Abstract
Group A streptococci (Streptococcus pyogenes or GAS) freshly isolated from individuals with streptococcal sore throat or invasive (“flesh-eating”) infection often grow as mucoid colonies on primary culture but lose this colony appearance after laboratory passage. The mucoid phenotype is due to abundant production of the hyaluronic acid capsular polysaccharide, a key virulence determinant associated with severe GAS infections. These observations suggest that signal(s) from the human host trigger increased production of capsule and perhaps other virulence factors during infection. Here we show that subinhibitory concentrations of the human antimicrobial cathelicidin peptide LL-37 stimulate expression of the GAS capsule synthesis operon (hasABC). Up-regulation is mediated by the CsrRS 2-component regulatory system: it requires a functional CsrS sensor protein and can be antagonized by increased extracellular Mg2+, the other identified environmental signal for CsrS. Up-regulation was also evident for other CsrRS-regulated virulence genes, including the IL-8 protease PrtS/ScpC and the integrin-like/IgG protease Mac/IdeS, findings that suggest a coordinated GAS virulence response elicited by this antimicrobial immune effector peptide. LL-37 signaling through CsrRS led to a marked increase in GAS resistance to opsonophagocytic killing by human leukocytes, an in vitro measure of enhanced GAS virulence, consistent with increased expression of the antiphagocytic capsular polysaccharide and Mac/IdeS. We propose that the human cathelicidin LL-37 has the paradoxical effect of stimulating CsrRS-regulated virulence gene expression, thereby enhancing GAS pathogenicity during infection. The ability of GAS to sense and respond to LL-37 may explain, at least in part, the unique susceptibility of the human species to streptococcal infection.
Keywords: CovRS, CsrRS, LL-37, Streptococcus pyogenes, 2-component system
Many species of microorganisms colonize the skin or mucosal surfaces of human beings with no apparent ill effects on the host. However, certain of these colonizers also have the capacity to produce local inflammation and injury or to breach the mucosal barrier and produce locally invasive infection or systemic disease. Group A Streptococcus (Streptococcus pyogenes or GAS) presents a striking example of such dualism: it colonizes the oropharynx of 15–20% of healthy children (1, 2); colonization is often entirely asymptomatic, but it can be associated with sore throat and fever in the self-limited syndrome of streptococcal pharyngitis; rarely, the organism invades locally to produce serious soft tissue infection or systemically to produce potentially life-threatening bloodstream infection and/or streptococcal toxic shock (3, 4). Clues that dynamic regulation of bacterial invasiveness occurs during infection come from observations that GAS freshly isolated from the blood or deep tissues often express large amounts of surface M protein and the hyaluronic acid (HA) capsular polysaccharide, 2 major virulence determinants, but that expression of these factors declines after culture of the organism in vitro (5, 6). Such observations suggest that specific stimuli in the host signal GAS to increase expression of factors that contribute to the development of symptomatic infection.
Bacterial 2-component systems (TCS) constitute an important regulatory mechanism through which GAS and other bacteria perceive and respond to environmental stimuli, including those encountered in the infected host (7). Among the several TCS described in GAS, CsrRS (also known as CovRS) has been most extensively characterized and most clearly shown to control expression of key virulence determinants including the HA capsule, streptokinase, streptolysin S, the antiphagocytic protein/IgG protease Mac/IdeS, and the IL-8 protease PrtS/ScpC, among others (8–12). The basic structure of CsrRS resembles that of the EnvZ/OmpR family of TCS: the sensor component CsrS is a histidine kinase predicted to be associated with the bacterial cell membrane. Interaction of the CsrS extracellular domain with environmental stimuli is thought to change the conformation of the cytoplasmic domain, thereby altering its autokinase activity and/or its activity as a phosphatase for the cognate transcriptional regulator CsrR. High concentrations of environmental Mg2+ signal through CsrS to repress expression of CsrR-regulated virulence genes (13, 14). By contrast, the low concentration of Mg2+ (with respect to CsrRS signaling) in human extracellular fluids is expected to result in relatively derepressed expression of these virulence factors. Until now, Mg2+ has been the only identified signal for the CsrRS system. However, it seemed likely that additional environmental cues encountered by GAS within the human host might also interact with CsrS to regulate virulence gene expression during human infection.
Among host molecules that might act as potential signals, antimicrobial peptides (AMPs) are a particularly attractive group. AMPs are 2- to 5-kDa peptides of animal or plant origin that have antimicrobial activity against viruses, bacteria, and/or fungi (15). Several human AMPs are produced, either constitutively or upon induction by microbial products or cell injury, by epithelial cells and neutrophils. They are relatively small, usually cationic molecules that exert their antimicrobial effect by damaging microbial cell membranes. At concentrations below those that mediate bacterial damage, AMPs might serve a signaling function to GAS on the skin or pharyngeal mucosa by binding to and signaling through CsrS or the sensor module of another TCS.
In the present investigation, we tested a large panel of AMPs for their ability to regulate virulence gene expression in GAS. We found that the human cathelicidin peptide LL-37, but not other AMPs, acts as a potent stimulus to up-regulate expression of multiple virulence genes by signaling through the CsrRS TCS.
Results
Subinhibitory Concentrations of LL-37 Stimulate GAS Capsule Gene Expression.
