Abstract
We investigated the role of the cdk inhibitor protein p21Cip-1/WAF1/MDA6 (p21) in the ability of MAPK pathway inhibition to enhance radiation-induced apoptosis in A431 squamous carcinoma cells. In carcinoma cells, ionizing radiation (2 Gy) caused both primary (0–10 min) and secondary (90–240 min) activations of the MAPK pathway. Radiation induced p21 protein expression in A431 cells within 6 h via secondary activation of the MAPK pathway. Within 6 h, radiation weakly enhanced the proportion of cells in G1 that were p21 and MAPK dependent, whereas the elevation of cells present in G2/M at this time was independent of either p21 expression or MAPK inhibition. Inhibition of the MAPK pathway increased the proportion of irradiated cells in G2/M phase 24–48 h after irradiation and enhanced radiation-induced apoptosis. This correlated with elevated Cdc2 tyrosine 15 phosphorylation, decreased Cdc2 activity, and decreased Cdc25C protein levels. Caffeine treatment or removal of MEK1/2 inhibitors from cells 6 h after irradiation reduced the proportion of cells present in G2/M phase at 24 h and abolished the ability of MAPK inhibition to potentiate radiation-induced apoptosis. These data argue that MAPK signaling plays an important role in the progression/release of cells through G2/M phase after radiation exposure and that an impairment of this progression/release enhances radiation-induced apoptosis. Surprisingly, the ability of irradiation/MAPK inhibition to increase the proportion of cells in G2/M at 24 h was found to be dependent on basal p21 expression. Transient inhibition of basal p21 expression increased the control level of apoptosis as well as the abilities of both radiation and MEK1/2 inhibitors to cause apoptosis. In addition, loss of basal p21 expression significantly reduced the capacity of MAPK inhibition to potentiate radiation-induced apoptosis. Collectively, our data argue that MAPK signaling and p21 can regulate cell cycle checkpoint control in carcinoma cells at the G1/S transition shortly after exposure to radiation. In contrast, inhibition of MAPK increases the proportion of irradiated cells in G2/M, and basal expression of p21 is required to maintain this effect. Our data suggest that basal and radiation-stimulated p21 may play different roles in regulating cell cycle progression that affect cell survival after radiation exposure.
INTRODUCTION
Ionizing radiation is used as a primary treatment for many types of carcinoma, including squamous, mammary, and prostate carcinomas. However, the mechanisms by which radiation can either increase cell death or alter the proliferative rate of surviving cells are not understood. Recently, radiation has been shown to activate multiple signaling pathways within cells that can alter cell survival or proliferation, depending on the radiation dose, the cell type, and the culture conditions (Xia et al., 1995; Santana et al., 1996; Chmura et al., 1997; Carter et al., 1998). Several groups have shown that the EGF receptor (EGFR, also called ErbB1) is activated in response to irradiation of squamous and mammary carcinoma cells (Balaban et al., 1996; Schmidt-Ullrich et al., 1997; Carter et al., 1998; Kavanagh et al., 1998). Radiation exposure via activation of the EGFR can activate the MAPK pathway to a level similar to that observed by physiological, growth stimulatory EGF concentrations (∼0.1 nM) (Schmidt-Ullrich et al., 1997; Suy et al., 1997; Carter et al., 1998; Kavanagh et al., 1998).
The proliferation of many carcinoma cells in vitro and in vivo is in part regulated by the synthesis and autocrine action of TGFα (Levenson et al., 1998). Irradiation of A431 and MBA-MB-231 cells can increase expression of TGFα and activate the EGFR; this has been proposed to increase the proliferative rate of surviving cells (Baselga et al., 1996; Schmidt-Ullrich et al., 1997). Increased proliferative rates and the poor prognosis of carcinomas in vivo are also correlated with increased expression of the EGFR (Putz et al., 1999). These findings argue that radiation may have a self-limiting effect on its toxicity via increased activity of EGFR and associated downstream signaling modules such as the MAPK pathway.
We recently demonstrated that increased signaling by the MAPK pathway is cytoprotective versus ionizing radiation and various cytotoxic drugs, although the precise mechanism(s) by which this occurred were unclear (Goldkorn et al., 1997; Schmidt-Ullrich et al., 1997; Carter et al., 1998; Kavanagh et al., 1998). This finding also supports the concept that certain cytotoxic stresses may have a self-limiting effect on their toxicity as a result of activation of the MAPK pathway. In contrast to the apparent protective role for radiation-induced MAPK signaling, radiation-induced activation of the c-Jun NH2-terminal kinase pathway has been linked in several cell types to apoptosis and clonogenic cell death (Xia et al., 1995; Santana et al., 1996). The ability of radiation to activate the c-Jun NH2-terminal kinase pathway has been linked to both sphingomyelinase enzymes and ceramide generation and to the activation of growth factor receptors such as EGFR (Rosette and Karin, 1996; Santana et al., 1996; Verheij et al., 1996; Dent et al., 1998, 1999).
The regulation of proliferation versus differentiation by MAPK signaling depends on the cell type and on the amplitude and duration of MAPK activation (Jakus and Yeudall, 1996; Sewing et al., 1997; Woods et al., 1997; Auer et al., 1998b; Tombes et al., 1998). A short activation of the MAPK cascade has been correlated with increased proliferation, potentially via coordinated increases in the expression of cyclin D1 and the cdk inhibitor protein p21Cip-1/WAF1/MDA6 (p21), whereas prolonged elevation of MAPK activity has been demonstrated to inhibit DNA synthesis, potentially via superinduction of p21. Recent studies have suggested that part of the mechanism by which radiation and signaling by ErbB family receptors can transiently increase p21 protein levels in nonfunctional p53 cells is via activation of the MAPK pathway (Carter et al., 1998; Fiddes et al., 1998). High levels of p21 expression would potentially lead to growth arrest at the G1/S and G2/M boundaries, as has been reported for cells exposed to ionizing radiation (Deng et al., 1995; Macleod et al., 1995; Morgan, 1995; Sherr and Roberts, 1995; O’Connor, 1997; Palmer et al., 1998; Reed et al., 1998; Xu et al., 1998). These data argue that radiation-induced MAPK signaling and p21 may play a role in the regulation of cell cycle progression after irradiation.
The mechanism by which inhibition of the MAPK pathway increases apoptosis and decreases the proliferative potential of carcinoma cells after irradiation is not known. Previously, we had demonstrated that MAPK inhibition decreased the ability of radiation to increase expression of the cdk inhibitor protein p21. This was correlated to the ability of MAPK inhibition to increase radiation-induced cell killing (Carter et al., 1998). The studies described here were undertaken to determine whether inhibition of MAPK signaling radiosensitized cells by altering p21 expression and altering cell cycle progression after irradiation.
MATERIALS AND METHODS
Materials
Anti-p42MAPK (sc-154), anti-p21Cip-1/WAF1/MDA6 (sc-817), anti-Cdc2 (sc-54), anti-cyclin D1 (sc-246), anti-cyclin E (sc-247), anti-cyclin B1 (sc-752), and anti-cyclin A (sc-239) were from Santa Cruz Biotechnology (Santa Cruz, CA) (Carter et al., 1998). Anti-phosphotyrosine 15 Cdc2 antibody (no. 9111) was from New England Biolabs (Beverly, MA). Radiolabeled [γ-32P]ATP was from New England Nuclear (Boston, MA). The novel MEK1/2 inhibitor U0126 was a kind gift from DuPont (Wilmington, DE) (Favata et al., 1998). The MEK1/2 inhibitor PD98059 was a kind gift from Parke-Davis (Ann Arbor, MI). Western immunoblotting was performed with the Amersham ECL system (Bucks, England). GST–c-Jun (amino acids 1–169) was synthesized in Escherichia coli and purified on glutathione-Sepharose. Other reagents were as described by Schmidt-Ullrich et al. (1997), Carter et al. (1998), and Kavanagh et al. (1998).
Methods
Generation of MDA-TR15-EGFR-CD533 and A431-TR25-EGFR-Antisense Cells.
Squamous and mammary carcinoma cell lines A431-TR25-EGFR-antisense (AS) and MDA-TR15-EGFR-CD533 were generated as described (Contessa et al., 1999). CD533 is the wild-type EGFR with the COOH-terminal 533 amino acids deleted. Treatment of A431-TR25-EGFR-AS or MDA-TR15-EGFR-CD533 cells with 1 μg/ml doxycycline for 48 h induces antisense EGFR-CD533 mRNA and sense EGFR-CD533 mRNA, respectively. Antisense EGFR-CD533 reduces expression of full-length wild-type EGFR protein expression by >100-fold, and sense EGFR-CD533 mRNA increases expression of EGFR-CD533 protein >100 fold (Contessa et al., 1999; Dent et al., 1999; our unpublished results).
Culture of MDA-TR15-EGFR-CD533 and A431-TR25-EGFR-AS Cells.
Asynchronous carcinoma cells were cultured in RPMI-1640 medium supplemented with 5% (vol/vol) FCS at 37°C in 95% (vol/vol) air/5% (vol/vol) CO2 (Schmidt-Ullrich et al., 1997; Carter et al., 1998; Kavanagh et al., 1998). Cells were plated at a density 3.2 × 104 cells/cm2 of plate area. For radiation-induced activation of protein kinases only, cells were cultured for 4 d in this medium, and for 2 h before irradiation cells were cultured in serum-reduced RPMI-1640 medium (0.5% [vol/vol] FCS) to lower basal kinase activity. For all other experiments involving p21 expression, promoter activity, flow cytometry, proliferation, and survival, cells were cultured for 4 d in RPMI-1640 medium (5.0% [vol/vol] FCS) and the medium was changed to fresh medium 2 h before irradiation.
Recombinant Adenoviral Vectors: Generation and Infection In Vitro.
The adenoviruses to express β-galactosidase and p21 antisense were prepared as described by Valerie and Singhal (1995). Cells were infected with adenoviruses in vitro at a multiplicity of infection (m.o.i.) of 100 (p21 sense virus was used at a m.o.i. of 5) and incubated at 37°C for another 24 h. To assess infection of cells, we performed staining for β-galactosidase 24 h after infection.
Infection of MDA-TR15-EGFR-CD533 and A431-TR25-EGFR-AS Cells with Poly-l-Lysine–conjugated Adenoviral Technology.
Cells were infected with poly-l-lysine–conjugated adenovirus as described by Auer et al. (1998b). The DNA-conjugated virus was added to cells at a m.o.i. of 250, and the cells were incubated for 4 h at 37°C. The cells were washed with medium to remove virus. Cells expressed transduced gene products 10–24 h after infection. Using a plasmid to express green fluorescent protein under control of the cytomegalovirus promoter, we determined that 1 μg of plasmid conjugated to virus particles and infected into cells at a m.o.i. of 250 gave 39 ± 7% infection, as judged by microscopic observation 24 h after infection.
Exposure of Cells to Ionizing Radiation and Cell Homogenization.
