Abstract
Hemorrhagic shock is a leading cause of death in trauma patients worldwide. Bleeding control, maintenance of tissue oxygenation with fluid resuscitation, coagulation support, and maintenance of normothermia remain mainstays of therapy for patients with hemorrhagic shock. Although now widely practised as standard in the USA and Europe, shock resuscitation strategies involving blood replacement and fluid volume loading to regain tissue perfusion and oxygenation vary between trauma centers; the primary cause of this is the scarcity of published evidence and lack of randomized controlled clinical trials. Despite enormous efforts to improve outcomes after severe hemorrhage, novel strategies based on experimental data have not resulted in profound changes in treatment philosophy. Recent clinical and experimental studies indicated the important influences of sex and genetics on pathophysiological mechanisms after hemorrhage. Those findings might provide one explanation why several promising experimental approaches have failed in the clinical arena. In this respect, more clinically relevant animal models should be used to investigate pathophysiology and novel treatment approaches. This review points out new therapeutic strategies, namely immunomodulation, cardiovascular maintenance, small volume resuscitation, and so on, that have been introduced in clinics or are in the process of being transferred from bench to bedside. Control of hemorrhage in the earliest phases of care, recognition and monitoring of individual risk factors, and therapeutic modulation of the inflammatory immune response will probably constitute the next generation of therapy in hemorrhagic shock. Further randomized controlled multicenter clinical trials are needed that utilize standardized criteria for enrolling patients, but existing ethical requirements must be maintained.
Introduction
Trauma is the leading cause of death worldwide in persons aged between 5 and 44 years, and it has an impact in every community regardless of demographics [1,2]. Up to 50% of early deaths are due to massive hemorrhage, which is a major contributor to the dilemmas associated with traumatic injury and its care [3]. Studies have shown that hemorrhagic shock is a predictor of poor outcome in the injured patient. Early hypotension with hemorrhage in the field or at initial hospital evaluation is associated with complications such as multiple organ failure (MOF) and the development of secondary infection such as pneumonia and sepsis [4-6].
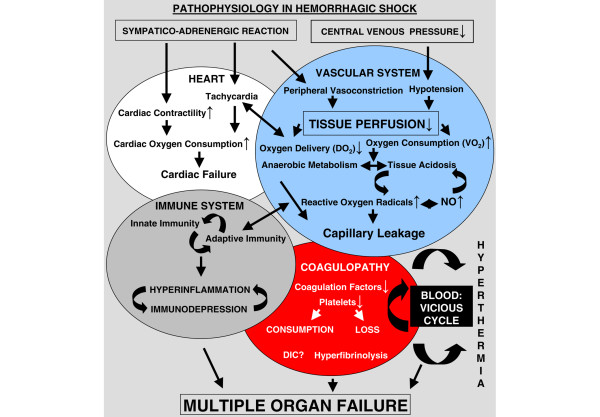
The complex pathophysiology of hemorrhagic shock is summarized in Figure 1. Briefly, the 'shock syndrome' during massive bleeding reflects an imbalance between systemic oxygen delivery and oxygen consumption [7,8]. Blood loss leads sequentially to hemodynamic instability, coagulopathy, decreased oxygen delivery, decreased tissue perfusion, and cellular hypoxia [3]. Such alterations lay the foundations for subsequent development of MOF, a systemic inflammatory process that leads to dysfunction of different vital organs and accounts for high mortality rates [9]. The pathogenesis of organ injury secondary to hypovolemic insult is still incompletely understood, but both experimental studies and clinical observations suggest that leukocytes, in particular macrophages, are activated by translocated bacterial endotoxin and hypoxia/reoxygenation [10,11]. Activated Kupffer cells release pathologically active substances such as inflammatory cytokines, reactive oxygen species, and nitric oxide, all of which may participate in the mechanisms of hemorrhagic shock [11,12]. Moreover, increased free radical production during hemorrhagic shock and resuscitation gives rise to increased oxidative stress, which contributes to organ damage [13,14]. In addition, data confirm that the biologic precondition (namely sex, age, and genetic background) plays an important role in the response to hemorrhage and therapy.
Figure 1.
Illustration of the pathophysiological changes in hemorrhagic shock. DIC, disseminated intravascular coagulopathy; NO, nitric oxide.
It is commonly accepted that bleeding control, damage control surgery using interventional radiology, maintenance of tissue oxygenation with fluid resuscitation, coagulation support, and maintenance of normothermia represent basic support measures in hemorrhage care that can pre-empt or rapidly reverse hypoxemia, hypovolemia, and the onset of shock [15-17]. Regarding current resuscitation strategies, management of hemorrhagic shock in the USA and Europe relies heavily on blood replacement and fluid volume loading to restore tissue perfusion [15-17]. Although resuscitation strategies for severely injured patients who present with shock have improved greatly, these transfusions are associated with development of MOF, and increased intensive care unit (ICU) admissions, ICU and hospital length of stay, and mortality [18-21]. Although prevention of injury is plausible and desirable, the complications of traditional treatment modalities should be recognized and further clinical trials conducted to evaluate potential new therapeutic strategies.