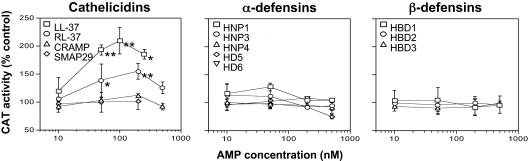
We screened subinhibitory concentrations of a variety of human AMPs for their ability to stimulate transcription of the genes required for HA capsule biosynthesis using a GAS reporter strain, 003CAT, in which cat encoding chloramphenicol acetyltransferase is expressed under the control of the hasABC (hyaluronic acid synthesis) operon promoter (16). The human cathelicidin LL-37 (17) stimulated a dose-dependent increase in CAT activity in 003CAT that was maximal at ≈100 nM (Fig. 1), a concentration >200 times lower than the minimum concentration required to inhibit GAS growth under similar conditions. By contrast, 8 human α- and β-defensins [supporting information (SI) Table S1] were inactive at concentrations up to 500 nM (Fig. 1). RC-101, an analog of primate θ-defensins, and the porcine θ-defensin-like protegrin PG-1 were also inactive (data not shown).
Fig. 1.
Hyaluronic acid capsule gene expression of GAS in the presence of subinhibitory concentrations of antimicrobial peptides (AMPs). The has operon reporter strain 003CAT was grown to midexponential phase in the presence of human (LL-37), rhesus macaque (RL-37), mouse (CRAMP), or sheep (SMAP-29) cathelicidin peptides (Left) or in the presence of human α- or β-defensins (Center and Right, respectively). CAT activity, reflecting capsule gene expression, was quantified in bacterial lysates and is expressed as a percentage of CAT activity in bacteria grown in unsupplemented medium. *, P < 0.03; **, P < 0.009 for comparison of CAT activity at 50, 100, and 250 nM versus 10 nM LL-37 or for comparison of activity at 50 and 200 nM versus 10 nM RL-37. Results shown are means ± SD from at least 3 independent experiments.
The results above indicated that the cathelicidin LL-37, but not other classes of human or nonhuman AMPs, stimulated GAS capsule gene expression. To test whether cathelicidins from other species might have similar activity, we measured CAT activity in strain 003CAT after exposure to nonhuman cathelicidins (Table S1). Among these, RL-37 from rhesus macaque (18) that shares 68% amino acid identity with LL-37 was the most active in stimulating has operon expression, but its maximal effect was lower than that of LL-37 (Fig. 1). The mouse cathelicidin CRAMP (19) (46% identity with LL-37) and the sheep cathelicidin SMAP-29 (20) (16% identity with LL-37) were inactive (Fig. 1). Thus, the activity of nonhuman cathelicidins paralleled their degree of similarity to LL-37. There was no correlation between signaling activities of the AMPs tested and their antimicrobial activity or net charge. Rather, the capacity to stimulate has capsule gene expression appeared to be specific to LL-37 and closely related cathelicidin peptides.
Mg2+ Antagonizes the LL-37 Stimulatory Effect on has Operon Expression.
Because expression of the has operon is controlled by the CsrRS TCS, the 003CAT reporter system is sensitive to CsrRS-mediated gene regulation (14). If LL-37 stimulates capsule gene expression by signaling through CsrRS, we reasoned that its effect might be antagonized by Mg2+, because high concentrations of extracellular Mg2+ have been shown to down-regulate has operon expression in a CsrRS-dependent fashion (13, 14). To test this idea, we measured CAT activity in lysates of 003CAT grown in the presence of 100 nM LL-37 and various concentrations of MgCl2. We found that stimulation of has operon expression by LL-37 could be inhibited by Mg2+ in a dose-dependent manner (Fig. S1). A concentration of 15 mM Mg2+ was required to block the stimulatory effect of 100 nM LL-37, a result that implies that LL-37 is a particularly potent signaling molecule compared with Mg2+. These results are consistent with a model in which LL-37 and Mg2+ function as mutually antagonistic ligands for CsrS, the membrane-bound sensor component of the CsrRS system, with opposite effects on CsrRS-mediated gene regulation.
LL-37 Stimulation of Multiple CsrRS-Regulated Genes Requires a Functional CsrS Protein.
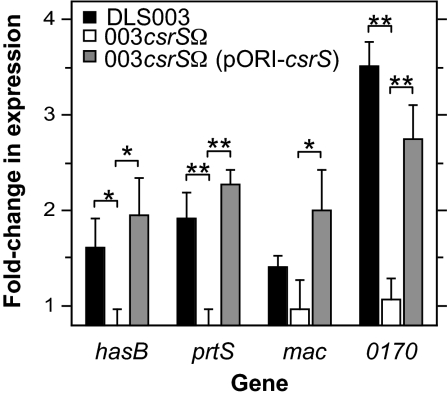
In addition to using the reporter strain 003CAT to demonstrate LL-37-stimulated up-regulation of the has operon, we used quantitative RT-PCR (qRT-PCR) to confirm LL-37 regulation of hasB, the second gene in the hasABC operon, and also investigated regulation of 3 additional genes shown previously to be strongly regulated by CsrRS: prtS encoding a putative IL-8 protease, mac encoding the antiphagocytic factor/IgG protease Mac/IdeS, and SPy0170 (spyM3_0132) encoding a hypothetical protein (13). For these experiments, wild-type strain DLS003 (from which reporter strain 003CAT was derived), its isogenic csrS mutant strain 003csrSΩ, and strain 003csrSΩ(pORI-csrS), in which wild-type csrS is expressed in trans from plasmid pORI23 under the control of the lactococcal P23 promoter (13), were grown to midexponential phase with or without 100 nM LL-37, and expression of hasB, prtS, mac, and SPy0170 was quantified by qRT-PCR. All 4 genes showed a similar up-regulation in wild-type strain DLS003, whereas no significant change in expression was observed in response to LL-37 in the CsrS mutant strain 003csrSΩ (Fig. 2). Notably, expression of CsrS in trans from pORI-csrS in the csrS-complemented strain 003csrSΩ(pORI-csrS) restored LL-37 stimulation of the 4 CsrRS-regulated genes to wild-type levels (Fig. 2). These data support the requirement of CsrS for LL-37 signaling and suggest that LL-37 interacts with CsrS to up-regulate CsrRS-dependent gene expression in GAS.