Cells were cultured in RPMI-1640 plus 5% (vol/vol) FCS as described above and were cultured in serum-reduced RPMI-1640 medium (0.5% [vol/vol]) for 2 h before irradiation. U0126 or PD98059 treatment was from a 100 mM stock solution, and the maximal concentration of vehicle (DMSO) in medium was 0.02% (vol/vol). Cells were irradiated with a 60Co source at a dose of 1.1 Gy/min (Schmidt-Ullrich et al., 1997; Carter et al., 1998; Kavanagh et al., 1998). Cells were maintained at 37°C throughout the experiment except during the irradiation itself. Zero time was designated as the time at which exposure to radiation ceased. After radiation treatment, cells were incubated for specified times followed by aspiration of medium and snap freezing at −70°C on dry ice. Cells were homogenized in 1 ml of ice-cold buffer A (25 mM HEPES, pH 7.4 at 4°C, 5 mM EDTA, 5 mM EGTA, 5 mM benzamidine, 1 mM PMSF, 1 mg/ml soybean trypsin inhibitor, 40 μg/ml pepstatin A, 1 μM microcystin-LR, 0.5 mM sodium orthovanadate, 0.5 mM sodium pyrophosphate, 0.05% [wt/vol] sodium deoxycholate, 1% [vol/vol] Triton X-100, 0.1% [vol/vol] 2-mercaptoethanol) with trituration with the use of a P1000 pipette to lyse the cells. Homogenates were stored on ice before clarification by centrifugation (4°C).
Immunoprecipitations from Lysates.
Fifty microliters of protein A–agarose slurry (25-μl bead volume) was washed twice with 1 ml of PBS containing 0.1% (vol/vol) Tween 20 and resuspended in 0.1 ml of the same buffer. Antibodies (2 μg, 20 μl) and serum (20 μl) were added to each tube and incubated (3 h, 4°C). For preconjugated antibodies, 10 μl of slurry (4 μg of antibody) was used. Clarified equal aliquots of lysates (0.25 ml, ∼100 μg of total protein) were mixed with agarose-conjugated antibodies in duplicate with gentle agitation (2.5 h, 4°C). Agarose-antibody-antigen complexes were recovered by centrifugation, the supernatant was discarded, and complexes were washed (10 min) sequentially with 0.5 ml of buffer A (twice), PBS, and buffer B (25 mM HEPES, pH 7.4, 0.1 mM Na3VO4).
Assay of p42MAPK Activity.
Immunoprecipitates were incubated (final volume, 50 μl) with 50 μl of buffer B containing 0.2 mM [γ-32P]ATP (5000 cpm/pmol), 1 μM microcystin-LR, and 0.5 mg/ml myelin basic protein (MBP), which initiated reactions at time zero. After 20 min, 40 μl of the reaction mixture was spotted onto a 2-cm circle of P81 paper (Whatman, Maidstone, England) and immediately placed into 180 mM phosphoric acid. Papers were washed four times (10 min each) with phosphoric acid and once with acetone, and 32P incorporation into MBP was quantified by liquid scintillation spectroscopy.
SDS-PAGE and Western Blotting.
Cells were irradiated, and at specified times/treatments, medium was aspirated and the plates were snap frozen. Cells were lysed with homogenization buffer and subjected to immunoprecipitation. Immunoprecipitates were solubilized with 100 μl of 5× SDS-PAGE sample buffer (10% [wt/vol] SDS), diluted to 250 μl with distilled water, and placed in a 100°C dry bath for 15 min. Aliquots of 100 μl from each time point were subjected to SDS-PAGE on 10% (wt/vol) acrylamide gels. Gels were transferred to nitrocellulose, and Western blotting with specific antibodies was performed as indicated. Blots were developed with ECL (Amersham) with the use of Fuji (Stanford, CT) RX x-ray film. Blots were digitally scanned with the use of Adobe (Mountain View, CA) Photoshop 4, their color was removed, and figures were created in Microsoft (Redmond, WA) PowerPoint.
Luciferase Assays for Promoter Activity.
Luciferase activity assays were performed with full-length (base pairs 1–2326) and truncated (base pairs 1–850) p21 promoters as described (Chinery et al., 1997; Auer et al., 1998a; Cram et al., 1998). Luciferase assays were performed with the use of a kit (Promega, Madison, WI) according to the manufacturer’s instructions. Luciferase activity was measured in a Bertold (Nashua, NH) LB9501 luminometer for 20 s per assay.
Terminal Uridyl-Nucleotide End Labeling for Apoptosis.
Cells were grown in 100-mm dishes as described, treated with or without varying concentrations of U0126/PD98059/DMSO control 30 min before irradiation, and irradiated (2 Gy). Cells were isolated 24 h after irradiation by trypsinization followed by centrifugation onto glass slides (cytospin). Terminal uridyl-nucleotide end labeling (TUNEL) was performed on these cells as described previously (Carter et al., 1998, Jarvis et al., 1998). Randomly selected fields of fixed cells (∼150 cells per field, n = 5 per slide) were counted initially with the use of propidium iodide counter stain, followed by examination and counting of TUNEL positive-staining cells of the same field under FITC/fluorescent light.
Cell Cycle Analysis: Propidium Iodide and Antibody Staining of Cells.
Cells were isolated by tryptic digestion at the indicated times after various treatments, and aliquots containing 1 × 106 cells were pelleted by centrifugation at 1500 rpm at 4°C for 5 min, resuspended in 1.5 ml of PBS followed by the addition of 3 ml of 100% (vol/vol) ethanol (67% [vol/vol] final ethanol concentration), and incubated on ice at 4°C for 3 h. Cells were pelleted by centrifugation as described above, and the supernatant was removed, resuspended in 1.0 ml of propidium iodide stain containing 3.8 mM sodium citrate, 0.5 mg/ml RNAse A, and 0.01 mg/ml propidium iodide, and incubated on ice at 4°C overnight. Cells were pelleted by centrifugation as described above, and the supernatant was removed and resuspended in 1.0 ml of PBS. In the indicated experiments, cells were then incubated with fluorescein-conjugated anti-cyclin B1 antibody for an additional 2 h, followed by washing in PBS. Cells were analyzed with a Becton-Dickinson (Franklin Lakes, NJ) FACScan flow cytometer and Verity (Topsham, ME) Winlist software.
Data Analysis.
Comparison of the effects of treatments was done with one-way analysis of variance and a two-tailed t test. Differences with a p value < 0.05 were considered statistically significant. Results shown, except where indicated, are the means of multiple individual points from multiple separate experiments (±SEM).
RESULTS
Radiation Induces Immediate Primary and Secondary Activation of the MAPK Pathway in A431-TR25-EGFR-AS and MDA-TR15-EGFR-CD533 Carcinoma Cells
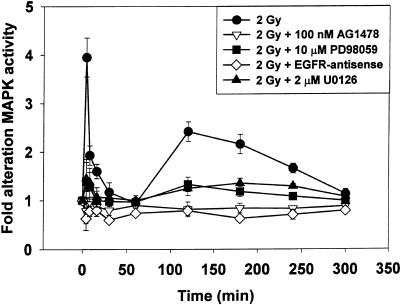
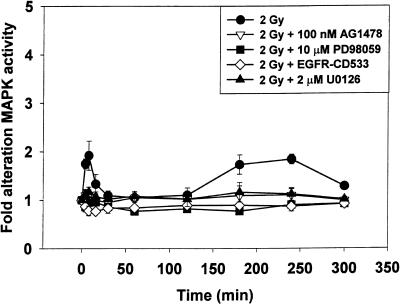
The ability of radiation (2 Gy) to modulate MAPK activity was investigated in A431-TR25-EGFR-AS and MDA-TR15-EGFR-CD533 carcinoma cells for a prolonged period (0–300 min) (Figures 1 and 2). Radiation caused immediate primary activation of the MAPK pathway (0–10 min), followed by a later secondary activation (90–240 min). Chemical inhibition of the EGFR function, by means of the ErbB1-specific tyrphostin AG1478, blocked MAPK activation, in agreement with previous data (Schmidt-Ullrich et al., 1997; Carter et al., 1998). Molecular inhibition of EGFR function by either inducible expression of antisense EGFR or dominant negative EGFR-CD533 also was able to block MAPK activation after irradiation. Incubation of cells with either of the chemically dissimilar specific inhibitors of MEK1/2, U0126 or PD98059, partially reduced basal MAPK activity and largely abolished the ability of radiation to activate MAPK (∼80%). These data demonstrate that radiation increases MAPK activity in carcinoma cells by an EGFR/MEK1/2-dependent mechanism.
Figure 1.
Radiation-induced activation of MAPK in A431-TR25-EGFR-AS cells is blocked by expression of antisense EGFR or incubation of cells with MEK1/2 inhibitors PD98059 or U0126. A431-TR25-EGFR-AS cells were treated with doxycycline 24 h before irradiation, EGFR inhibitor tyrphostin AG1478 (100 nM) 30 min before irradiation, or MEK1/2 inhibitors (U0126, 2 μM; PD98059, 10 μM) 30 min before irradiation. Cells were irradiated (2 Gy), and MAPK activity was determined from 0 to 300 min. Cells were lysed, and portions (∼100 μg) from each plate were used to immunoprecipitate MAPK, followed by immune complex kinase assays. MAPK activity data are shown as -fold increases in 32P incorporation into MBP substrate and are normalized to activity at 0 min from the means ± SEM of three independent experiments.
Figure 2.
Radiation-induced activation of MAPK in MDA-TR15-EGFR-CD533 cells is blocked by expression of dominant negative EGFR-CD533 or incubation of cells with MEK1/2 inhibitors PD98059 or U0126. MDA-TR15-EGFR-CD533 cells were treated with doxycycline 24 h before irradiation, EGFR inhibitor tyrphostin AG1478 (100 nM) 30 min before irradiation, or MEK1/2 inhibitors (U0126, 2 μM; PD98059, 10 μM) 30 min before irradiation. Cells were irradiated (2 Gy), and MAPK activity was determined from 0 to 300 min. Cells were lysed, and portions (∼100 μg) from each plate were used to immunoprecipitate MAPK, followed by immune complex kinase assays. MAPK activity data are shown as -fold increases in 32P incorporation into MBP substrate and are normalized to activity at 0 min from the means ± SEM of three independent experiments.
Inhibition of the Secondary MAPK Activation Blunts Radiation-induced Expression of p21 in A431-TR25-EGFR-AS and MDA-TR15-EGFR-CD533 Cells
The MAPK-dependent modulation of p21 expression has been the subject of considerable research (Sewing et al., 1997; Woods et al., 1997; Auer et al., 1998b; Tombes et al., 1998). Previously, we correlated reduced p21 expression to the potentiation of radiation-induced apoptosis by MAPK inhibition (Carter et al., 1998). Thus, we next examined the mechanism(s) by which radiation-induced MAPK signaling regulates p21 expression and whether this modulation has an impact on MAPK inhibition to alter radiation-induced apoptosis.