Biologic precondition: sex, age, and genetics
It was recently shown that the biologic precondition of individuals (namely sex, age, and genetics) has a profound impact on immune response after trauma/hemorrhage [22]. Although sex-specific differences in susceptibility to and morbidity resulting from sepsis have been observed in several epidemiologic and clinical studies [23-26], little attention has been given to sex-specific differences in the immune response to trauma and severe blood loss. Furthermore, experimental studies investigating alterations in immune functions after trauma have historically used male laboratory animals. In this respect, a number of studies have demonstrated diverse immune responses in male and female mice after trauma/hemorrhage [27]. Higher plasma estradiol and/or higher plasma prolactin levels might contribute to enhanced immune response after hemorrhage in pro-estrus females. Administration of estrogen in castrated male mice, even with testosterone supplementation, improved the depressed immune responses after trauma/hemorrhage [28]. Moreover, treatment of intact male mice with estradiol normalized the depressed immune responses after trauma and hemorrhage, and improved the survival rate after subsequent sepsis [29]. A prospective study conducted in more than 4,000 trauma patients recently demonstrated that hormonally active women tolerate trauma/shock better than men [30].
Dehydroepiandrosterone (DHEA) has also been reported to have estrogenic effects [31]. Treatment of male mice with DHEA after trauma and blood loss prevented immuno-suppression and decreased susceptibility of those mice to subsequent sepsis [32]. Because DHEA is used clinically as a long-term immuno-enhancing drug, this hormone might represent a useful therapy for preventing immunodepression in surgical patients. Recently, DHEA was found to stimulate the release of proinflammatory cytokines from human peripheral blood mononuclear cells after major abdominal surgery in vitro [33]. A prospective clinical trial, however, must be conducted to validate the immunoprotective effects of DHEA in patients after severe blood loss. In addition to female sex steroids, the lower levels of male hormones in female animals as compared with males might also contribute to the divergent immunoresponsiveness observed after injury and blood loss. Depletion of testosterone by castration before hemorrhage or by administration of flutamide after hemorrhage and resuscitation normalized the depressed immune responses and maintained cardiovascular function in male animals [27]. Because flutamide is used clinically without major side effects, this might represent a clinically relevant therapeutic approach in trauma victims.
In addition, age must be taken into account when extrapolating experimental results into clinics. This is because recent studies have indicated that aged male and female mice exhibit different sex-specific immune responses than do young animals [34,35].
The availability of improved techniques for molecular diagnosis has allowed investigation of the roles played by genetic variations in the inflammatory responses to trauma/hemorrhage. New diagnostic tools have revealed the importance of genetic background on pathophysiological mechanisms and their modulation after shock [36]. In this respect, clinical studies revealed that IL-6-174G/C polymorphism is associated with increased severity of post-traumatic systemic inflammatory response syndrome [37], suggesting an adverse outcome in those patients. Furthermore, it has been shown that the 1,082 IL-10 polymorphism is associated with acute respiratory failure after major trauma [38]. In addition, specific genetic variations in the mitochondrial DNA that influence energy production and free radical generation appear to increase the risk for inhospital mortality after severe injury [39]. To date, genomics findings in patients have only been used as prognostic factors, and have not been used to direct therapy after hemorrhage. Larger scale studies are needed to improve our understanding of how genetic variability influences responses to post-traumatic complications and pharmacotherapy.
In summary, the above-mentioned studies collectively suggest that sex, age, and genetics reveal potential clinically relevant therapeutic options. However, several promising experimental approaches such as tumor necrosis factor (TNF) antibody and others [9,40] have not been successful in the clinical arena for the treatment of shock because of the redundancy in the inflammatory pathways. Other pathways and cytokines may activate and/or inhibit the system at the same point, and different mediators may function as co-determinants of the inflammatory system in parallel. Therefore, modulating rather than neutralizing a cytokine is the desired objective in the treatment of trauma victims. At present, however, such strategies are in the early stages of investigation. Furthermore, because of the complexity of genetic patterns, it remains difficult to apply genetic preconditions to develop individualized hemorrhage therapy.
