria. Annu Rev Genet. 1992;26:29–50. doi: 10.1146/annurev.ge.26.120192.000333. [DOI] [PubMed] [Google Scholar]
- 4.Jakubowski H, Goldman E. Editing of errors in selection of amino acids for protein synthesis. Microbiol Rev. 1992;56:412–429. doi: 10.1128/mr.56.3.412-429.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nureki O, et al. Enzyme structure with two catalytic sites for double-sieve selection of substrate. Science. 1998;280:578–582. doi: 10.1126/science.280.5363.578. [DOI] [PubMed] [Google Scholar]
- 6.Dock-Bregeon A, et al. Transfer RNA-mediated editing in threonyl-tRNA synthetase: The class II solution to the double discrimination problem. Cell. 2000;103:877–884. doi: 10.1016/s0092-8674(00)00191-4. [DOI] [PubMed] [Google Scholar]
- 7.Beuning PJ, Musier-Forsyth K. Hydrolytic editing by a class II aminoacyl-tRNA synthetase. Proc Natl Acad Sci USA. 2000;97:8916–8920. doi: 10.1073/pnas.97.16.8916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lincecum TL, et al. Structural and mechanistic basis of pre- and posttransfer editing by leucyl-tRNA synthetase. Mol Cell. 2003;11:951–963. doi: 10.1016/s1097-2765(03)00098-4. [DOI] [PubMed] [Google Scholar]
- 9.Ferri-Fioni ML, et al. Structure of crystalline d-Tyr-tRNATyr deacylase: A representative of a new class of tRNA-dependent hydrolases. J Biol Chem. 2001;276:47285–47290. doi: 10.1074/jbc.M106550200. [DOI] [PubMed] [Google Scholar]
- 10.LaRiviere FJ, Wolfson AD, Uhlenbeck OC. Uniform binding of aminoacyl-tRNAs to elongation factor Tu by thermodynamic compensation. Science. 2001;294:165–168. doi: 10.1126/science.1064242. [DOI] [PubMed] [Google Scholar]
- 11.Murphy FVR, Ramakrishnan V, Malkiewicz A, Agris PF. The role of modifications in codon discrimination by tRNALysUUU. Nat Struct Mol Biol. 2004;11:1186–1191. doi: 10.1038/nsmb861. [DOI] [PubMed] [Google Scholar]
- 12.Cochella L, Green R. An active role for tRNA in decoding beyond codon:anticodon pairing. Science. 2005;308:1178–1180. doi: 10.1126/science.1111408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen GN, Munier R. Incorporation of structural analogues of amino acids in bacterial proteins. Biochim Biophys Acta. 1956;21:592–593. doi: 10.1016/0006-3002(56)90207-4. [DOI] [PubMed] [Google Scholar]
- 14.Wang L, Xie J, Schultz PG. Expanding the genetic code. Annu Rev Biophys Biomol Struct. 2006;35:225–249. doi: 10.1146/annurev.biophys.35.101105.121507. [DOI] [PubMed] [Google Scholar]
- 15.Kohrer C, Sullivan EL, RajBhandary UL. Complete set of orthogonal 21st aminoacyl-tRNA synthetase-amber, ochre, and opal suppressor tRNA pairs: Concomitant suppression of 3 different termination codons in an mRNA in mammalian cells. Nucleic Acids Res. 2004;32:6200–6211. doi: 10.1093/nar/gkh959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu W, Brock A, Chen S, Chen S, Schultz PG. Genetic incorporation of unnatural amino acids into proteins in mammalian cells. Nat Methods. 2007;4:239–244. doi: 10.1038/nmeth1016. [DOI] [PubMed] [Google Scholar]
- 17.Min B, et al. Protein synthesis in Escherichia coli with mischarged tRNA. J Bacteriol. 2003;185:3524–3526. doi: 10.1128/JB.185.12.3524-3526.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woese CR. On the evolution of the genetic code. Proc Natl Acad Sci USA. 1965;54:1546–1552. doi: 10.1073/pnas.54.6.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nangle LA, Motta CM, Schimmel P. Global effects of mistranslation from an editing defect in mammalian cells. Chem Biol. 2006;13:1091–1100. doi: 10.1016/j.chembiol.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 20.Lee JW, et al. Editing-defective tRNA synthetase causes protein misfolding and neurodegeneration. Nature. 2006;443:50–55. doi: 10.1038/nature05096. [DOI] [PubMed] [Google Scholar]