To determine whether the primary or secondary phase of MAPK activation was responsible for increased p21 protein levels, the chemically dissimilar MEK1/2 inhibitors, either PD98059 or U0126, were added to culture medium either before radiation exposure or 30 min after exposure. Inhibition of either the primary or the secondary MAPK activation by PD98059 or U0126 blocked the induction of p21 by radiation (Figure 3, A and B). Identical data were obtained with both A431-TR25-EGFR-AS cells expressing antisense EGFR mRNA and MDA-TR15-EGFR-CD533 cells expressing dominant negative EGFR-CD533. These data argue that the mechanism by which low-dose radiation increases p21 expression requires the secondary, and not the primary, EGFR/MAPK activation.
Figure 3.
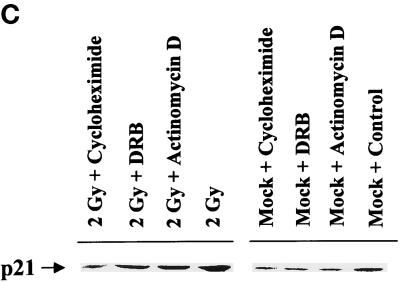
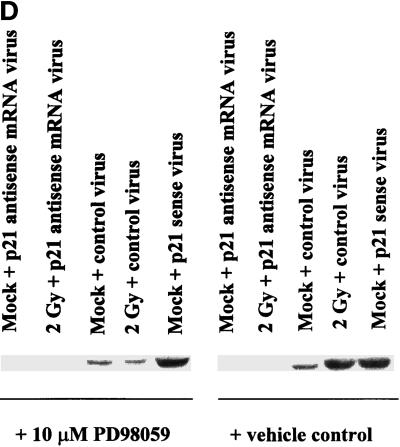
Radiation-induced p21 expression in A431-TR25-EGFR-AS cells and MDA-TR15-EGFR-CD533 cells is dependent on EGFR function and the second phase of MAPK activation. A431-TR25-EGFR-AS cells (A) and MDA-TR15-EGFR-CD533 cells (B) were either treated with doxycycline 24 h before irradiation or pretreated for 30 min before irradiation with the MEK1/2 inhibitor PD98059 (10 μM) or U0126 (2 μM). In parallel, other plates of cells were also treated 30 min after irradiation with the MEK1/2 inhibitor PD98059 or U0126. Cells were irradiated (2 Gy), and the protein expression of p21 was determined from 0 to 300 min. Cells were lysed, and equal portions (∼100 μg) from each plate were subjected to SDS-PAGE, followed by immunoblotting to determine p21 protein levels. Ponceau S staining revealed equal protein loading on the nitrocellulose (our unpublished results). Exposure was for 1 min. A representative experiment is shown (n = 3). (C) A431-TR25-EGFR-AS cells were infected with a control adenovirus to express β-galactosidase, a virus to express antisense p21 mRNA, or a virus to express p21 protein. Twenty-four hours after infection, cells were pretreated for 30 min before irradiation with PD98059 (10 μM) orvehicle control (DMSO). Cells were irradiated (2 Gy) or mock exposed, and the protein expression of p21 was determined after 4 h. Cells were lysed, and portions (∼400 μg) from each plate were subjected to SDS-PAGE, followed by immunoblotting to determine p21 protein levels. Ponceau S staining revealed equal protein loading on the nitrocellulose (our unpublished results). Exposure was for 3 min. A representative experiment for each condition is shown (n = 5). (D) A431-TR25-EGFR-AS cells were pretreated for 5 min before irradiation with DRB (30 μM), actinomycin D (5 μM), or cycloheximide (20 μg/ml) for 4 h. Cells were irradiated (2 Gy) or mock exposed, and the protein expression of p21 was determined after 4 h. Cells were lysed, and portions (∼400 μg) from each plate were subjected to SDS-PAGE, followed by immunoblotting to determine p21 protein levels. Exposure was for 3 min. A representative experiment for each condition is shown (n = 5).
Because MAPK inhibition blocked radiation-induced p21 expression, we next determined whether its inhibition also modulated basal levels of p21 (Figure 3C). Cells were infected with either control adenovirus or recombinant adenovirus to express antisense p21 mRNA and then treated with either vehicle control or MEK1/2 inhibitor for 4 h. Fourfold greater protein loading was used in panel C, compared with panels A and B, to accurately visualize basal p21 protein levels. Antisense p21 mRNA virus abolished basal p21 expression. However, although MAPK inhibition blocked radiation-induced expression of p21, it did not reduce basal p21 expression. These data suggest that multiple signaling pathways may regulate basal and stimulated p21 protein levels, including the MAPK pathway.
Studies with UV irradiation of cells have argued that this stimulus increases p21 protein expression by a posttranscriptional mechanism (Butz et al., 1998). To investigate whether ionizing radiation also increases p21 protein expression at a posttranscriptional level in carcinoma cells, we irradiated cells in the presence of the inhibitors of transcription and translation, either actinomycin D or 5,6-dichloro-1-β-d-ribofuranosylbenzimidazole (DRB) and cycloheximide, respectively. Irradiation of A431-TR25-EGFR-AS cells increased protein expression of p21 approximately fourfold within 4 h, which was reduced by ∼50% when cells were incubated in the presence of either actinomycin D or DRB (Figure 3D). These findings argue that radiation increases protein levels of p21 via both an increased rate of transcription and posttranscriptional stabilization of the p21 mRNA/protein, in general agreement with studies that used growth factors in other cell types (Bromberg et al., 1998; Johannessen et al., 1999).
Radiation-induced MAPK Signaling Regulates the p21 Promoter in Carcinoma Cells
To further examine the transcriptional mechanism(s) by which radiation increases protein levels of p21, we examined whether radiation regulates transcription from the p21 promoter in a MAPK-dependent manner. Cells were infected via poly-l-lysine–conjugated adenovirus with both a full-length (base pairs 1–2326) and a truncated (base pairs 1–850) p21 promoter coupled to a construct encoding the luciferase gene product. Twenty-four hours after infection, cells were pretreated with either MEK1/2 inhibitor or DMSO, after which they were irradiated or left unirradiated. Seven hours after irradiation, cells were processed to determine luciferase activity.
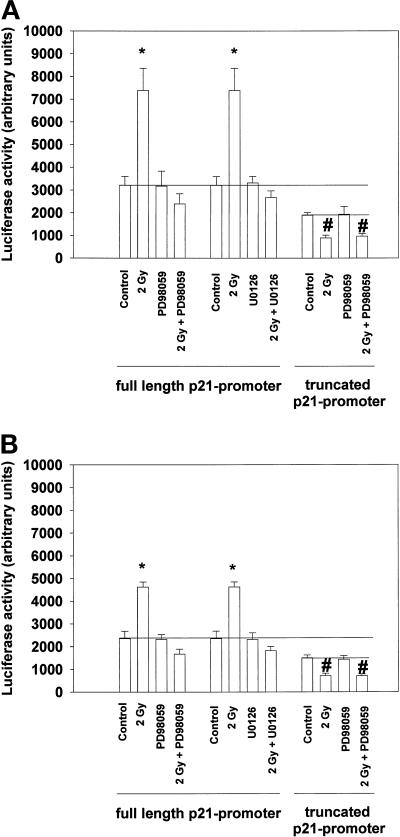
Radiation caused an approximately threefold increase in luciferase activity from the full-length p21 promoter within 7 h, which was blocked by treatment of cells with either PD98059 or U0126 (Figure 4A). However, inhibition of the MAPK pathway did not reduce basal promoter activity, which is in agreement with our data in Figure 3 showing a lack of effect on basal protein levels. In contrast to these findings, radiation reduced promoter activity from the truncated p21 promoter construct, and this reduction was MAPK independent. Identical data were obtained in MDA-TR15-EGFR-CD533 cells (Figure 4B). These data suggest that irradiation of cells initiates at least one positive MAPK-dependent signal and one negative MAPK-independent signal toward the p21 promoter. However, MAPK inhibition did not alter basal promoter activity. Thus, multiple pathways may mediate basal and stimulated p21 promoter activity.
Figure 4.
Radiation increases transcriptional activity of a full-length p21 promoter via MAPK signaling. A431-TR25-EGFR-AS cells and MDA-TR15-EGFR-CD533 cells were cultured and infected via a poly-l-lysine–conjugated adenovirus with constructs containing the full-length p21 promoter or a truncated p21 promoter. Twenty-four hours after infection, cells were pretreated with either PD98059 (10 μM) or control (DMSO), followed by irradiation (2 Gy). The ability of radiation to alter full-length and truncated p21 promoter activity by luciferase activity in either A431-TR25-EGFR-AS cells (A) or MDA-TR15-EGFR-CD533 cells (B) is shown. Data represent the means of multiple individual points from six separate experiments (±SEM); *p < 0.01 greater than control value; #p < 0.01 less than control value.
Inhibition of the MAPK Pathway Increases the Proportion of Cells in G2/M Phase 24 h after Irradiation and Potentiates Radiation-induced Apoptosis
Exposure of cells to ionizing radiation or overexpression of p21 has been shown to cause growth arrest at both G1/S and G2/M transitions of the cell cycle. Cells unable to express p21 have a reduced ability to undergo a radiation-induced G2/M arrest compared with wild-type cells (Rigberg et al., 1998, 1999), whereas the radiation-induced G1/S arrest is largely abolished (Deng et al., 1995). Because MAPK inhibition is associated with a reduced induction of p21 expression, we next examined the ability of MAPK inhibition to modify radiation-induced growth arrest responses at G1/S and G2/M.