Fig. 2.
LL-37 stimulation of virulence gene expression requires a functional CsrS protein. Regulation of gene expression by LL-37 was assessed by qRT-PCR in wild-type GAS strain DLS003, the isogenic CsrS mutant 003csrSΩ, or the CsrS mutant complemented strain 003csrSΩ(pORI-csrS) expressing native CsrS from plasmid pORI-csrS. Data represent mean fold change in expression of 4 CsrRS-regulated genes (hasB, prtS, mac, and SPy0170) during growth in the presence of 100 nM LL-37 compared with that in control cultures of the same strain in unsupplemented medium. *, P < 0.02; **, P < 0.005. Results shown are means ± SD from at least 3 independent experiments.
LL-37 Stimulates CsrRS-Dependent Virulence Gene Expression in 4 Independent GAS Isolates.
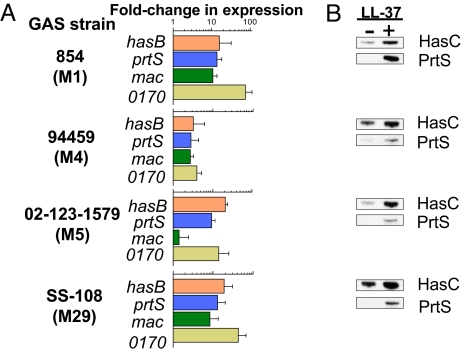
The CsrRS system is expressed in nearly all clinical isolates of GAS; spontaneous inactivating mutations have been described but are uncommon (21, 22). To test whether LL-37 acts as a CsrRS signal in GAS strains other than DLS003, we tested the effect of LL-37 on CsrRS-regulated gene expression by qRT-PCR in 4 additional GAS strains: strain 854, 94459, 02-123-1579, and SS-108. These strains represented M-types 1, 4, 5, and 29, respectively, and 3 of them were isolated from patients with invasive infections such as bacteremia or necrotizing fasciitis (Table S2). The regulation pattern of hasB, prtS, mac, and SPy0170 observed in these 4 strains in response to LL-37 was consistent with that observed in strain DLS003. Moreover, the magnitude of up-regulation in response to LL-37 was greater in these strains with levels of induction ranging from 2.8- to 73.9-fold (Fig. 3A). The only exception was mac in strain 02-123-1579, which, unlike the other 3 target genes, was not up-regulated in response to LL-37. It should be noted, however, that the baseline mac transcript level in this strain was very low, so it is unclear whether mac is actually expressed in strain 02-123-1579.
Fig. 3.
LL-37 stimulates CsrRS-regulated gene expression in 4 independent GAS isolates. (A) Up-regulation of gene expression by LL-37 as measured by qRT-PCR in wild-type GAS strains 854, 94459, 02-123-1579, and SS-108. Data represent fold change in expression of hasB, prtS, mac, and SPy0170 during growth in the presence of 100 nM LL-37 compared with that in control cultures of the same strain in unsupplemented medium. Results shown are means ± SD from at least 3 independent experiments. LL-37-induced increases in gene expression were statistically significant (P < 0.05) for all 4 genes in all 4 strains except for mac in strain 02-123-1579, in which baseline expression was extremely low. (B) Validation of qRT-PCR data by assessment of protein expression. GAS protein preparations were obtained from bacteria grown to midexponential phase in the presence or absence of LL-37 (100 nM), fractionated by SDS/PAGE, and then immunoblotted with specific antisera to HasC or PrtS. Note the increase in expression of both HasC and PrtS in the presence of LL-37 consistent with increased mRNA levels shown by qRT-PCR. Results shown are from a representative experiment.
The strong up-regulation of CsrRS-regulated gene expression detected by qRT-PCR was verified by immunoblotting for 2 CsrRS-regulated gene products: HasC, representing the product of the third gene of the has operon, and PrtS. Specific antisera were used to detect these proteins in lysates of GAS grown in the presence or absence of 100 nM LL-37. As predicted by qRT-PCR data, expression of both protein products increased in response to LL-37 in strains 854, 94459, 02-123-1579, and SS-108 (Fig. 3B).
To verify that the marked up-regulation of LL-37-induced gene expression observed in these additional GAS strains was mediated through CsrRS, we constructed a csrS mutant in the background of the M-type 1 strain 854, using the mutagenesis plasmid and methods described previously (14). As expected, mutant strain 854csrSΩ showed no change in expression of hasB, prtS, mac, or SPy0170 in response to LL-37 (Fig. S2), results that further support an absolute requirement for CsrS in LL-37-mediated gene regulation. Taken together, these findings show that LL-37 signals through CsrRS to up-regulate virulence gene expression in several independent GAS isolates. Thus, it is likely that such a signaling mechanism is widespread among GAS strains.
LL-37 Up-Regulates Capsular Polysaccharide Production in Multiple GAS Strains.