- 21.Seburn KL, Nangle LA, Cox GA, Schimmel P, Burgess RW. An active dominant mutation of glycyl-tRNA synthetase causes neuropathy in a Charcot–Marie–Tooth 2D mouse model. Neuron. 2006;51:715–726. doi: 10.1016/j.neuron.2006.08.027. [DOI] [PubMed] [Google Scholar]
- 22.Jordanova A, et al. Disrupted function and axonal distribution of mutant tyrosyl-tRNA synthetase in dominant intermediate Charcot–Marie–Tooth neuropathy. Nat Genet. 2006;38:197–202. doi: 10.1038/ng1727. [DOI] [PubMed] [Google Scholar]
- 23.Roy H, Ling J, Irnov M, Ibba M. Posttransfer editing in vitro and in vivo by the β subunit of phenylalanyl-tRNA synthetase. EMBO J. 2004;23:4639–4648. doi: 10.1038/sj.emboj.7600474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams AM, Martinis SA. Mutational unmasking of a tRNA-dependent pathway for preventing genetic code ambiguity. Proc Natl Acad Sci USA. 2006;103:3586–3591. doi: 10.1073/pnas.0507362103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ling J, Roy H, Ibba M. Mechanism of tRNA-dependent editing in translational quality control. Proc Natl Acad Sci USA. 2007;104:72–77. doi: 10.1073/pnas.0606272104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.SternJohn J, Hati S, Siliciano PG, Musier-Forsyth K. Restoring species-specific posttransfer editing activity to a synthetase with a defunct editing domain. Proc Natl Acad Sci USA. 2007;104:2127–2132. doi: 10.1073/pnas.0611110104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bacher JM, Waas WF, Metzgar D, de Crécy-Lagard V, Schimmel P. Genetic code ambiguity confers a selective advantage on Acinetobacter baylyi. J Bacteriol. 2007;189:6494–6496. doi: 10.1128/JB.00622-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yao P, et al. Unique residues crucial for optimal editing in yeast cytoplasmic leucyl-tRNA synthetase are revealed by using a novel knockout yeast strain. J Biol Chem. 2008;283:22591–22600. doi: 10.1074/jbc.M801181200. [DOI] [PubMed] [Google Scholar]
- 29.Korencic D, et al. A freestanding proofreading domain is required for protein synthesis quality control in Archaea. Proc Natl Acad Sci USA. 2004;101:10260–10265. doi: 10.1073/pnas.0403926101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murgola EJ. tRNA, suppression, and the code. Annu Rev Genet. 1985;19:57–80. doi: 10.1146/annurev.ge.19.120185.000421. [DOI] [PubMed] [Google Scholar]
- 31.Michaels ML, Kim CW, Matthews DA, Miller JH. Escherichia coli thymidylate synthase: Amino acid substitutions by suppression of amber nonsense mutations. Proc Natl Acad Sci USA. 1990;87:3957–3961. doi: 10.1073/pnas.87.10.3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahel I, et al. Cysteine activation is an inherent in vitro property of prolyl-tRNA synthetases. J Biol Chem. 2002;277:34743–34748. doi: 10.1074/jbc.M206928200. [DOI] [PubMed] [Google Scholar]
- 33.Yanofsky C, Horn V. Tryptophan synthetase chain positions affected by mutations near the ends of the genetic map of trpA of Escherichia coli. J Biol Chem. 1972;247:4494–4498. [PubMed] [Google Scholar]
- 34.Lapointe J, Duplain L, Proulx M. A single glutamyl-tRNA synthetase aminoacylates tRNAGlu and tRNAGln in Bacillus subtilis and efficiently misacylates Escherichia coli tRNA1Gln in vitro. J Bacteriol. 1986;165:88–93. doi: 10.1128/jb.165.1.88-93.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Núñez H, Lefimil C, Min B, Söll D, Orellana O. In vivo formation of glutamyl-tRNAGln in Escherichia coli by heterologous glutamyl-tRNA synthetases. FEBS Lett. 2004;557:133–135. doi: 10.1016/s0014-5793(03)01460-1. [DOI] [PubMed] [Google Scholar]