Monitoring
In patients who have or have had hemorrhagic shock, heart rate, urine output, capillary refill, systolic blood pressure, and mental status represent the basic parameters that define cardiovascular stability. Appropriate additional monitoring primarily includes arterial pressure (via arterial catheter), arterial hemoglobin oxygen saturation (via pulse oximetry), and central venous pressure (via central venous line) monitoring. Measurement of arterial blood gases with lactate and base deficit determinations indicate the severity of shock, and serial hemoglobin measurements allow assessment of ongoing bleeding [15-17]. The goal is to return blood pressure and heart rate to normal, and establish urine output while maintaining central venous pressure in a moderate range (8 to 15 mmHg) [41]. How specific those traditional parameters are in the clinical arena and which values represent the end-point that should be reached are still under extensive discussion. In particular, restoration of vital signs to normal while uncontrolled hemorrhage is ongoing may be detrimental to the patient, causing volume overload and rebleeding, and thus creating a vicious cycle of events. Therefore, hypotensive resuscitation, whereby safe but subnormal blood pressure is achieved, may represent a future strategy to avoid the problems that result from use of traditional resuscitation strategies [2,42].
Studies indicate that oxygen debt and lactate appear to play important roles in defining the severity of hemorrhagic shock; thus, normalizing these parameters might be appropriate therapeutic end-points [41,43]. Currently, indirect measurement techniques that reflect cellular oxygen utilization and perfusion, either systemically (lactate and base deficit) or locally (gastric intramucosal pH and microdialysis), are primarily used [44]. Experimental and clinical studies have indicated that evaluation of tissue perfusion with measurement of cutaneous and transcutaneous partial oxygen tension (PO2), skeletal muscle PO2, gastric tonometry, pH determination using fiberoptic technology, infrared spectroscopy, and minimally invasive monitoring with measurement of bladder mucosa pH and partial carbon dioxide tension (PCO2) may represent new techniques for monitoring patients with hemorrhagic shock [45-50]. However, at present these technologies require the use of relatively complex or invasive methodologies. Moreover, newer experimental results demonstrate that the findings of continuous monitoring of skeletal muscle and subcutaneous pH, PO2, and PCO2 – as potential surrogates of impaired tissue metabolism – vary among tissues and according to the phase of hemorrhage or resuscitation [51]. New bioimpedance technology to monitor cardiac output has successfully been applied to direct trauma resuscitation in the emergency department setting [52]. Buccal PCO2 measurement and high intensity focused ultrasound also represent new and promising techniques; they have proven to be practical and reliable measurements in the diagnosis of circulatory failure states and to serve as useful indicators of the severity of these states [53,54].
Although these techniques may move the end-points of hemorrhagic shock to the organic, cellular, and subcellular levels, large clinical studies are scarce. More precise techniques for monitoring hemorrhagic shock are thus required, which can be easily and repeatedly applied in the clinical setting.
It is well known that shock initiates dysfunctional inflammation that causes MOF. Resuscitation is a vital intervention that decreases the severity of the shock insult, but current strategies are not directed at modulating the immune response; in fact, they may worsen it. Those parameters that are routinely monitored in the clinical setting do not adequately reflect the immune status of the patient (hyperinflammation versus immunoparalysis), which is important when starting traditional and immunomodulatory therapies.
Proinflammatory cytokine levels correlate with prognosis; in this respect, elevated levels of TNF-α, IL-1, and IL-6 in plasma have been well described in both animal experiments [55-57] and patient studies [58-62] after trauma and severe blood loss. Measurement of soluble inflammatory parameters can be routinely performed in many institutions. However, there are several important limitations because determination of soluble inflammatory mediators does not entirely reflect cytokine tissue levels; they therefore may not represent a good indicator for the extent of tissue inflammation and organ damage. Furthermore, a single mediator may not be an adequate indicator of the inflammatory response because of the complexity of the underlying pathways. In this regard, newer biotechnological methods may be employed to identify immune cell surface markers (HLA-DR); expression of proinflammatory and anti-inflammatory mediators at the immune cell level (intracellular cytokine staining, cytokine secretion assay) and cytokine gene expression via array technology may demonstrate new tools for diagnosis and monitoring of critically ill patients with bleeding [63-65]. Nonetheless, proinflammatory cytokine levels might soon become important in the management of trauma patients in the ICU. Knowledge of the patient's cytokine levels may yield some indicator of the intracellular milieu and possibly provide insight into the cellular changes taking place. This information might provide the clinician with a better understanding of how to treat such a critically ill trauma patient. However, the techniques require refinement to allow rapid and online measurement of cytokines before the full benefit of such information can be effectively translated into better management of trauma patients.
Although various cytokine therapies in critically ill patients have not yet yielded satisfactory results, the lack of beneficial effect might be related to timing and dose of anticytokine administered. It is our hypothesis that total blockade/neutralization of cytokines will not be helpful to the host. Rather, modulation of cytokine production/release by immune cells (macrophages and T cells), leading to restoration of cellular homeostasis, might be a better way to decrease the susceptibility of trauma victims to subsequent sepsis and infection. However, further basic research to investigate inflammatory pathways and identify co-determinants will represent an inescapable prerequisite for progress in this area.