Among the multiple virulence determinants regulated by CsrRS, the HA capsule may be the most critical for pathogenesis (23, 24). To investigate further the generality of LL-37 signaling among GAS strains and also the effect of has operon up-regulation on the amount of cell-associated HA capsule produced by GAS, we measured HA capsular polysaccharide levels in response to LL-37 in 10 GAS isolates. The strains varied widely in their degree of encapsulation under standard growth conditions and included the 5 GAS strains studied above. Nine of 10 strains showed a 1.5- to 76-fold increase in capsular polysaccharide production in response to LL-37 (Fig. S3). Four strains were poorly encapsulated under standard growth conditions (0.1–1 fg/cfu) but produced >10 fg/cfu HA when grown in the presence of LL-37, HA levels that are comparable to that produced by mucoid (i.e., highly encapsulated) strains. In general, a larger fold increase in capsule production was observed for strains that were poorly encapsulated under standard growth conditions. Mucoid strain 950771 was the only isolate that did not produce increased capsule in response to LL-37. This observation prompted us to investigate the possibility of a defect in CsrRS signaling in 950771 because mutations in CsrR or CsrS have been reported previously in highly encapsulated isolates from invasive GAS infections (21, 22, 25, 26). Sequencing of the 950771 csrRS locus revealed a point mutation (K102R) in a highly conserved consensus motif of the CsrR receiver domain (11). The lysine residue of the receiver motif is absolutely conserved in the regulator proteins of TCSs from diverse bacterial species (27). Mutation of this conserved residue in strain 950771 is likely to account for disrupted CsrRS signaling because the same arginine substitution at the corresponding site in the CheY response regulator abolished regulation through the CheA/CheY TCS in Salmonella typhimurium (28). The relatively modest up-regulation of capsule expression observed in 1 or more of the other mucoid GAS strains studied might also be due to csrRS mutation(s). Alternatively, it is possible that in mucoid strains an increase in capsule beyond the high level produced under standard growth conditions is constrained by substrate availability.
LL-37 Enhances GAS Resistance to Phagocytic Killing.
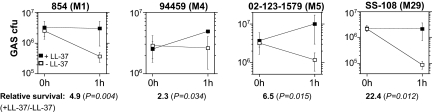
The striking increase in HA capsule production observed in several GAS strains in response to LL-37 could, by itself, result in increased resistance to phagocytosis, enhanced invasion across host epithelial barriers, and increased overall virulence (29, 30). Similar changes in expression of other CsrRS-regulated genes, such as the antiphagocytic Mac/IdeS and the IL-8 protease PrtS, are likely to further enhance pathogenicity (31–33). To test this prediction, we assessed the effect of LL-37 on GAS resistance to complement-mediated opsonophagocytic killing using 4 GAS isolates that exhibited moderate to strong up-regulation of capsule and other CsrRS-regulated genes in response to LL-37: strains 854, 94459, 02-123-1579, and SS-108. Each strain was grown to early exponential phase with or without 100 nM LL-37 and then incubated with freshly isolated human peripheral blood leukocytes for 1 h in the presence of 10% human serum as a complement source. As shown in Fig. 4, bacteria grown in the absence of LL-37 showed moderate to high susceptibility to phagocytic killing with survival rates of the inoculum as low as 4% at 1 h. In contrast, prior growth in the presence of LL-37 resulted in increased resistance to phagocytic killing, with survival increases ranging from 2.3- to 22.4-fold as compared with survival of untreated bacteria. It seems unlikely that increased phagocytic resistance was attributable to a change in expression of M protein, because LL-37 treatment had no significant effect on emm expression (as assessed by qRT-PCR) in strains 94459 and SS-108. LL-37 treatment was associated with a 1.5- to 2-fold increase in emm expression in strains 854 and 02-123-1579, so it is possible that increased M protein expression makes some contribution to LL-37-induced resistance to phagocytosis in the latter strains. In control assays performed in the absence of serum or leukocytes, no killing was observed for either LL-37-treated or untreated GAS (data not shown), a result that is consistent with the requirement of complement for GAS opsonization and phagocytosis by human leukocytes. Thus, LL-37-induced up-regulation of virulence gene expression was associated with a marked increase in GAS resistance to phagocytic killing, an indicator of enhanced bacterial pathogenicity in the infected host.
Fig. 4.
LL-37 signaling enhances GAS resistance to phagocytic killing. The 4 indicated GAS strains were grown to early exponential phase in the presence (filled squares) or absence (open squares) of 100 nM LL-37 and then mixed with human peripheral blood leukocytes for 1 h in the presence of 10% human serum as a source of complement. Exposure to LL-37 was associated with increased GAS survival (i.e., resistance to phagocytic killing) for all 4 strains compared with bacteria grown in the absence of the peptide. Results shown are means ± SD from at least 3 independent experiments.
Discussion
These studies provide evidence that the human AMP LL-37 triggers a coordinated transcriptional response through the GAS CsrRS TCS that results in increased expression of CsrRS-regulated gene products including the HA capsule biosynthetic enzymes, the IL-8 protease Prts/ScpC, and the antiphagocytic protein/IgG protease Mac/IdeS, all of which contribute to GAS virulence (10, 30, 31, 34, 35). Up-regulation of virulence gene expression was associated with a striking increase in resistance to complement-mediated opsonophagocytic killing by human blood leukocytes, an in vitro correlate of GAS pathogenicity. Signaling by LL-37 appears to be remarkably specific; we observed little or no activity in response to other human AMPs, and even the closely related rhesus macaque cathelicidin RL-37 was less active than human LL-37 although the bactericidal activities of the nonhuman cathelicidins were comparable to their human counterpart. LL-37 stimulated increased expression of CsrRS-regulated virulence factors in multiple GAS isolates, a finding that suggests that the CsrRS-mediated response to LL-37 is widespread, if not universal, among GAS strains. The apparently unique signaling capacity of LL-37 for this key virulence control system may contribute to the narrow host range of GAS for human beings. The mouse cathelicidin CRAMP has been shown to confer relative resistance to cutaneous GAS infection in mice (36). Such a result is consistent with the known antibacterial activity of CRAMP and with our finding that CRAMP is inactive in CsrRS signaling. By contrast, in human beings, the protective effect attributable to the antimicrobial effect of LL-37 may be outweighed by the virulence-enhancing activity of LL-37 during GAS infection.