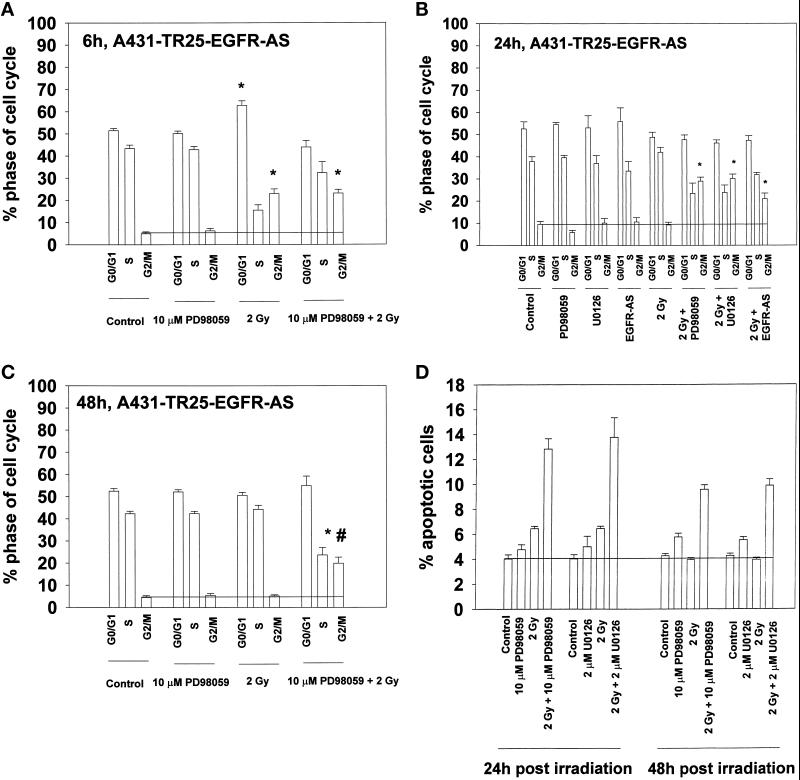
Six hours after irradiation, a significant (∼300%) increase in the number of A431-TR25-EGFR-AS cells (Figure 5A) and MDA-TR15-EGFR-CD533 cells (our unpublished results) present in G2/M was observed. A much smaller (∼5%), although significant, increase was also observed in the number of A431-TR25-EGFR-AS cells present in G1 phase. This increase was not observed in MDA-TR15-EGFR-CD533 cells (our unpublished results). Irradiation combined with inhibition of MAPK signaling by PD98059 exhibited similar numbers of cells present in G2/M and reduced the numbers of cells present in G1 and S phases (Figure 5A). Twenty-four and 48 h after irradiation, the numbers of cells present in each phase of the cell cycle had returned to preirradiated control levels in control-treated, MAPK-inhibited, EGFR-inhibited, and irradiated cells (Figure 5, B and C). In contrast, in irradiated cells that had either their EGFR or MAPK activity suppressed, no decline in the proportion of cells present in G2/M was observed. The enhanced proportion of A431-TR25-EGFR-AS cells in G2/M phase at 24 and 48 h after exposure correlated with increased apoptosis as determined by TUNEL assays (Figure 5D). The total increase in apoptosis was ∼2.5-fold; the increase in radiation-induced apoptosis after MAPK inhibition was ∼5-fold. These data suggest that an inhibition of MAPK activity after irradiation increases the number of cells observed in G2/M phase; an accumulation of cells in G2/M correlated with an increase in radiation-induced apoptosis.
Figure 5.
Inhibition of the MAPK pathway increases the proportion of cells in G2/M after irradiation. A431-TR25-EGFR-AS cells were pretreated for 30 min before irradiation with MEK1/2 inhibitors (PD98059, 10 μM; U0126, 2 μM), EGFR inhibitor AG1478 (100 nM), or DMSO control. Cells were irradiated (2 Gy), and the cell cycle profiles under each condition were determined 6, 24, and 48 h after irradiation by flow cytometry. Apoptosis determination at 24 h was by TUNEL staining. (A) Cell cycle profiles of A431-TR25-EGFR-AS cells 6 h after irradiation. (B) Cell cycle profiles of A431-TR25-EGFR-AS cells 24 h after irradiation. (C) Cell cycle profiles of A431-TR25-EGFR-AS cells 48 h after irradiation. (D) Determination of apoptosis by TUNEL assay in A431-TR25-EGFR-AS cells 24 and 48 h after irradiation. Data are the means of five separate experiments (±SEM); *p < 0.01 greater than control value; #p < 0.05 less than corresponding value at 24 h.
Transient Mimosine Treatment Does Not Alter the Ability of MAPK Inhibition to Potentiate Radiation-induced Apoptosis
Inhibition of MAPK decreased both p21 expression and the number of cells in the G1 phase of the cell cycle after irradiation. This correlated with increased apoptosis. Thus, one potential mechanism by which increased apoptosis occurred was by blunting p21 expression, thereby permitting entry of cells with damaged DNA into S phase. To determine whether an increased capacity of cells to enter S phase after irradiation was directly responsible for the increased apoptosis, we artificially induced a transient G1/S arrest by use of the amino acid mimosine. Mimosine has been proposed to increase p21 levels in a p53-independent manner (Bissonnette and Hunting, 1998). Cells were irradiated and, 3 h later, at the time of radiation-induced p21 expression, treated with 2.0 mM mimosine to mimic the radiation-induced increase in p21 protein levels. Six hours after exposure, mimosine was removed by several washes with warm medium. The cell cycle profiles of mimosine-treated cells were determined 6 and 24 h after irradiation. Twenty-four hours after irradiation, the numbers of apoptotic cells were also determined (Figure 6).
Figure 6.
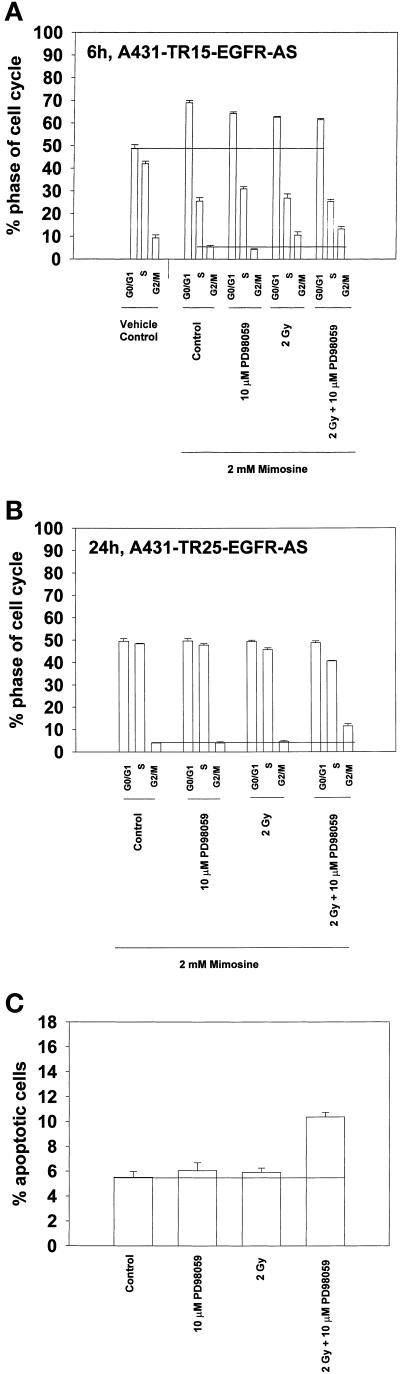
Transient mimosine treatment neither alters the increased numbers of irradiated/MAPK-inhibited cells found in G2/M nor modifies the ability of MAPK inhibition to potentiate radiation-induced apoptosis. A431-TR25-EGFR-AS cells were pretreated for 30 min before irradiation with a MEK1/2 inhibitor (PD98059, 10 μM) or DMSO control as indicated. Cells were irradiated (2 Gy), and 3 h after irradiation, 2.0 mM mimosine (final concentration) was added to the medium. At 6 h, mimosine was removed by washing with warm medium three times. The cell cycle profiles under each condition were determined 6 and 24 h after irradiation by flow cytometry. Apoptosis determination at 24 h was by TUNEL staining. (A) Cell cycle profiles of A431-TR25-EGFR-AS cells 6 h after irradiation. (B) Cell cycle profiles of A431-TR25-EGFR-AS cells 24 h after irradiation. (C) Determination of apoptosis by TUNEL assay in A431-TR25-EGFR-AS cells 24 h after irradiation. Data are the means of three separate experiments (±SEM).
Mimosine treatment increased the percentage of G1 cells under all conditions within 6 h from ∼50 to ∼70% (Figure 6A). Mimosine treatment decreased the numbers of cells present in S phase and G2/M under control conditions, relative to nontreated control cells (Figure 6A). However, although the numbers of cells present in G2/M were reduced by mimosine, this did not prevent a radiation-induced enhancement in the numbers of cells present in G2/M at 6 h (Figure 6A; compare with Figure 5A). For example, inhibition of the MAPK pathway combined with irradiation increased the proportion of cells present in G2/M in mimosine-treated cells from ∼5 to ∼12%.
Washing to remove mimosine at 6 h permitted a relaxation by 24 h in the number of cells present in G1 phase, and little difference in the cell cycle profiles of control, MAPK-inhibited, and irradiated cells was seen compared with those of nontreated control cells (Figure 6B; compare with Figure 5B). Inhibition of the MAPK pathway increased the proportion of irradiated cells present in G2/M phase at 24 h from ∼3 to 10%, demonstrating that mimosine treatment does not alter the enhancement of G2/M cell numbers under these conditions. In addition, mimosine treatment did not alter the potentiation of radiation-induced apoptosis caused by MAPK inhibition (Figure 6C; compare with Figure 5D). These data argue that the ability of MAPK inhibition/loss of p21 expression to potentiate radiation-induced apoptosis is not due to inappropriate entry of cells with damaged DNA into S phase.
Inhibition of the MAPK Pathway Enhances and Maintains Radiation-induced Cdc2 Tyrosine 15 Phosphorylation 24 h after Irradiation
Because we observed a potentiation of G2/M arrest in irradiated/MAPK-inhibited cells, we next examined the ability of this treatment to alter the phosphorylation and activity of the cdk Cdc2. Cdc2 activation is required for cell cycle progression through G2/M, and one mechanism by which radiation has been suggested to cause G2/M arrest is increased phosphorylation of Cdc2 at tyrosine 15. We investigated whether MAPK inhibition after irradiation altered the phosphorylation state of Cdc2 tyrosine 15 (Figure 7).
Figure 7.
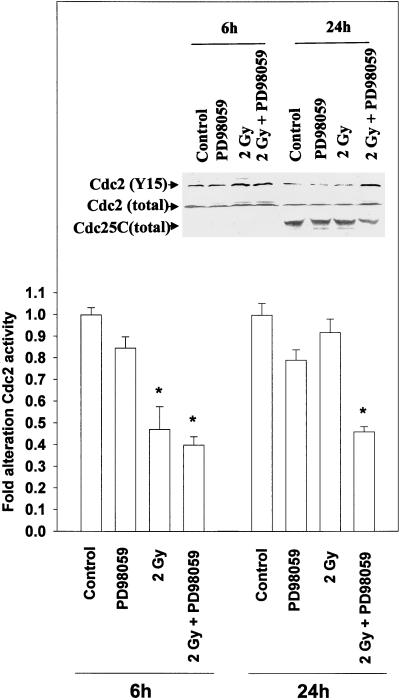
Inhibition of the MAPK pathway maintains radiation-induced Cdc2 tyrosine 15 phosphorylation 24 h after irradiation. A431-TR25-EGFR-AS cells were pretreated for 30 min before irradiation with a MEK1/2 inhibitor (PD98059, 10 μM) or DMSO control. Cells were irradiated (2 Gy), and the phosphorylation and activity of Cdc2, and the total expression of Cdc2 and Cdc25C, were determined at the indicated times after irradiation. For kinase activity, cells were lysed, and portions (∼100 μg) from each plate were subjected to immunoprecipitation, followed by immune complex kinase assays with the use of histone H1 as substrate. Results from a representative experiment (±SEM) are shown (n = 3); *p < 0.01 less than control value. (Inset) The phosphorylation of Cdc2 tyrosine 15 was determined after Cdc2 immunoprecipitation 6 and 24 h after irradiation. Cells were lysed, and portions (∼100 μg) from each plate were subjected to immunoprecipitation for Cdc2. Immunoprecipitates were resolved via SDS-PAGE, followed by immunoblotting to determine Cdc2 tyrosine 15 phosphorylation levels. Exposure time was 5 min. The total protein levels of Cdc2 and Cdc25C were also determined by immunoblotting 24 h after radiation exposure. Cells were lysed, and equal portions (∼100 μg) from each plate were subjected to SDS-PAGE, followed by immunoblotting to determine Cdc2 and Cdc25C protein levels. Exposure time was 4 min. Results of a representative experiment for each condition are shown (n = 3).