Resuscitation
For the past four decades, the standard approach to the trauma victim who is hypotensive from presumed hemorrhage has been to infuse large volumes of isotonic crystalloids as early and as rapidly as possible [15-17,66]. The goals of this treatment strategy are rapid restoration of intravascular volume and vital signs, and maintenance of vital organ perfusion. The most efficient solution for use in resuscitation is still under debate. Lactated Ringer's (LR) and normal isotonic saline solution (NS) remain the most commonly used isotonic fluids [16]. Although colloid solutions, including hyperosmolar colloid and hypertonic electrolyte compounds, have been approved for use as volume expanders, their administration is still under debate in the USA and Europe [16,17].
It is generally believed that LR is better than NS because it provides a better buffer for metabolic acidosis, but investigators have still not validated this hypothesis. Experimental studies have revealed that resuscitation with NS in the setting of massive hemorrhage requires significantly greater volume and is associated with increased physiologic derangements (for example, hyperchloremic acidosis and dilutional coagulopathy) and higher mortality as compared with LR [67,68]. In contrast, results from a recent preclinical study [69] indicated increased endothelial dysfunction after resuscitation with LR alone. Alternative crystalloid solutions have also been developed (for instance, Ringer's ethyl pyruvate) that exhibit anti-inflammatory properties [70], but a recent experimental study [71] showed that resuscitation with Ringer's ethyl pyruvate was not associated with improved early hemodynamics or tissue energetics as compared with LR.
The first prospective randomized controlled trials were initiated during the mid-1970s to compare semisynthetic colloid solutions (gelatins, dextrans, and hydroxyethyl starches) as plasma volume expanders in critically ill patients, with pulmonary dysfunction as the primary end-point because excessive administration of isotonic fluids resulted in a new entity termed 'shock lung' [72-74]. However, the results of the studies were not definitive because of differences in study design, small numbers of patients included, heterogeneous populations, differences in resuscitation end-points, and difficulties in defining pulmonary dysfunction. Nonetheless, if mortality is used as the end-point and the data are subgrouped, then the use of crystalloids in trauma patients is associated with improved survival [74]. Several additional systematic reviews were conducted, but the large variation in patient populations impeded clear interpretation. It was finally concluded that because there was no evidence that one colloid solution was safer than another, and because no reduction in risk for death was evident in critically ill patients, continued use of these agents in these patients could not be justified outside the setting of randomized controlled trials [74,75]. Although experimental results indicated that resuscitation with LR and colloids have equivalent effects on indices of inflammation in the lungs [76], reported data from several studies conducted in critically ill patients have indicated that use of colloid solutions has a significant impact on hemorrhage, hemostasis, and inflammatory response [77-79].
Because human serum albumin (HSA) is responsible for 80% of the colloid osmotic pressure of plasma, it may represent a possible resuscitation fluid in hemorrhagic shock. However, a meta-analysis of studies using albumin-containing fluids revealed that their use is associated with a 1.68-fold increase in the relative risk for death when compared with a crystalloid solution [80]. This effect is related to an increased leakage of albumin into the extravascular spaces, worsening the edema that takes place during hypovolemia and exacerbating respiratory and cardiac failure [81]; possible contamination of the preparation with prion/viral components poses additional risk to users. Nonetheless, in a large-scale clinical trial conducted in Australia and New Zealand, in which 4% albumin was compared with NS for intravascular fluid resuscitation, no difference in mortality was reported [82]. Recombinant HSA may offer a new perspective, because reported data indicate a high retention rate in circulating blood and lower vascular permeability than with the native HSA [81]; however, potential immunological alteration after the use of recombinant HSA in critically ill patients must be investigated.
The most recent studies of hemorrhagic shock and resuscitation suggest that our current protocols are far from ideal, and may in fact be harmful [41]. During the past decade, several studies have investigated different strategies in resuscitation. In the 1980s the first report of use of hypertonic saline was published [83]. Small volume hypertonic saline was shown to be as effective as large volume crystalloids in expanding plasma volume; enhancing cardiac output and microcirculation; limiting acute lung injury, neutrophil activation, and red blood cell injury; and restoring renal performance in animals with hemorrhagic shock [84,85]. However, the increased microcirculation after resuscitation with hypertonic saline in animals was associated with increased bleeding, but mortality was model dependent and the best survival was obtained when saline was given with high-volume crystalloid [83]. A further experimental study demonstrated enhanced resuscitation effects with a combination of hypertonic saline and dextran [86]. Several clinical studies followed, and a meta-analysis of those studies [87] revealed that hypertonic saline is not better than the current standard of care, namely isotonic fluids, but the combination of hypertonic saline with dextran might be superior. Trauma patients with combined head injury and hemorrhagic shock profited the most because data showed that resuscitation with this combination increased cerebral perfusion, and decreased intracranial pressure and brain edema. Therefore, hypertonic saline solutions have evolved as an alternative to mannitol. However, caution is advised with high osmolar loads because they carry increased risk for potentially deleterious consequences of hypernatremia, or they may induce osmotic blood-brain barrier disruption, with possibly harmful extravasation of the hypertonic solution into the brain tissue [88]. Moreover, a randomized controlled trial [89] indicated that prehospital resuscitation with hypertonic saline in patients with hypotension and severe traumatic brain injury led to almost identical neurological function 6 months after injury as in patients who received conventional fluids. Nevertheless, investigation of the optimal resuscitation fluid is an ongoing field of interest.