Other bacterial species have evolved systems for detecting and responding to AMPs. In Salmonella enterica serovar Typhimurium, exposure to subinhibitory concentrations of any of several cationic AMPs results in gene expression changes involving regulons controlled by the PhoPQ TCS and by the alternative σ factor RpoS (37, 38). A consequence of altered gene expression is modification of outer membrane lipopolysaccharide, rendering the organisms more resistant to killing by AMPs (39). Exposure to the cationic peptide C18G has been shown to induce PhoQ-mediated phosphorylation of PhoP in an in vitro system using purified components, evidence that AMP signaling in S. typhimurium reflects direct interaction of AMPs with PhoQ (38). Among Gram-positive bacteria, staphylococci have been shown to respond to the anionic AMP dermcidin with changes in expression of the agr, sarA, and saeRS and increased proteolytic activity against dermcidin (40). The system that senses dermcidin has not been defined. Staphylococcus epidermidis and Staphylococcus aureus also sense and respond to multiple cationic peptides through the Aps 3-component system, up-regulating expression of proteins involved in cell surface charge and thereby conferring enhanced resistance to the antimicrobial action of cationic AMPs (41, 42).
The GAS CsrRS system shares certain structural features with PhoPQ of S. typhimurium, and there are some similarities in AMP signaling between the 2 systems. Like PhoPQ, CsrRS senses environmental Mg2+ to regulate virulence gene expression (13, 14). The predicted extracellular domain of CsrS is similar in size to the periplasmic domain of PhoQ and shares with PhoQ 10 of 20 negatively charged amino acids, some of which have been implicated in cation binding to PhoQ (11, 38, 43). We attempted to evaluate the role of acidic amino acids in binding to LL-37 by substituting a cluster of 3 negatively charged amino acids in the predicted extracellular domain of CsrS with 3 uncharged residues (D148N, E151Q, and D152N). Whereas expression of the native CsrS in the csrS mutant complemented LL-37 signaling (Fig. 2), expression of the 3-aa mutant CsrS did not (data not shown). However, Western blots of the 2 complemented strains showed a higher level of CsrS expression for the mutant construct compared with that expressed by the wild type. Therefore, before reaching a definitive conclusion about the role of these acidic residues in LL-37 sensing, further studies will be required to assure that a negative result with the mutant construct is not simply an artifact of the CsrS expression level. As in the case of PhoPQ, we found that Mg2+ could block the stimulatory effect of LL-37 on CsrRS-regulated gene expression in a dose-dependent manner (38).
There are also important differences between AMP signaling through CsrRS in GAS and PhoPQ in S. typhimurium or ApsXRS in staphylococci. First, whereas the PhoPQ and Aps systems respond to multiple cationic peptides, CsrRS appears to have exquisite specificity for the human cathelicidin LL-37. Second, maximum signaling effects of LL-37 for CsrRS were observed at a concentration of 100 nM, 10-fold lower than the maximum stimulatory concentration of cationic AMPs for PhoPQ or ApsXRS. The fact that CsrRS senses and responds to concentrations of LL-37 that are >200 times lower than those required for antimicrobial activity implies that the system could trigger changes in virulence factor expression sufficiently early in infection to counteract effective bacterial killing by host defense mechanisms. Third, whereas the Aps system in staphylococci appears primarily to regulate loci involved in cationic peptide resistance, LL-37 signaling through CsrRS up-regulates expression of multiple factors that mediate resistance to clearance by the host. This pattern of response suggests a sentinel function for CsrRS sensing of LL-37 as an indicator of activation of host innate immunity. Up-regulation of virulence determinants as a consequence of CsrRS signaling serves to enhance GAS resistance to several host defenses including deployment of chemotactic cytokines and opsonophagocytic killing by leukocytes.
The precise mechanism by which LL-37 stimulates expression of CsrRS-regulated virulence genes remains undefined. Because expression of the regulated genes is repressed by high concentrations of Mg2+, it has been suggested that Mg2+ binding to CsrS stimulates CsrS autokinase activity and downstream phosphorylation of CsrR to repress target gene expression (14). In this model, LL-37 could compete for Mg2+ binding to CsrS, further reducing CsrS phosphorylation at the relatively low extracellular Mg2+ concentration (≈1 mM) found in human extracellular fluids. It is also possible that LL-37 does not compete for Mg2+ binding, but inhibits CsrS autokinase activity through an independent mechanism. It has also been proposed that CsrS functions as a phosphatase, catalyzing the dephosphorylation of phospho-CsrR (44). In the latter model, LL-37 could function as an activator of the phosphatase activity of CsrS for phospho-CsrR.
The concentration of LL-37 that induces maximal CsrRS signaling is well within the range that GAS is likely to encounter on the pharyngeal mucosa as LL-37 has been detected in human saliva at a median concentration of 660 nM (45). LL-37 production is undetectable in resting skin keratinocytes, but expression is rapidly up-regulated in response to injury, inflammation, or microbial products, and LL-37 has been found in human sweat at ≈100 nM (46, 47). We propose that LL-37 signaling through CsrRS is a critical element in the dynamics of GAS colonization and symptomatic infection: the transition from asymptomatic colonization of the pharynx or a superficial skin site to symptomatic infection may be initiated by epithelial cell injury from GAS products. Host cell injury induces release of LL-37, which, in turn, is sensed by the CsrRS system, triggering up-regulation of GAS virulence factors that heighten bacterial resistance to host clearance mechanisms and contribute to tissue invasion. Thus, the production of LL-37 as an innate defense mechanism in response to GAS infection appears, paradoxically, to increase GAS pathogenicity during human infection. Tissue samples from patients with invasive GAS disease showed colocalization of LL-37 with GAS in soft tissues, an observation consistent with local stimulation of LL-37 production (48). The capacity of GAS to sense and respond to subinhibitory concentrations of a human innate immune effector represents a remarkable host-specific adaptation that enhances the virulence of a uniquely human pathogen. This insight into GAS pathogenesis may be exploited through the development of specific antagonists of LL-37 signaling to prevent or treat streptococcal infection.