Cdc2 tyrosine 15 phosphorylation was increased 6 h after irradiation of cells (Figure 7, inset), in agreement with data showing an increase in the number of cells present in G2/M at this time (Figure 5A). Inhibition of MAPK did not significantly alter the radiation-induced increase in Cdc2 tyrosine 15 phosphorylation. Twenty-four hours after irradiation, the level of Cdc2 tyrosine 15 phosphorylation had returned to control levels in control-treated, PD98059-treated, or irradiated cells. In contrast, in irradiated cells in which MAPK had been inhibited, Cdc2 tyrosine 15 phosphorylation remained elevated (Figure 7, inset). No alteration was observed in total Cdc2 protein levels during this time. These data argue that MAPK inhibition prevents Cdc2 tyrosine 15 dephosphorylation 24 h after irradiation.
In agreement with elevated levels of Cdc2 tyrosine 15 phosphorylation, immune complex kinase assays demonstrated that radiation reduced Cdc2 kinase activity versus histone H1 6 h after irradiation, which returned to control levels during the next 18 h. In contrast, in irradiated cells in which MAPK had been inhibited, Cdc2 activity did not return to control levels 24 h after irradiation (Figure 7). One possible mechanism for increased Cdc2 tyrosine 15 phosphorylation may be loss of Cdc25C function and/or expression. Twenty-four hours after exposure, Cdc25C protein levels were reduced in irradiated cells in which MAPK had been inhibited (Figure 7, inset). The negative modulation of Cdc2 activity was not due to an increase in p21 expression or to a lack of cyclin B1 or cyclin A expression, which did not significantly change under any treatment condition during the 24 h (our unpublished results).
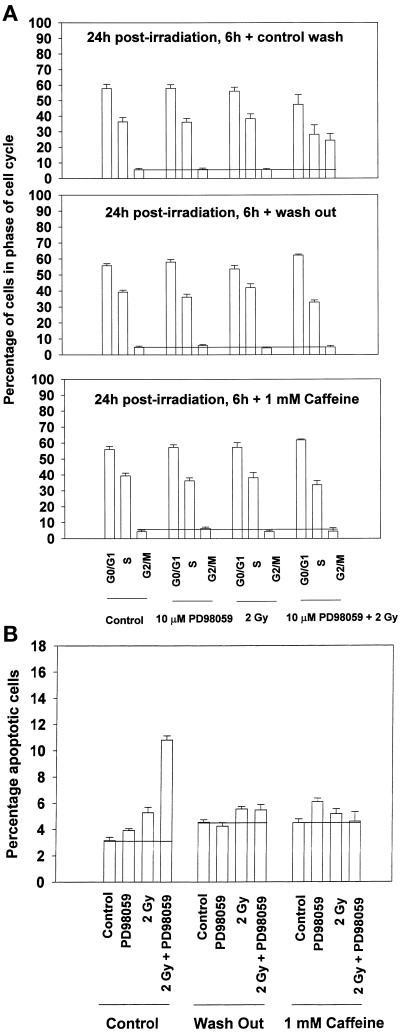
Caffeine Treatment of Cells, or Removal of the MEK1/2 Inhibitor, Abrogates the Potentiation of Radiation-induced G2/M Growth Arrest and Reduces Apoptosis
Prolonged growth arrest in G2/M has been correlated to the ability of taxanes to cause apoptosis (Poon et al., 1997; Wang et al., 1998). Because we observed an increased proportion of cells in G2/M phase in MAPK-inhibited/irradiated cells, which correlated with increased apoptosis, we sought to determine whether the increased numbers of cells in G2/M played a causal role in the apoptotic response. To achieve this, cells were washed free of the MEK1/2 inhibitor 6 h after irradiation. We then examined both the cell cycle profiles of washed cells 18 h after washing and their levels of apoptosis (Figure 8). Other investigators have demonstrated that caffeine can abrogate radiation-induced G2/M arrest (Poon et al., 1997; Blasina et al., 1999). In our studies, caffeine was added to the medium 6 h after irradiation, a time corresponding to the peak radiation-induced G2/M arrest and several hours after the cells have completed DNA damage repair (Eckardt-Schupp and Klaus, 1999). We then examined the cell cycle profiles of caffeine-treated cells 18 h after treatment and their levels of apoptosis (Figure 8).
Figure 8.
Caffeine treatment of cells, or washing to remove the MEK1/2 inhibitor, 6 h after irradiation abrogates the ability of MAPK inhibition to potentiate enhanced G2/M cell numbers and reduces the level of apoptosis at 24 h. A431-TR25-EGFR-AS cells were pretreated for 30 min before irradiation with a MEK1/2 inhibitor (PD98059, 10μM) or DMSO control. (A) Cells were irradiated (2 Gy), and 6 h laterthey were subjected to medium replacement containing PD98059 (control wash), medium replacement with no PD98059 addition in fresh medium (wash out), or medium replacement containing PD98059 supplemented with 1 mM caffeine. The cell cycle profiles under each condition were determined 18 h after washing (24 h after irradiation) by flow cytometry. Data are the means of four separate experiments (±SEM). (B) Cells from each treatment and condition in A were examined for apoptosis 18 h after washing (24 h after irradiation) by TUNEL assay. Cells were trypsinized from their dishes, and portions (∼10,000 cells) were spun onto glass slides, followed by TUNEL staining for double-stranded DNA breaks. Randomly selected fields of fixed cells (n = 5 per slide) were counted initially with the use of propidium iodide counter stain, followed by examination and counting of TUNEL positive-staining cells of the same field under FITC/florescent light. Data are the means of multiple individual points from three separate experiments (±SEM).
Combined irradiation and MAPK inhibition caused an increase in the percentage of cells present in G2/M phase in control-treated cells (Figure 8A). However, washing of cells to remove the MEK1/2 inhibitor returned the percentage of cells present in G2/M under all conditions to near control levels by 24 h (Figure 8A). Treatment of irradiated/MAPK-inhibited cells with caffeine also returned the percentage of cells present in G2/M under all conditions to near control levels by 24 h (Figure 8A). Furthermore, either washing of cells or treatment of cells with caffeine also abolished the potentiation of radiation-induced apoptosis by MAPK inhibition (Figure 8B). Loss of elevated G2/M cell numbers correlated with a decrease in Cdc2 tyrosine 15 phosphorylation and an increase in Cdc2 activity, in agreement with the findings of Poon et al. (1997) (our unpublished results). These data suggest that the ability of MAPK inhibition to potentiate radiation-induced cell killing is linked to its ability to cause an increase in the proportion of cells found in G2/M phase.
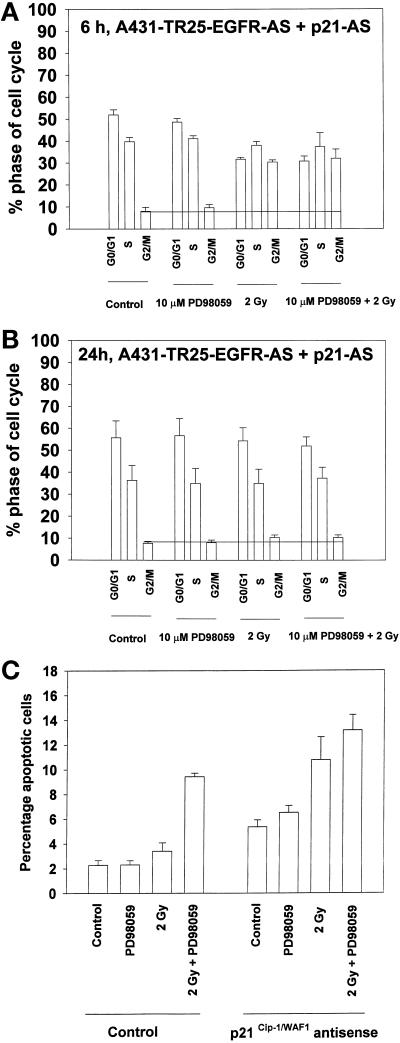
Loss of Basal p21 Expression Abrogates the Ability of MAPK Inhibition to Increase the Percentage of G2/M Phase Cells and to Potentiate Apoptosis
Previous experiments examined the role of stimulated p21 expression on cell cycle control after irradiation. We next explored possible roles for interactions between MAPK signaling and basal p21 expression in regulating cell cycle progression after exposure to radiation.
Inhibition of p21 expression by infection of A431-TR25-EGFR-AS cells with a recombinant antisense p21 mRNA adenovirus did not alter the radiation-induced increase in the numbers of cells present in G2/M after 6 h, but it did reduce the numbers of cells present in G1/S (Figure 9A). This was very similar to the ability of the MEK1/2 inhibitors PD98059 and U0126 (see Figure 5). These data argue that elevated p21 does not play an essential role in the radiation-induced G2/M growth arrest at 6 h. However, and in contrast to studies performed in the presence of basal p21 levels, inhibition of basal p21 expression abolished the capacity of MAPK inhibition to increase the numbers of irradiated cells in G2/M at 24 h (Figure 9B). Identical data were obtained in MDA-TR15-EGFR-CD533 cells (our unpublished results). These data suggest that basal levels of p21 play a different role in the regulation of cell cycle control compared with radiation-stimulated p21 expression.
Figure 9.
Loss of basal p21 expression abrogates the ability of MAPK inhibition to increase the percentage of G2/M phase cells and to potentiate apoptosis. A431-TR25-EGFR-AS cells were infected with either a recombinant adenovirus to express antisense p21 mRNA or β-galactosidase virus (100 m.o.i.) 24 h before irradiation. Infected cells were pretreated 30 min before irradiation with a MEK1/2 inhibitor (PD98059, 10 μM) or DMSO control. Cells were irradiated (2 Gy), and the cell cycle profiles under each condition were determined 6 and 24 h after irradiation by flow cytometry. Apoptosis determination at 24 h was by TUNEL staining. (A) Cell cycle profiles of A431-TR25-EGFR-AS cells 6 h after irradiation. (B) Cell cycle profiles of A431-TR25-EGFR-AS cells 24 h after irradiation. (C) Determination of apoptosis by TUNEL assay in A431-TR25-EGFR-AS cells 24 h after irradiation. Data are the means of five separate experiments (±SEM).