Other current experimental data indicate that titrated hypertonic solutions combined with 10% hydroxyethyl starch are superior to LR without increasing blood loss [90]. Moreover, the optimal fluid composition appears to play an important role because a single bolus of 3% saline with 6% dextran-70 was able to raise mean arterial pressure and tissue oxygen saturation while attenuating post-traumatic hypercoagulability in an animal model of uncontrolled hemorrhagic shock [91]. Further benefit may result from the combination of hypertonic saline and the immunoactive phosphodiesterase inhibitor pentoxifylline [92]. Resuscitation with this combination has been shown to prevent lung injury and bacterial translocation, downregulate inducible nitric oxide synthase, and decrease end-organ damage [93-95]. However, those resuscitation strategies must prove their efficacy in the clinical arena, and therefore further randomized clinical trials are needed. In this regard, two prehospital intervention trials were recently successfully designed and implemented to compare hypertonic saline resuscitation with or without dextran versus conventional isotonic resuscitation in patients with hypovolemic shock or traumatic brain injury [96]. The results of these studies will hopefully advance and improve the early care of severely injured patients.
Transfusion
Early administration of blood is one potential treatment to decrease the need for massive crystalloid solution in hemorrhagic shock; however, the limited supply of stored blood and potential adverse effects make this option logistically difficult and possibly harmful [15-17]. In addition, potential infectious complications (hepatitis, HIV, and bacterial contamination), immune suppression [97-99], and metabolic complications (hyperkalemia, hypocalcemia, and citrate toxicity) and risk for mistransfusion [100] are well documented. Transfusion-related risks include possible development of MOF, increased ICU admissions and length of stay, increased hospital length of stay, and mortality [18-21]. As a result, intensive investigations were conducted to develop a new artificial oxygen carrier (Table 1).
Table 1.
Artificial oxygen carriers
| Category | Product | Type | MW (Daltons) | Phase of testing |
| Perfluorocarbons | Oxygent™ | Perfluoroctylbromide | 450 to 500 | Up to clinical phase III, discontinued |
| Hemoglobin-based oxygen carrier | HemAssist™ | Diaspirin-crosslinked hemoglobin (human) | 65,000 | Up to clinical phase III, discontinued |
| Hemopure™ | Polymerized bovine hemoglobin (bovine) | 250,000 | Up to clinical phase III | |
| Polyheme™ | Pyridoxylated glutaraldehyde-polymerized hemoglobin (human) | 150,000 | Up to clinical phase III | |
| Hemospan™ | Maleimide-activated polyethylene-glycol-modified hemoglobin (human) | 95,000 | Up to clinical phase II, phase III planned | |
| Hemoglobin vesicles | Oxygenix™ | Hemoglobin containing liposomes (OXY-0301) | Unpublished | Experimental, up to phase I |
Presented are the physiochemical characteristics and state of clinical research on artificial oxygen carrier. The manufacturers are as follows: Oxygent™, Alliance Pharmaceutical Corp., San Diego, CA, USA; HemAssist™, Baxter Healthcare, Round Lake, IL, USA; Hemopure™, Biopure Corp., Cambridge, MA, USA; Polyheme™, Northfield Lab Inc., Evanston, IL, USA; Hemospan™, Sangart Inc., San Diego, CA, USA; and Oxygenix™, Oxygenix Co. Ltd., Tokyo, Japan.
Perfluorocarbons (PFCs) are an interesting development, representing simply constructed molecules from cyclic or straight-chain hydrocarbons with hydrogen atoms replaced by halogens (fluorine or bromide), which are characterized by a linear relationship between arterial PO2 and oxygen content [101]. PFCs are insoluble in water and must be emulsified for intravenous administration. The oxygen release from PFCs to tissue is almost complete in the presence of a high PO2 gradient between arterial blood and tissue. However, administration of PFCs is restricted to low dosages because studies have indicated that PFC emulsion droplets are rapidly taken up by the reticuloendothelial system and subsequently cause immunosuppression [101,102]. Experimental studies have demonstrated that use of PFCs allows the extension of acute normovolemic anemia (hematocrit 21% to 8%) without signs of impaired tissue oxygenation or compromised myo-cardial contractility [103,104]. Clinical application of PFCs has been shown to maintain gastrointestinal tissue oxygenation at hemoglobin levels below 7 g/dl, to delay the interval until transfusion of allogenic blood was required, and to decrease the number of transfused red blood cell units in patients undergoing cardiac and noncardiac surgery [105-107]. However, enrollment in a phase III study investigating administration of PFCs in cardiac surgical patients was discontinued because of an increased rate of neurological complications. A recent multicenter phase III study conducted in patients undergoing noncardiac surgery and suffering high-volume blood loss demonstrated an increased incidence of postoperative ileus, in addition to typical mild side effects (flu-like symptoms, fever, headache, nausea, and myalgia) [101,107].