Materials and Methods
Bacterial Strains and Growth Conditions.
GAS strains used in this study (Table S2) were grown at 37°C in Todd–Hewitt broth (Difco) supplemented with 0.5% yeast extract (THY) or on THY agar or trypticase-soy agar, both supplemented with 5% defibrinated sheep blood. When appropriate, antibiotics were added at the following concentrations: 300 μg/mL kanamycin and 0.5 μg/mL erythromycin.
GAS RNA Isolation and qRT-PCR.
Total GAS RNA was isolated by using the RNeasy mini kit (Qiagen). qRT-PCR was performed on an ABI PRISM 7000 Sequence Detection System (Applied Biosystems) using the QuantiTect SYBR green RT-PCR kit (Qiagen) as described (13). Primers are described in Table S3.
Chloramphenicol Acetyltransferase (CAT) Assays.
Cytoplasmic extracts for CAT assays were prepared from GAS grown to midexponential phase (A600 = 0.3) in unsupplemented THY broth or broth supplemented with MgCl2, LL-37, or both, as described (13, 49).
AMPs.
Mouse cathelicidin CRAMP was purchased from Anaspec. Other AMPs were synthesized as described previously, and their purity was confirmed by high-performance liquid chromatography and mass spectrometry (50–53).
Measurement of GAS Cell-Associated Capsular Polysaccharide.
The amount of cell-associated capsular polysaccharide isolated from GAS grown to midexponential phase (A600 = 0.4) was measured as described (13).
GAS Protein Preparation and Immunoblotting.
GAS protein preparations were obtained from cultures grown to midexponential phase (A600 = 0.4). SDS/PAGE analysis and immunoblotting were performed by using HasC- or PrtS-specific mouse serum (gift of Giuliano Bensi, Novartis Vaccines) (13).
Phagocytosis Assays.
GAS sensitivity to opsonophagocytic killing was assessed as described (30) by using a protocol approved by the Children's Hospital Boston institutional review board. Approximately 2–3 × 106 GAS cfu were mixed with an equivalent number of human peripheral blood leukocytes in the presence of 10% human serum as a source of complement and were rotated end-over-end at 37°C for 1 h. Aliquots were withdrawn for quantitative culture at time 0 and 1 h.
emm Genotyping.
Strains 854 and 02-123-1579 were emm-typed as described by the Centers for Disease Control and Prevention (www.cdc.gov/ncidod/biotech/strep/M-ProteinGene_typing.htm) except that GAS chromosomal DNA template was prepared by boiling (54).
Statistical Calculations.
Statistical significance of differences between groups was evaluated by using Student's t test.
Supplementary Material
Acknowledgments.
This work was supported in part by National Institutes of Health Grants AI43934 and AI37945 (to R.I.L.), AI61482 (to W.L.), and AI29952 (to M.R.W.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. FJ358497).
This article contains supporting information online at www.pnas.org/cgi/content/full/0803815105/DCSupplemental.
References
- 1.Danchin MH, et al. Burden of acute sore throat and group A streptococcal pharyngitis in school-aged children and their families in Australia. Pediatrics. 2007;120:950–957. doi: 10.1542/peds.2006-3368. [DOI] [PubMed] [Google Scholar]
- 2.Wannamaker LW. Perplexity and precision in the diagnosis of streptococcal pharyngitis. Am J Dis Child. 1972;124:352–358. doi: 10.1001/archpedi.1972.02110150050009. [DOI] [PubMed] [Google Scholar]
- 3.Bisno AL, Stevens DL. Streptococcal infections of skin and soft tissues. N Engl J Med. 1996;334:240–245. doi: 10.1056/NEJM199601253340407. [DOI] [PubMed] [Google Scholar]
- 4.Stevens DL. Invasive group A streptococcus infections. Clin Infect Dis. 1992;14:2–11. doi: 10.1093/clinids/14.1.2. [DOI] [PubMed] [Google Scholar]
- 5.Ji Y, McLandsborough L, Kondagunta A, Cleary PP. C5a peptidase alters clearance and trafficking of group A streptococci by infected mice. Infect Immun. 1996;64:503–510. doi: 10.1128/iai.64.2.503-510.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raeder R, Harokopakis E, Hollingshead S, Boyle MD. Absence of SpeB production in virulent large capsular forms of group A streptococcal strain 64. Infect Immun. 2000;68:744–751. doi: 10.1128/iai.68.2.744-751.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beier D, Gross R. Regulation of bacterial virulence by two-component systems. Curr Opin Microbiol. 2006;9:143–152. doi: 10.1016/j.mib.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 8.Dalton TL, Collins JT, Barnett TC, Scott JR. RscA, a member of the MDR1 family of transporters, is repressed by CovR and required for growth of Streptococcus pyogenes under heat stress. J Bacteriol. 2006;188:77–85. doi: 10.1128/JB.188.1.77-85.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graham MR, et al. Virulence control in group A Streptococcus by a two-component gene regulatory system: Global expression profiling and in vivo infection modeling. Proc Natl Acad Sci USA. 2002;99:13855–13860. doi: 10.1073/pnas.202353699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lei B, et al. Evasion of human innate and acquired immunity by a bacterial homolog of CD11b that inhibits opsonophagocytosis. Nat Med. 2001;7:1298–1305. doi: 10.1038/nm1201-1298. [DOI] [PubMed] [Google Scholar]