In addition to the observed impact on cell cycle progression, loss of basal p21 expression increased the basal level of apoptosis twofold and enhanced the ability of either MAPK inhibition or radiation to cause apoptosis 24 h after exposure (Figure 9C). Loss of basal p21 expression also blunted the ability of MAPK inhibition to potentiate radiation-induced apoptosis. These data demonstrate that basal expression of p21 inhibits apoptosis and that loss of basal p21 increases the ability of stresses such as MAPK inhibition and irradiation to cause apoptosis.
DISCUSSION
These studies were initiated to further our understanding of the role of p21 in the mechanism by which inhibition of the MAPK pathway sensitizes squamous and mammary carcinoma cells to the cytotoxic effects of ionizing radiation. Our studies demonstrated that radiation causes immediate primary and prolonged secondary activation of the MAPK pathway via the EGFR. Activation of the MAPK pathway was dependent on MEK1/2, as judged by the abilities of the chemically dissimilar MEK1/2 inhibitors PD98059 and U0126 to block activation.
Previously, we had shown that MAPK inhibition blocked radiation-induced expression of the cdk inhibitor p21 and that this correlated with increased apoptosis (Carter et al., 1998). Thus, further investigations were initiated to examine in more detail the interactions between radiation, MAPK signaling, p21 expression, cell cycle control, and apoptosis. We demonstrated that the prolonged second-phase MAPK activation was responsible for increased expression of p21 protein and that a portion of this radiation-induced increase in p21 expression required de novo transcription, as judged by a MAPK-dependent increase in full-length p21 promoter activity. However, at least 50% of this increase in p21 protein expression was independent of an altered transcription rate, in partial agreement with the study by Johannessen et al. (1999). In contrast, Bromberg et al. (1998) have suggested that EGFR signaling increases p21 protein levels in A431 cells via transcriptional regulation of the p21 promoter by the Stat1 transcription factor. The differences in mechanism between our studies may be attributable to the fact that low-dose ionizing radiation activates the EGFR and MAPK pathway to a much lesser extent than the high concentrations of natural ligand used in these studies. In addition, it is probable that continual exposure to cytotoxic growth-inhibiting concentrations of EGF will recruit different cassettes of signaling pathways in cells than a single exposure of low-dose ionizing radiation.
Surprisingly, radiation also was found to reduce the activity of a truncated p21 promoter. This truncated promoter lacked the enhancer region binding sites for p53 and C/EBP transcription factors but contained consensus binding proximal sequences for the factors Stat1 and Sp1. Recently, several groups have argued that signaling from the stress-regulated Rho GTPase increases Sp1 DNA-binding ability and mediates a negative signal toward protein expression of p21 (Adnane et al., 1998; Auer et al., 1998b; Olson et al., 1998). We have found that dominant negative Rho N19 blunted the ability of radiation to reduce truncated p21 promoter activity (our unpublished results). Collectively, these data argue that radiation initiates both positive and negative signals toward the p21 promoter. Further studies beyond the scope of this paper will be required to determine how radiation and MAPK signaling control p21 promoter function and alter the stability of p21 mRNA/protein levels.
Several studies have demonstrated that a loss of p21 expression correlates with increased radiosensitivity (Deng et al., 1995; Macleod et al., 1995; Palmer et al., 1998; Reed et al., 1998; Xu et al., 1998). Because the ability of MAPK inhibition to potentiate radiation-induced apoptosis correlated with a loss of p21 expression, we also examined cell cycle profiles of cells exposed to radiation during a 48-h time course. Radiation caused a very modest but significant (5%) increase in the percentage of A431-TR25-EGFR-AS cells in G1 6 h after irradiation. This arrest was dependent on the function of increased expression of p21. This arrest was not observed in MDA-TR15-EGFR-CD533 cells, even though p21 protein levels were increased by radiation. This may be due to a smaller induction of MAPK/p21 in the MDA-TR15-EGFR-CD533 cell line. In contrast, radiation increased the percentage of cells in G2/M at 6 h by 300% in both cell types, and this increase did not appear to be dependent on expression of p21. Recently, Bunz et al. (1998) argued that the ability of radiation to cause G2/M arrest is dependent on the functions of both p53 and p21 in fibroblasts. In contrast, the epithelial carcinoma cells used in this study express a nonfunctional p53 protein, and a reduced ability of radiation to increase p21 protein levels did not significantly alter the ability of radiation to induce G2/M arrest at 6 h (Poon et al., 1996).
We found that 24 h after irradiation, the percentage of cells found in each phase of the cell cycle had returned to near control levels. However, in irradiated cells that had their ability to activate MAPK blocked, the percentage of cells present in G2/M phase remained elevated, in agreement with data of Vrana et al. (1999) and Abbott and Holt (1999). Washing of cells to remove MEK1/2 inhibitors or caffeine treatment of cells 6 h after irradiation abrogated the ability of MAPK inhibition to both enhance radiation-stimulated increases in the number of G2/M phase cells and potentiate radiation-induced apoptosis. Bonner et al. (1998) recently suggested that MAPK activation does not influence survival of irradiated squamous carcinoma cells. The most likely explanation for differences between the present study and that of Bonner et al. is that those authors performed their experiments with the use of growth-arrested cells and removed MEK inhibitor within 6 h of irradiation. These conditions would preclude a prolonged increase in the number of cells present in G2/M, in contrast to our observations with proliferating cells.
Both positive and negative roles for MAPK signaling in mediating the G2/M transition, including a role in the dephosphorylation and activation of Cdc2, have been documented (Tamemoto et al., 1992; Watanabe et al., 1995; Abrieu et al., 1997; Laird and Shalloway, 1997; Walter et al., 1997; Bitangcol et al., 1998; Cross and Smythe, 1998; Takenaka et al., 1998). Our results suggest that low doses of radiation induce a transient increase in the numbers of G2/M cells, which subsequently relaxes, allowing normal cell cycle progression at 24 h. Inhibition of MAPK pathway activation modified this relaxation process, either by causing an irreversible growth arrest in a portion of the cells or by slowing G2/M progression itself. This elevation was associated with increased Cdc2 tyrosine 15 phosphorylation/reduced Cdc2 activity and lower Cdc25C expression (Poon et al., 1996, 1997; Leach et al., 1998; Yu et al., 1998). This finding also correlated with increased apoptosis in the G2/M population of cells, as judged by the correlation of apoptotic DNA fragments and cyclin B1 staining with the use of bivariate FACScan analysis (our unpublished data). However, it is still possible that apoptosis occurs in other portions of the cell cycle. Further studies will be needed to definitively answer this question. We suggest that interruption of the MAPK pathway increases radiation-induced cell death in carcinoma cells through a cell cycle–related mechanism within G2 and M phases.
Our data tend to favor a mechanism in which MAPK inhibition modifies the numbers of cells present in G2/M arrest by inhibiting the function of the Cdc2 tyrosine 15 phosphatase (Cdc25C). We saw a reduction in Cdc25C protein levels. This is in general agreement with recent studies by Blasina et al. (1997, 1999), who argued that ionizing radiation can inhibit the activities of Cdc2 and Cdc25C. The protein kinases that phosphorylate and inactivate Cdc25C, Chk1 and Chk2, were not investigated in our study (Furnari et al., 1997; Matsuoka et al., 1998). Another potential mechanism to increase Cdc2 tyrosine 15 phosphorylation is to increase the activity of the protein kinase that phosphorylates this site (Wee1) (Watanabe et al., 1995; Leach et al., 1998). Further studies will be required to understand MAPK’s role, if any, in Wee1 function and regulation.
Surprisingly, we found that loss of basal p21 expression abrogated the ability of irradiated/MAPK-inhibited cells to maintain cell numbers in G2/M phase at 24 h, in partial agreement with the findings of Bunz et al. (1998). This is in contrast to the ability of MAPK inhibition to block stimulation of p21 levels, which did not play a role in the increased number of cells observed in G2/M at 6 h (Dulic et al., 1998). Loss of basal p21 function doubled the basal rate of apoptosis and increased the ability of stresses to cause apoptosis, in general agreement with the findings of Ruan et al. (1999). In addition, the increased rate of apoptosis attributable to lack of stimulated p21 expression was not caused by an increased ability of cells to enter S phase after exposure to radiation, as judged by our data with mimosine to mimic G1/S phase arrest. Recently, our group has also argued that loss of basal p21 expression sensitizes leukemic cells to cytotoxic stimuli and that MAPK inhibition could not potentiate drug-induced apoptosis in these cells (Wang et al., 1998). Collectively, these data suggest that basal expression of p21 plays a general antiapoptotic role in carcinoma cells. In contrast, radiation-stimulated p21 levels appear to play a lesser role in blunting radiation-induced apoptosis. Furthermore, our data imply that findings obtained with cells that are either p21 null or stably transfected with p21 antisense mRNA may not be identical to data obtained with cells that have their p21 expression held at basal levels.
Several groups have argued that p21 may function both as a cyclin kinase inhibitor protein and as a scaffold protein (Chen et al., 1995, 1996; Morgan, 1995; Sherr and Roberts, 1995). No large increase in coimmunoprecipitating p21 was seen with Cdc2 during the time course of our studies (our unpublished observations). In contrast, it has been suggested that p21, functioning as a scaffold protein, can both increase and/or decrease the interactions of cyclin molecules with cdks, thereby regulating kinase function (Chellappan et al., 1998). Ongoing studies are addressing whether basal p21 expression plays a role in the regulation of cyclin-Cdc2 complex formation and Cdc2 activity in the increased numbers of cells in G2/M at 24 h.
ACKNOWLEDGMENTS
The authors thank Mrs. Julie Farnsworth for the expansion of the p21Cip-1/WAF1/MDA6 antisense adenovirus and Drs. R. Chinery and W. El Diery for the use of p21-luciferase constructs. The authors also thank Drs. J. Sebolt-Leopold and J. Trzaskos for providing PD98059 and U0126, respectively. This work was funded by Public Health Service grant R01DK52825, Department of Defense Career Development Award BC980148, a fellowship from the Jim Valvano V Foundation, and grant J-464 from the Jeffress Research Foundation to P.D.; by Public Health Service grants P01CA72955 and R01CA65896 to R.S.-U.; and by National Cancer Institute grants R01CA35675 and R01CA74468 and a gift from the Chernow Endowment to P.B.F. This manuscript is dedicated to the memory of Alfred Wight, MRCVS, in the hope of a cure for all creatures great and small.