Hemoglobin-based oxygen carriers (HBOCs) for trauma resuscitation may provide a workable compromise, because their use in experimental studies of severe hemorrhagic shock demonstrated stabilization of hemodynamic status and tissue oxygenation, and decreased mortality [108-111]. Moreover, the postischemic interactions between leukocytes and endothelium were attenuated by infusion of human and bovine HBOC [112-114]. Surprisingly, an interim analysis of clinical application of the long-term favorite HBOC diaspirin-crosslinked hemoglobin (HemAssist™; Baxter Healthcare, Round Lake, IL, USA) for treatment of severe trauma-hemorrhagic shock [115] demonstrated increased rates of 24-hour and 48-hour mortality; therefore, the trauma study was discontinued. In contrast, pyridoxylated glutaraldehyde-polymerized hemoglobin (PolyHeme™; Northfield Labs Inc., Evanston, IL, USA) proved to be an effective resuscitation fluid in 171 patients suffering from massive hemorrhage [116]. Although experimental studies indicated that administration of HBOC-201 (polymerized bovine hemoglobin; Hemopure™; Biopure Corp., Cambridge, MA, USA) in hemorrhagic shock is effective and improved outcome, the potential vasoconstrictive properties of all HBOCs must be taken into account if their use is considered [117-120]. The clinical impact is not yet fully understood, but possible underlying mechanisms include scavenging of nitric oxide, augmented release of endothelin, and stimulation of endothelin receptors and adrenoreceptors [117-120]. In this respect, a nonvasoactive HBOC was recently developed (maleimide-activated polyethylene-glycol-modified hemoglobin; Hemospan™) and proven to provide sufficient tissue oxygenation at the microcirculatory level in an experimental model of hemorrhagic shock [121]. Hemospan™ (Sangart Inc., San Diego, CA, USA) has finished testing in phases I and II, and a phase III prehospital trial is underway [122].
Another promising development representing hemoglobin vesicles involves use of phospholipid vesicles (liposomes) that encapsulate purified human hemoglobin. Because experimental studies in hemorrhagic shock have shown that fluid resuscitation with hemoglobin vesicles maintained systemic oxygenation and did not induce either vasoconstriction or activation of the immune system, their further investigation in hemorrhagic shock is needed [123,124].
Vasoactive substances
The primary goal of treatment in hemorrhagic shock is prompt restoration of tissue perfusion. However, this concept has come under debate, particularly in uncontrolled and advanced hemorrhage [125]. In the initial phase of hemorrhagic shock, vasopressors are widely used to maintain blood pressure while resuscitation is ongoing. However, during the late phase of hemorrhagic shock, replacement of fluids and blood may become ineffective, even when supported by traditional vasopressors such as norepinephrine, which indicates a paralyzed vasculature [125].
Theorized mechanisms of vasomotor paralysis partly involve tissue acidosis, hypoxia, and ATP depletion. The ATP-sensitive potassium channels in particular represent an important regulator of the vascular tone of arterioles, and thus blood supply of most organs is regulated in part via these channels [126,127]. However, if too many organs try to obtain additional perfusion, which is simply not possible during severe shock, then regional perfusion decreases and a vicious shock cycle results. Adrenal insufficiency in hemorrhagic shock may also represent a contributing factor [128]. Most recently, vasopressin – a recognized endogenous stress hormone – has been found to represent a potential drug therapy that can prevent this vicious shock cycle. Experimental studies demonstrated that vasopressin can inhibit both ATP-sensitive potassium channels and nitric oxide induced accumulation of cGMP [129]. In critically ill patients such as those suffering out-of-hospital cardiac arrest or septic shock, vasopressin has been identified to be highly supportive [130,131]. Experimental studies conducted in trauma and hemorrhagic shock have also indicated that administration of vasopressin minimized fluid resuscitation volume, improved cardiopulmonary parameters, led to a markedly improved neurologic outcome if brain injury was present, and improved survival [132-134]. Regarding potential benefits in patients with hemorrhage, only case reports are currently available, but a multicenter trial was recently initiated [135]. A recent retrospective data analysis revealed that caution should be exercised with vasopressor treatment in hemorrhagic shock, because increased mortality in blunt injured adults with hemorrhagic shock was observed after use of vasopressors as compared with aggressive early crystalloid resuscitation [136]. However, that study had several limitations. Specifically, it was a secondary analysis, and those patients who required early vasopressor treatment were older, more severely injured, had worse initial shock parameters, and required greater resuscitation after injury, more invasive monitoring, and treatment in the ICU. Therefore, further prospective randomized trials are needed to address this issue adequately and identify potential confounders.