- 11.Levin JC, Wessels MR. Identification of csrR/csrS, a genetic locus that regulates hyaluronic acid capsule synthesis in group A Streptococcus. Mol Microbiol. 1998;30:209–219. doi: 10.1046/j.1365-2958.1998.01057.x. [DOI] [PubMed] [Google Scholar]
- 12.von Pawel-Rammingen U, Johansson BP, Bjorck L. IdeS, a novel streptococcal cysteine proteinase with unique specificity for immunoglobulin G. EMBO J. 2002;21:1607–1615. doi: 10.1093/emboj/21.7.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gryllos I, et al. Mg2+ signalling defines the group A streptococcal CsrRS (CovRS) regulon. Mol Microbiol. 2007;65:671–683. doi: 10.1111/j.1365-2958.2007.05818.x. [DOI] [PubMed] [Google Scholar]
- 14.Gryllos I, Levin JC, Wessels MR. The CsrR/CsrS two-component system of group A Streptococcus responds to environmental Mg2+ Proc Natl Acad Sci USA. 2003;100:4227–4232. doi: 10.1073/pnas.0636231100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jenssen H, Hamill P, Hancock RE. Peptide antimicrobial agents. Clin Microbiol Rev. 2006;19:491–511. doi: 10.1128/CMR.00056-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Albertí S, Ashbaugh CD, Wessels MR. Structure of the has operon promoter and regulation of hyaluronic acid capsule expression in group A Streptococcus. Mol Microbiol. 1998;28:343–353. doi: 10.1046/j.1365-2958.1998.00800.x. [DOI] [PubMed] [Google Scholar]
- 17.Lehrer RI, Ganz T. Cathelicidins: A family of endogenous antimicrobial peptides. Curr Opin Hematol. 2002;9:18–22. doi: 10.1097/00062752-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Zhao C, et al. RL-37, an alpha-helical antimicrobial peptide of the rhesus monkey. Antimicrob Agents Chemother. 2001;45:2695–2702. doi: 10.1128/AAC.45.10.2695-2702.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gallo RL, et al. Identification of CRAMP, a cathelin-related antimicrobial peptide expressed in the embryonic and adult mouse. J Biol Chem. 1997;272:13088–13093. doi: 10.1074/jbc.272.20.13088. [DOI] [PubMed] [Google Scholar]
- 20.Skerlavaj B, Benincasa M, Risso A, Zanetti M, Gennaro R. SMAP-29: A potent antibacterial and antifungal peptide from sheep leukocytes. FEBS Lett. 1999;463:58–62. doi: 10.1016/s0014-5793(99)01600-2. [DOI] [PubMed] [Google Scholar]
- 21.Engleberg NC, Heath A, Miller A, Rivera C, DiRita VJ. Spontaneous mutations in the CsrRS two-component regulatory system of Streptococcus pyogenes result in enhanced virulence in a murine model of skin and soft tissue infection. J Infect Dis. 2001;183:1043–1054. doi: 10.1086/319291. [DOI] [PubMed] [Google Scholar]
- 22.Sumby P, Whitney AR, Graviss EA, DeLeo FR, Musser JM. Genome-wide analysis of group a streptococci reveals a mutation that modulates global phenotype and disease specificity. PLoS Pathog. 2006;2:e5. doi: 10.1371/journal.ppat.0020005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Engleberg NC, Heath A, Vardaman K, DiRita VJ. Contribution of CsrR-regulated virulence factors to the progress and outcome of murine skin infections by Streptococcus pyogenes. Infect Immun. 2004;72:623–628. doi: 10.1128/IAI.72.2.623-628.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wessels MR, Bronze MS. Critical role of the group A streptococcal capsule in pharyngeal colonization and infection in mice. Proc Natl Acad Sci USA. 1994;91:12238–12242. doi: 10.1073/pnas.91.25.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ravins M, et al. Characterization of a mouse-passaged, highly encapsulated variant of group A streptococcus in in vitro and in vivo studies. J Infect Dis. 2000;182:1702–1711. doi: 10.1086/317635. [DOI] [PubMed] [Google Scholar]
- 26.Miyoshi-Akiyama T, et al. Use of DNA arrays to identify a mutation in the negative regulator, csrR, responsible for the high virulence of a naturally occurring type M3 group A streptococcus clinical isolate. J Infect Dis. 2006;193:1677–1684. doi: 10.1086/504263. [DOI] [PubMed] [Google Scholar]
- 27.Varughese KI. Molecular recognition of bacterial phosphorelay proteins. Curr Opin Microbiol. 2002;5:142–148. doi: 10.1016/s1369-5274(02)00305-3. [DOI] [PubMed] [Google Scholar]
- 28.Lukat GS, Lee BH, Mottonen JM, Stock AM, Stock JB. Roles of the highly conserved aspartate and lysine residues in the response regulator of bacterial chemotaxis. J Biol Chem. 1991;266:8348–8354. [PubMed] [Google Scholar]
- 29.Cywes C, Wessels MR. Group A Streptococcus tissue invasion by CD44-mediated cell signalling. Nature. 2001;414:648–652. doi: 10.1038/414648a. [DOI] [PubMed] [Google Scholar]