Abbreviations:
- AS
antisense
- EGFR
epidermal growth factor receptor
- MBP
myelin basic protein
- m.o.i.
multiplicity of infection
- TUNEL
terminal uridyl-nucleotide end labeling
REFERENCES
- Abbott DW, Holt JT. Mitogen-activated protein kinase kinase 2 activation is essential for progression through the G2/M checkpoint arrest in cells exposed to ionizing radiation. J Biol Chem. 1999;274:2732–2742. doi: 10.1074/jbc.274.5.2732. [DOI] [PubMed] [Google Scholar]
- Abrieu A, Fisher D, Simon MN, Doree M, Picard A. MAPK inactivation is required for the G2 to M-phase transition of the first mitotic cell cycle. EMBO J. 1997;16:6407–6413. doi: 10.1093/emboj/16.21.6407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adnane J, Bizouarn FA, Qian Y, Hamilton AD, Sebti SM. p21(WAF1/CIP1) is up-regulated by the geranylgeranyltransferase I inhibitor GGTI-298 through a transforming growth factor beta- and Sp1-responsive element: involvement of the small GTPase rhoA. Mol Cell Biol. 1998;18:6962–6970. doi: 10.1128/mcb.18.12.6962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auer K, et al. The Ras/Rac1/Cdc42/SEK/JNK/c-Jun cascade is a key pathway by which agonists stimulate DNA synthesis in primary cultures of rat hepatocytes. Mol Biol Cell. 1998a;9:561–573. doi: 10.1091/mbc.9.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auer KL, Seth P, Coffey RJ, DePinho R, Fisher PB, Dent P. Prolonged activation of the mitogen-activated protein kinase pathway promotes DNA synthesis in primary hepatocytes from p21Cip-1/WAF1-null mice, but not in hepatocytes from p16INK4a-null mice. Biochem J. 1998b;336:551–560. doi: 10.1042/bj3360551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaban N, Moni J, Shannon M, Dang L, Murphy E, Goldkorn T. The effect of ionizing radiation on signal transduction: antibodies to EGF receptor sensitize A431 cells to radiation. Biochim Biophys Acta. 1996;1314:147–156. doi: 10.1016/s0167-4889(96)00068-7. [DOI] [PubMed] [Google Scholar]
- Baselga J, Mendelson J, Kim Y-M, Pandiella A. Autocrine regulation of membrane transforming growth factor-α cleavage. J Biol Chem. 1996;271:3279–3284. doi: 10.1074/jbc.271.6.3279. [DOI] [PubMed] [Google Scholar]
- Bissonnette N, Hunting DJ. p21-induced cycle arrest in G1 protects cells from apoptosis induced by UV-irradiation or RNA polymerase II blockage. Oncogene. 1998;16:3461–3469. doi: 10.1038/sj.onc.1201899. [DOI] [PubMed] [Google Scholar]
- Bitangcol JC, Chau AS, Stadnick E, Lohka MJ, Dicken B, Shibuya EK. Activation of the p42 mitogen-activated protein kinase pathway inhibits Cdc2 activation and entry into M-phase in cycling Xenopus egg extracts. Mol Biol Cell. 1998;9:451–467. doi: 10.1091/mbc.9.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasina A, de Weyer IV, Laus MC, Luyten WHML, Parker AE, McGowan CH. A human homologue of the checkpoint kinase cds1 directly inhibits cdc25 phosphatase. Curr Biol. 1999;14:1–10. doi: 10.1016/s0960-9822(99)80041-4. [DOI] [PubMed] [Google Scholar]
- Blasina A, Paegle ES, McGowan CH. The role of inhibitory phosphorylation of CDC2 following DNA replication block and radiation-induced damage in human cells. Mol Biol Cell. 1997;8:1013–1023. doi: 10.1091/mbc.8.6.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner JA, Vroman BT, Christianson TJ, Karnitz LM. Ionizing radiation-induced MEK and Erk activation does not enhance survival of irradiated human squamous carcinoma cells. Int J Radiat Oncol Biol Phys. 1998;42:921–925. doi: 10.1016/s0360-3016(98)00325-3. [DOI] [PubMed] [Google Scholar]
- Bromberg JF, Fan Z, Brown C, Mendelsohn J, Darnell JE., Jr Epidermal growth factor-induced growth inhibition requires Stat1 activation. Cell Growth Differ. 1998;9:505–512. [PubMed] [Google Scholar]
- Bunz F, Dutriaux A, Lengauer C, Waldman T, Zhou S, Brown JP, Sedivy JM, Kinzler KW, Vogelstein B. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science. 1998;282:1497–1501. doi: 10.1126/science.282.5393.1497. [DOI] [PubMed] [Google Scholar]
- Butz K, Geisen C, Ullmann A, Zentgraf H, Hoppe-Seyler F. Uncoupling of p21WAF1/CIP1/SDI1 mRNA and protein expression upon genotoxic stress. Oncogene. 1998;17:781–787. doi: 10.1038/sj.onc.1201995. [DOI] [PubMed] [Google Scholar]
- Carter S, Auer KL, Birrer M, Fisher PB, Schmidt-Ullrich R, Valerie K, Mikkelsen R, Dent P. Inhibition of mitogen activated protein kinase cascade potentiates cell killing by low dose ionizing radiation in A431 human squamous carcinoma cells. Oncogene. 1998;16:2787–2796. doi: 10.1038/sj.onc.1201802. [DOI] [PubMed] [Google Scholar]
- Chellappan SP, Giordano A, Fisher PB. Role of cyclin-dependent kinases and their inhibitors in cellular differentiation and development. Curr Top Microbiol Immunol. 1998;227:57–103. doi: 10.1007/978-3-642-71941-7_4. [DOI] [PubMed] [Google Scholar]
- Chen J, Jackson PK, Kirschner MW, Dutta A. Separate domains of p21 involved in the inhibition of Cdk kinase and PCNA. Nature. 1995;374:386–388. doi: 10.1038/374386a0. [DOI] [PubMed] [Google Scholar]
- Chen U, Chen S, Saha P, Dutta A. p21Cip1/Waf1 disrupts the recruitment of human Fen1 by proliferating-cell nuclear antigen into the DNA replication complex. Proc Natl Acad Sci USA. 1996;93:11597–11602. doi: 10.1073/pnas.93.21.11597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinery R, Brockman JA, Peeler MO, Shyr Y, Beauchamp RD, Coffey RJ. Antioxidants enhance the cytotoxicity of chemotherapeutic agents in colorectal cancer: a p53-independent induction of p21WAF1/CIP1 via C/EBPbeta. Nat Med. 1997;3:1233–1241. doi: 10.1038/nm1197-1233. [DOI] [PubMed] [Google Scholar]
- Chmura SJ, Mauceri HJ, Advani S, Heimann R, Nodzenski E, Quintans J, Kufe DW, Weichselbaum RR. Decreasing the apoptotic threshold of tumor cells through protein kinase C inhibition and sphingomyelinase activation increases tumor cell killing by ionizing radiation. Cancer Res. 1997;57:4340–4347. [PubMed] [Google Scholar]
- Contessa JN, Reardon DB, Todd D, Dent P, Mikkelsen RB, Valerie K, Bower GD, Schmidt-Ullrich RK. The inducible expression of dominant negative epidermal growth factor receptor CD533 in radiosensitization of human mammary carcinoma cells. Clin Cancer Res. 1999;5:405–411. [PubMed] [Google Scholar]
- Cram EJ, Ramos RA, Wang EC, Cha HH, Nishio Y, Firestone GL. Role of the CCAAT/enhancer binding protein-alpha transcription factor in the glucocorticoid stimulation of p21waf1/cip1 gene promoter activity in growth-arrested rat hepatoma cells. J Biol Chem. 1998;273:2008–2014. doi: 10.1074/jbc.273.4.2008. [DOI] [PubMed] [Google Scholar]
- Cross DA, Smythe C. PD 98059 prevents establishment of the spindle assembly checkpoint and inhibits the G2-M transition in meiotic but not mitotic cell cycles in Xenopus. Exp Cell Res. 1998;241:12–22. doi: 10.1006/excr.1998.4023. [DOI] [PubMed] [Google Scholar]
- Deng C, Zhang P, Harper JW, Elledge SJ, Leder P. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell. 1995;82:675–684. doi: 10.1016/0092-8674(95)90039-x. [DOI] [PubMed] [Google Scholar]
- Dent P, Jarvis WD, Birrer MJ, Fisher PB, Schmidt-Ullrich RK, Grant S. The roles of signaling by the p42/44 mitogen activated protein kinase pathway: a potential route to radio- and chemo-sensitization of tumor cells resulting in the induction of apoptosis and loss of clonogenicity. Leukemia. 1998;12:1843–1850. doi: 10.1038/sj.leu.2401222. [DOI] [PubMed] [Google Scholar]
- Dent P, Reardon DB, Park JS, Bowers G, Logsdon C, Valerie K, Schmidt-Ulrich R. Radiation-induced release of transforming growth factor α activates the epidermal growth factor receptor and mitogen-activated protein kinase pathway in carcinoma cells, leading to increased proliferation and protection from radiation-induced cell death. Mol Biol Cell. 1999;10:2493–2506. doi: 10.1091/mbc.10.8.2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulic V, Stein GH, Far DF, Reed SI. Nuclear accumulation of p21Cip1 at the onset of mitosis: a role at the G2/M-phase transition. Mol Cell Biol. 1998;18:546–557. doi: 10.1128/mcb.18.1.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckardt-Schupp F, Klaus C. Radiation inducible DNA repair processes in eukaryotes. Biochimie. 1999;81:161–171. doi: 10.1016/s0300-9084(99)80049-2. [DOI] [PubMed] [Google Scholar]
- Favata MF, et al. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem. 1998;273:18623–18632. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- Fiddes RJ, Janes PW, Sivertsen SP, Sutherland RL, Musgrove EA, Daly RJ. Inhibition of the MAP kinase cascade blocks heregulin-induced cell cycle progression in T-47D human breast cancer cells. Oncogene. 1998;16:2803–2813. doi: 10.1038/sj.onc.1201815. [DOI] [PubMed] [Google Scholar]
- Furnari B, Rhind N, Russell P. Cdc25 mitotic inducer targeted by chk1 DNA damage checkpoint kinase. Science. 1997;277:1495–1497. doi: 10.1126/science.277.5331.1495. [DOI] [PubMed] [Google Scholar]
- Goldkorn T, Balaban N, Shannon M, Matsukuma K. EGF receptor phosphorylation is affected by ionizing radiation. Biochim Biophys Acta. 1997;1358:289–299. doi: 10.1016/s0167-4889(97)00063-3. [DOI] [PubMed] [Google Scholar]
- Jakus J, Yeudall WA. Growth inhibitory concentrations of EGF induce p21 (WAF1/Cip1) and alter cell cycle control in squamous carcinoma cells. Oncogene. 1996;12:2369–2376. [PubMed] [Google Scholar]