- 36.Dalbadie-McFarland G, Neitzel JJ, Richards JH. Active-site mutants of β-lactamase: Use of an inactive double mutant to study requirements for catalysis. Biochemistry. 1986;25:332–338. doi: 10.1021/bi00350a008. [DOI] [PubMed] [Google Scholar]
- 37.Johnson EJ, Cohen GN, Saint-Girons I. Threonyl-transfer ribonucleic acid synthetase and the regulation of the threonine operon in Escherichia coli. J Bacteriol. 1977;129:66–70. doi: 10.1128/jb.129.1.66-70.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shirvanee L, Horn V, Yanofsky C. Escherichia coli mutant trpA34 has an Asp–Asn change at active-site residue 60 of the tryptophan synthetase α chain. J Biol Chem. 1990;265:6624–6625. [PubMed] [Google Scholar]
- 39.Yura T, Kanemori M, Morita MT. The heat shock response: regulation and function. In: Storz G, Hengge-Aronis R, editors. Bacterial Stress Responses. Washington, DC: Am Soc Microbiol; 2000. pp. 3–18. [Google Scholar]
- 40.Kanemori M, Yanagi H, Yura T. The ATP-dependent HslVU/ClpQY protease participates in turnover of cell division inhibitor SulA in Escherichia coli. J Bacteriol. 1999;181:3674–3680. doi: 10.1128/jb.181.12.3674-3680.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Doring V, Mootz HD, Nangle LA, Hendrickson TL, de Crécy-Lagard V, Schimmel P, Marliere P. Enlarging the amino acid set of Escherichia coli by infiltration of the valine coding pathway. Science. 2001;292:501–504. doi: 10.1126/science.1057718. [DOI] [PubMed] [Google Scholar]
- 42.Roy H, Ibba M. RNA-dependent lipid remodeling by bacterial multiple peptide resistance factors. Proc Natl Acad Sci USA. 2008;105:4667–4672. doi: 10.1073/pnas.0800006105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ling J, Yadavalli SS, Ibba M. Phenylalanyl-tRNA synthetase editing defects result in efficient mistranslation of phenylalanine codons as tyrosine. RNA. 2007;13:1881–1886. doi: 10.1261/rna.684107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bouadloun F, Donner D, Kurland CG. Codon-specific missense errors in vivo. EMBO J. 1983;2:1351–1356. doi: 10.1002/j.1460-2075.1983.tb01591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rennell D, Bouvier SE, Hardy LW, Poteete AR. Systematic mutation of bacteriophage T4 lysozyme. J Mol Biol. 1991;222:67–88. doi: 10.1016/0022-2836(91)90738-r. [DOI] [PubMed] [Google Scholar]
- 46.Lovmar M, Ehrenberg M. Rate, accuracy and cost of ribosomes in bacterial cells. Biochimie. 2006;88:951–961. doi: 10.1016/j.biochi.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 47.Lue SW, Kelley SO. An aminoacyl-tRNA synthetase with a defunct editing site. Biochemistry. 2005;44:3010–3016. doi: 10.1021/bi047901v. [DOI] [PubMed] [Google Scholar]
- 48.Ruan B, et al. Cysteinyl-tRNACys formation in Methanocaldococcus jannaschii: The mechanism is still unknown. J Bacteriol. 2004;186:8–14. doi: 10.1128/JB.186.1.8-14.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salazar JC, et al. Coevolution of an aminoacyl-tRNA synthetase with its tRNA substrates. Proc Natl Acad Sci USA. 2003;100:13863–13868. doi: 10.1073/pnas.1936123100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sabina J, et al. Interfering with different steps of protein synthesis explored by transcriptional profiling of Escherichia coli K-12. J Bacteriol. 2003;185:6158–6170. doi: 10.1128/JB.185.20.6158-6170.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.