- 30.Wessels MR, Moses AE, Goldberg JB, DiCesare TJ. Hyaluronic acid capsule is a virulence factor for mucoid group A streptococci. Proc Natl Acad Sci USA. 1991;88:8317–8321. doi: 10.1073/pnas.88.19.8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hidalgo-Grass C, et al. A streptococcal protease that degrades CXC chemokines and impairs bacterial clearance from infected tissues. EMBO J. 2006;25:4628–4637. doi: 10.1038/sj.emboj.7601327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lei B, et al. Opsonophagocytosis-inhibiting mac protein of group A streptococcus: Identification and characteristics of two genetic complexes. Infect Immun. 2002;70:6880–6890. doi: 10.1128/IAI.70.12.6880-6890.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soderberg JJ, Engstrom P, von Pawel-Rammingen U. Intrinsic IgG endopeptidase activity of streptococcal Mac-2 proteins implies a unique role for the enzymatically impaired Mac-2 protein of M28 serotype strains. Infect Immun. 2008;76:2183–2188. doi: 10.1128/IAI.01422-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dale JB, Washburn RG, Marques MB, Wessels MR. Hyaluronate capsule and surface M protein in resistance to opsonization of group A streptococci. Infect Immun. 1996;64:1495–1501. doi: 10.1128/iai.64.5.1495-1501.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Edwards RJ, et al. Specific C-terminal cleavage and inactivation of interleukin-8 by invasive disease isolates of Streptococcus pyogenes. J Infect Dis. 2005;192:783–790. doi: 10.1086/432485. [DOI] [PubMed] [Google Scholar]
- 36.Nizet V, et al. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature. 2001;414:454–457. doi: 10.1038/35106587. [DOI] [PubMed] [Google Scholar]
- 37.Bader MW, et al. Regulation of Salmonella typhimurium virulence gene expression by cationic antimicrobial peptides. Mol Microbiol. 2003;50:219–230. doi: 10.1046/j.1365-2958.2003.03675.x. [DOI] [PubMed] [Google Scholar]
- 38.Bader MW, et al. Recognition of antimicrobial peptides by a bacterial sensor kinase. Cell. 2005;122:461–472. doi: 10.1016/j.cell.2005.05.030. [DOI] [PubMed] [Google Scholar]
- 39.Guo L, et al. Lipid A acylation and bacterial resistance against vertebrate antimicrobial peptides. Cell. 1998;95:189–198. doi: 10.1016/s0092-8674(00)81750-x. [DOI] [PubMed] [Google Scholar]
- 40.Lai Y, et al. The human anionic antimicrobial peptide dermcidin induces proteolytic defence mechanisms in staphylococci. Mol Microbiol. 2007;63:497–506. doi: 10.1111/j.1365-2958.2006.05540.x. [DOI] [PubMed] [Google Scholar]
- 41.Li M, et al. Gram-positive three-component antimicrobial peptide-sensing system. Proc Natl Acad Sci USA. 2007;104:9469–9474. doi: 10.1073/pnas.0702159104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li M, et al. The antimicrobial peptide-sensing system aps of Staphylococcus aureus. Mol Microbiol. 2007;66:1136–1147. doi: 10.1111/j.1365-2958.2007.05986.x. [DOI] [PubMed] [Google Scholar]
- 43.Vescovi EG, Ayala YM, Di Cera E, Groisman EA. Characterization of the bacterial sensor protein PhoQ. Evidence for distinct binding sites for Mg2+ and Ca2+ J Biol Chem. 1997;272:1440–1443. doi: 10.1074/jbc.272.3.1440. [DOI] [PubMed] [Google Scholar]
- 44.Dalton TL, Scott JR. CovS inactivates CovR and is required for growth under conditions of general stress in Streptococcus pyogenes. J Bacteriol. 2004;186:3928–3937. doi: 10.1128/JB.186.12.3928-3937.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tao R, et al. Salivary antimicrobial peptide expression and dental caries experience in children. Antimicrob Agents Chemother. 2005;49:3883–3888. doi: 10.1128/AAC.49.9.3883-3888.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dorschner RA, et al. Cutaneous injury induces the release of cathelicidin anti-microbial peptides active against group A Streptococcus. J Invest Dermatol. 2001;117:91–97. doi: 10.1046/j.1523-1747.2001.01340.x. [DOI] [PubMed] [Google Scholar]
- 47.Murakami M, et al. Cathelicidin anti-microbial peptide expression in sweat, an innate defense system for the skin. J Invest Dermatol. 2002;119:1090–1095. doi: 10.1046/j.1523-1747.2002.19507.x. [DOI] [PubMed] [Google Scholar]
- 48.Johansson L, et al. Cathelicidin LL-37 in severe Streptococcus pyogenes soft tissue infections in humans. Infect Immun. 2008;76:3399–3404. doi: 10.1128/IAI.01392-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shaw WV. Choramphenicol acetyltransferase from choramphenicol-resistant bacteria. Methods Enzymol. 1975;43:737–755. doi: 10.1016/0076-6879(75)43141-x. [DOI] [PubMed] [Google Scholar]
- 50.Sawai MV, et al. Impact of single-residue mutations on the structure and function of ovispirin/novispirin antimicrobial peptides. Protein Eng. 2002;15:225–232. doi: 10.1093/protein/15.3.225. [DOI] [PubMed] [Google Scholar]
- 51.Wu Z, Ericksen B, Tucker K, Lubkowski J, Lu W. Synthesis and characterization of human alpha-defensins 4–6. J Pept Res. 2004;64:118–125. doi: 10.1111/j.1399-3011.2004.00179.x. [DOI] [PubMed] [Google Scholar]
- 52.Wu Z, et al. Engineering disulfide bridges to dissect antimicrobial and chemotactic activities of human beta-defensin 3. Proc Natl Acad Sci USA. 2003;100:8880–8885. doi: 10.1073/pnas.1533186100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu Z, Powell R, Lu W. Productive folding of human neutrophil alpha-defensins in vitro without the pro-peptide. J Am Chem Soc. 2003;125:2402–2403. doi: 10.1021/ja0294257. [DOI] [PubMed] [Google Scholar]
- 54.Tewodros W, Kronvall G. M protein gene (emm type) analysis of group A beta-hemolytic streptococci from Ethiopia reveals unique patterns. J Clin Microbiol. 2005;43:4369–4376. doi: 10.1128/JCM.43.9.4369-4376.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.