- Jarvis WD, Fornari FA, Tombes RM, Martin HA, Erukulla RK, Bittman R, Schwartz GK, Dent P, Grant S. Evidence for involvement of mitogen activated protein kinase, rather than stress activated protein kinase, in potentiation of 1-β-d-arabinofuranosylcytosine-induced apoptosis by interruption of protein kinase C signaling. Mol Pharmacol. 1998;54:844–856. doi: 10.1124/mol.54.5.844. [DOI] [PubMed] [Google Scholar]
- Johannessen LE, Knardal SL, Madshus IH. Epidermal growth factor increases the level of the cyclin-dependent kinase (CDK) inhibitor p21/CIP1 (CDK-interacting protein 1) in A431 cells by increasing the half-lives of the p21/CIP1 transcript and the p21/CIP1 protein. Biochem J. 1999;337:599–606. [PMC free article] [PubMed] [Google Scholar]
- Kavanagh BD, Dent P, Schmidt-Ullrich RK, Chen P, Mikkelsen RB. Calcium-dependent stimulation of mitogen-activated protein kinase activity in A431 cells by low doses of ionizing radiation. Radiat Res. 1998;149:579–587. [PubMed] [Google Scholar]
- Laird AD, Shalloway D. Oncoprotein signaling and mitosis. Cell Signal. 1997;9:249–255. doi: 10.1016/s0898-6568(96)00176-3. [DOI] [PubMed] [Google Scholar]
- Leach SD, Scatena CD, Keefer CJ, Goodman HA, Song SY, Yang L, Pietenpol JA. Negative regulation of Wee1 expression and Cdc2 phosphorylation during p53-mediated growth arrest and apoptosis. Cancer Res. 1998;58:3231–3236. [PubMed] [Google Scholar]
- Levenson AS, Tonetti DA, Jordan VC. The estrogen-like effect of 4-hydroxytamoxifen on induction of transforming growth factor alpha mRNA in MDA-MB-231 breast cancer cells stably expressing the estrogen receptor. Br J Cancer. 1998;77:1812–1819. doi: 10.1038/bjc.1998.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macleod KF, Sherry N, Hannon G, Beach D, Tokino T, Kinzler K, Vogelstein B, Jacks T. p53-dependent and independent expression of p21 during cell growth, differentiation, and DNA damage. Genes Dev. 1995;9:935–944. doi: 10.1101/gad.9.8.935. [DOI] [PubMed] [Google Scholar]
- Matusoka S, Huang M, Elledge SJ. Linkage of ATM to cell cycle regulation by the Chk2 protein kinase. Science. 1998;282:1893–1897. doi: 10.1126/science.282.5395.1893. [DOI] [PubMed] [Google Scholar]
- Morgan DO. Principles of CDK regulation. Nature. 1995;374:131–134. doi: 10.1038/374131a0. [DOI] [PubMed] [Google Scholar]
- O’Connor PM. Mammalian G1 and G2 phase checkpoints. Cancer Surv. 1997;29:151–182. [PubMed] [Google Scholar]
- Olson MF, Paterson HF, Marshall CJ. Signals from Ras and Rho GTPases interact to regulate expression of p21 Waf1/Cip1. Nature. 1998;394:295–299. doi: 10.1038/28425. [DOI] [PubMed] [Google Scholar]
- Palmer A, Gavin AC, Nebreda AR. A link between MAP kinase and p34(cdc2)/cyclin B during oocyte maturation: p90(rsk) phosphorylates and inactivates the p34(cdc2) inhibitory kinase Myt1. EMBO J. 1998;17:5037–5047. doi: 10.1093/emboj/17.17.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon RYC, Chau MS, Yamashita K, Hunter T. The role of Cdc2 feedback loop control in the DNA damage checkpoint in mammalian cells. Cancer Res. 1997;57:5168–5178. [PubMed] [Google Scholar]
- Poon RYC, Jiang W, Toyoshima H, Hunter T. Cyclin-dependent kinases are inactivated by a combination of p21 and Thr-14/Tyr-15 phosphorylation after UV-induced DNA damage. J Biol Chem. 1996;271:13283–13291. doi: 10.1074/jbc.271.22.13283. [DOI] [PubMed] [Google Scholar]
- Putz T, Culig Z, Eder IE, Nessler-Menardi C, Bartsch G, Grunicke H, Uberall F, Klocker H. Epidermal growth factor (EGF) receptor blockade inhibits the action of EGF, insulin-like growth factor I, and a protein kinase A activator on the mitogen-activated protein kinase pathway in prostate cancer cell lines. Cancer Res. 1999;59:227–233. [PubMed] [Google Scholar]
- Reed MF, Liu VF, Ladha MH, Ando K, Griffin JD, Weaver DT, Ewen ME. Enforced CDK4 expression in a hematopoietic cell line confers resistance to the G1 arrest induced by ionizing radiation. Oncogene. 1998;17:2961–2971. doi: 10.1038/sj.onc.1202450. [DOI] [PubMed] [Google Scholar]
- Rigberg DA, Blinman TA, Kim FS, Cole MA, McFadden DW. Antisense blockade of p21/WAF1 decreases radiation-induced G2 arrest in esophageal squamous cell carcinoma. J Surg Res. 1999;81:6–10. doi: 10.1006/jsre.1998.5483. [DOI] [PubMed] [Google Scholar]
- Rigberg DA, Kim FS, Blinman TA, Cole MA, Lane JS, So J, McFadden DW. p21 expression is increased by irradiation in esophageal squamous carcinoma. J Surg Res. 1998;76:137–142. doi: 10.1006/jsre.1998.5308. [DOI] [PubMed] [Google Scholar]
- Rosette C, Karin M. UV light and osmotic stress: activation of the JNK cascade through multiple growth factor and cytokine receptors. Science. 1996;274:1194–1197. doi: 10.1126/science.274.5290.1194. [DOI] [PubMed] [Google Scholar]
- Ruan S, Okcu MF, Pong RC, Andreeff M, Levin V, Hsieh JT, Zhang W. Attenuation of WAF1/Cip1 expression by an antisense adenovirus expression vector sensitizes glioblastoma cells to apoptosis induced by chemotherapeutic agents 1,3-bis(2-chloroethyl)-1-nitrosourea and cisplatin. Clin Cancer Res. 1999;5:197–202. [PubMed] [Google Scholar]
- Santana P, Pena LA, Haimovitz-Friedman A, Martin S, Green D, McLoughlin M, Cordon-Cardo C, Schuchman EH, Fuks Z, Kolesnik R. Acid sphingomyelinase-deficient human lymphoblasts and mice are defective in radiation-induced apoptosis. Cell. 1996;86:189–199. doi: 10.1016/s0092-8674(00)80091-4. [DOI] [PubMed] [Google Scholar]
- Schmidt-Ullrich RK, Mikkelsen RB, Dent P, Todd DG, Valerie K, Kavanagh BD, Contessa JN, Rorrer WK, Chen PB. Radiation-induced proliferation of the human A431 squamous carcinoma cells is dependent on EGFR tyrosine phosphorylation. Oncogene. 1997;15:1191–1197. doi: 10.1038/sj.onc.1201275. [DOI] [PubMed] [Google Scholar]
- Sewing A, Wiseman B, Lloyd AC, Land H. High-intensity Raf signal causes cell cycle arrest mediated by p21Cip1. Mol Cell Biol. 1997;17:5588–5597. doi: 10.1128/mcb.17.9.5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr CJ, Roberts JM. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995;9:1149–1163. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- Suy S, Anderson WB, Dent P, Chang E, Kasid U. Association of Grb2 with Sos and Ras with Raf-1 upon gamma irradiation of breast cancer cells. Oncogene. 1997;15:53–61. doi: 10.1038/sj.onc.1201165. [DOI] [PubMed] [Google Scholar]
- Takenaka K, Moriguchi T, Nishida E. Activation of the protein kinase p38 in the spindle assembly checkpoint and mitotic arrest. Science. 1998;280:599–602. doi: 10.1126/science.280.5363.599. [DOI] [PubMed] [Google Scholar]
- Tamemoto H, Kadowaki T, Tobe K, Ueki K, Izumi T, Chatani Y, Kohno M, Kasuga M, Yazaki Y, Akanuma Y. Biphasic activation of two mitogen-activated protein kinases during the cell cycle in mammalian cells. J Biol Chem. 1992;267:20293–20297. [PubMed] [Google Scholar]
- Tombes R, Auer KL, Brenz-Verca S, Marshall CJ, McMahon M, Mikkelsen RS, Valerie K, Wymann MP, Dent P. The mitogen-activated protein (MAP) kinase cascade can either stimulate or inhibit DNA synthesis in primary cultures of rat hepatocytes depending upon whether its activation is acute/phasic or chronic. Biochem J. 1998;330:1451–1460. doi: 10.1042/bj3301451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valerie K, Singhal A. Host-cell reactivation of reporter genes introduced into cells by adenovirus as a convenient way to measure cellular DNA repair. Mutat Res. 1995;336:91–100. doi: 10.1016/0921-8777(94)00046-9. [DOI] [PubMed] [Google Scholar]
- Verheij M, et al. Requirement for ceramide-initiated SAPK/JNK signaling in stress-induced apoptosis. Nature. 1996;380:75–79. doi: 10.1038/380075a0. [DOI] [PubMed] [Google Scholar]
- Vrana J, Grant S, Dent P. Inhibition of the MAPK pathway abrogates BCL-2-mediated survival of leukemia cells after exposure to low-dose ionizing radiation. Radiat Res. 1999;151:559–569. [PubMed] [Google Scholar]
- Walter SA, Guadango TM, Ferrell JE. Induction of a G2-phase arrest in Xenopus egg extracts by activation of p42 mitogen-activated protein kinase. Mol Biol Cell. 1997;8:2157–2169. doi: 10.1091/mbc.8.11.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Guo CY, Castillo A, Dent P, Grant S. Effect of bryostatin 1 on taxol-induced apoptosis and cytotoxicity in human leukemia cells (U937) Biochem Pharmacol. 1997;56:635–644. doi: 10.1016/s0006-2952(98)00188-9. [DOI] [PubMed] [Google Scholar]
- Watanabe N, Broome M, Hunter T. Regulation of the human WEE1Hu CDK tyrosine 15-kinase during the cell cycle. EMBO J. 1997;14:1878–1891. doi: 10.1002/j.1460-2075.1995.tb07180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods D, Parry D, Cherwinski H, Bosch E, Lees E, McMahon M. Raf-induced proliferation or cell cycle arrest is determined by the level of Raf activity with arrest mediated by p21Cip1. Mol Cell Biol. 1997;17:5598–5611. doi: 10.1128/mcb.17.9.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- Xu Y, Yang EM, Brugarolas J, Jacks T, Baltimore D. Involvement of p53 and p21 in cellular defects and tumorigenesis in Atm-/- mice. Mol Cell Biol. 1998;18:4385–4390. doi: 10.1128/mcb.18.7.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Orlandi L, Wang P, Orr MS, Senderowicz AM, Sausville EA, Silvestrini R, Watanabe N, Piwnica-Worms H, O’Connor PM. UCN-01 abrogates G2 arrest through a Cdc2-dependent pathway that is associated with inactivation of the Wee1Hu kinase and activation of the Cdc25C phosphatase. J Biol Chem. 1998;273:33455–33464. doi: 10.1074/jbc.273.50.33455. [DOI] [PubMed] [Google Scholar]