In contrast, a newly identified vasodilatory peptide, namely adrenomedullin, which is depressed after severe blood loss because of downregulation of adrenomedullin binding protein (AMBP)-1 [137], acts as a circulating hormone that elicits various biologic activities in a paracrine and autocrine manner. Human AMBP-1 has been shown to potentiate adrenomedullin-induced vascular relaxation in the aorta under normal and pathophysiological conditions [138]. Experimental studies revealed that administration of adrenomedullin in combination with AMBP-1 in hemorrhagic shock improved cardiac performance and tissue perfusion, attenuated hepatic and renal injury, prevented metabolic acidosis, down-regulated proinflammatory cytokines, and reduced mortality [137,139,140]. The beneficial effect of adrenomedullin/AMBP-1 is associated with downregulation of endothelin-1, which is normally upregulated in hemorrhagic shock [140]. However, clinical studies are necessary to evaluate the precise underlying mechanisms and potential benefits of adrenomedullin and its binding protein in traumatic injury and hemorrhagic shock.
Coagulation support
Excessive hemorrhage is associated with coagulopathy, which is proportional to the volume of blood loss [141]. Analysis of adult patients receiving more than 10 units of red blood cells in a major trauma center identified acidosis (pH < 7.1), hypothermia (<34°C), Injury Severity Score above 25, and systolic blood pressure below 70 mmHg as independent risk factors for coagulopathy [142]. Nonetheless, coagulopathy is generally multifactorial and additional contributing factors include the following [143]: hemodilution, hypovolemia, hypothermia-associated platelet dysfunction, activation of the coagulation and fibrinolytic cascades, endothelial cell damage leading to disseminated intravascular coagulation, impaired hepatic synthesis of coagulation factors, decreased clearance of activated factors, and release of tissue factors into the circulation. In addition to rewarming, coagulation factor replacement with fresh frozen plasma, prothrombin complex concentrates, fibrinogen concentrates, antifibrinolytics (tranexamic acid and ε-aminocaproic acid), and platelet concentrates play an important role in the management of bleeding [15,17,143].
An additional attractive candidate for maintaining coagulation in hemorrhagic shock is recombinant factor VIIa (rFVIIa), which has been approved for treatment of bleeding in patients with hemophilia or vitamin K-antagonist therapy [144]. Supraphysiological doses of rFVIIa bind to phospholipid structures of activated platelets at the site of injury, which may underlie the localized effects of this compound [144]. In various animal models of hemorrhage, rFVIIa has been shown to be an effective procoagulant adjunct for controlling bleeding [145,146]. In addition to numerous promising case studies that reported decreased mortality in patients with severe trauma [144,147], only one randomized clinical trial is available at present [148], which indicated beneficial effects in the treatment of coagulopathic bleeding after trauma. Although a recent systematic review of the efficacy of rFVIIa in treatment of bleeding in patients without hemophilia [149] revealed no difference in thromboembolic events compared with placebo, there was also no difference observed for death, total blood loss, and transfusion. Currently, the rationale for using rFVIIa to treat massive bleeding is only as an adjunct to the surgical control of bleeding if conventional therapies have failed [150]. Although European and US recommendations indicate that rFVIIa should be used in massive bleeding [15,17], further randomized clinical trials are warranted. A major randomized double-blind study is currently being conducted in the USA, and the results will hopefully verify the therapeutic benefits of rFVIIa in traumatic hemorrhage as well as its potential adverse effects.
Future therapeutic approaches
Based on advances in the characterization of pathophysiological mechanisms and identification of new mediators after hemorrhagic shock, new therapeutic strategies have been developed in clinically relevant animal models. An incomplete selection of promising approaches is presented in the following discussion. Clinical studies using these agents must be conducted in the future to determine their effectiveness in patients.
High-mobility group protein (HMGB)1 has been identified as a late-acting mediator of lipopolyaccharide-induced or sepsis-induced lethality in mice [151,152]. Moreover, HMGB1 is a cytokine-like molecule that can promote TNF release from mononuclear cells [153]. In addition, HMGB1 is actively secreted by immunostimulated macrophages [154]. Similarly, elevated plasma levels of HMGB1 were evident in patients with trauma-induced hemorrhagic shock [155]. The above studies collectively suggest that HMGB1 is involved in the disease process after blood loss in patients and animals. In view of this, anti-HMGB1 neutralizing antibodies used after hemorrhagic shock and resuscitation in a clinically relevant animal model demonstrate improved survival rates [155]. Moreover, anti-HMGB1 neutralizing antibodies decreased bacterial translocation to mesenteric lymph nodes, associated with lower circulating levels of IL-6 and IL-10. Anti-HMGB1 neutralizing antibodies warrant further evaluation as a therapeutic option in patients who have suffered trauma and hemorrhage.
α1 Acid glycoprotein (AAG), one of the acute phase proteins, has been shown to protect endothelial barrier function and to decrease leukocyte-endothelium interaction following adverse circulatory conditions [156,157]. Endothelial cell dysfunction, capillary leakage, and leukocyte accumulation play key roles in initiating the pathophysiological mechanisms that become active after trauma and hemorrhage. In view of this, studies were conducted in which AAG was administered during resuscitation after trauma and blood loss in rodents [158]. The results indicate that AAG reduced edema formation, capillary leakage, and neutrophil accumulation after trauma and blood loss. Although the precise underlying mechanisms for the protective effects of AAG under those conditions are unknown, the findings suggest that various mediators are affected. Because there are many redundant pathways for mediators, AAG appears to be a promising adjunct in the clinical arena.
Additional pathways and mediators that might yield promising therapeutic options are hydrogen sulfide and the use of its blocker [159]. Additionally, blockade of reactive oxygen species with hemigramicidin-TEMPO conjugate (a novel mitochondria-targeted antioxidant) or 5-aminoisoquinolinone (a poly-ADP-ribose synthase inhibitor) may provide cyto-protection during hemorrhagic shock and after resuscitation [160,161]. Moreover, complement depletion or inhibition demonstrated beneficial effects in animal models of hemorrhage, and therefore they represent an interesting novel treatment option [162] in the clinical arena.
Studies have also shown that the use of 17β-estradiol (estrogen) after trauma/hemorrhage in male or ovariectomized female rodents improved cardiovascular and immunologic functions and decreased mortality from subsequent sepsis [22,27,163-170]. Because only a single dose of estrogen was used, this was not a hormone replacement therapy and thus should be considered as a safe and effective adjunct for maintaining cardiovascular and immunologic responses following low-flow conditions in male and female trauma patients. Studies have also shown that flutamide (androgen receptor antagonist) after trauma/hemorrhage in males increased aromatase activity, increased estrogen levels, upregulated estrogen receptors, and improved cell/organ function under those conditions [171,172]. Additional studies have shown that the use of DHEA, metoclopramide, prolactin, or progesterone [172-174] as adjuncts to resuscitation after trauma/hemorrhage also produced salutary effects on cardiovascular and immunologic functions after trauma/hemorrhage, and so their use in the clinical arena also appears to be warranted [172-174].
Conclusion
Hemorrhagic shock is a leading cause of death in trauma patients worldwide. Despite improved resuscitation strategies for severely injured patients who present in shock, these transfusions are associated with development of MOF, increased ICU admissions and length of stay, increased hospital length of stay, and increased mortality. An important contributing factor may be the marked suppression of cell-mediated immunity after hemorrhage treated with adequate fluid resuscitation, which is associated with an increased susceptibility to subsequent sepsis. Other factors that may profoundly influence outcomes after hemorrhage include age, sex, nutritional status, socioeconomic background, and pre-existing disease or infection.
Bleeding control, maintenance of tissue oxygenation with fluid resuscitation, coagulation support, and maintaining normothermia remain mainstays of therapy for patients with hemorrhagic shock. However, further benefits are anticipated from new technologies that are being brought into clinical use, especially hypertonic colloid saline, HBOCs, recombinant factor VIIa, and less invasive early monitors. In addition, promising results from experimental studies have demonstrated that recombinant HSA as a plasma expander as well as administration of vasopressin, adrenomedullin, estradiol, metoclopramide, prolactin, flutamide, or DHEA to maintain cardiac output, tissue perfusion and oxygenation may be beneficial in hemorrhagic shock. Furthermore, prevention of capillary leakage due to blockade of reactive oxygen formation, maintenance of endothelial barrier function, and attenuation of the hyperinflammatory immunological response are additional experimental approaches that are of therapeutic interest in combating hemorrhage-associated complications.
Hemorrhagic shock can be rapidly fatal. It is self-evident that prevention of injury is preferable and feasible, but control of hemorrhage in the earliest phases of care, recognition and monitoring of individual risk factors, and therapeutic modulation of the inflammatory immune response may represent the next generation of care in hemorrhagic shock. Further randomized controlled clinical trials are needed, but existing ethical requirements must be maintained and standardized criteria for enrolling comparable patient populations must be utilized.
Abbreviations
AAG = α1 acid glycoprotein; AMBP = adrenomedullin binding protein; DHEA = dehydroepiandrosterone; HBOC = hemoglobin-based oxygen carrier; HMBG = high-mobility group protein; HSA = human serum albumin; ICU = intensive care unit; IL = interleukin; LR = lactated Ringer's; MOF = multiple organ failure; NS = normal isotonic saline solution; rFVIIa = recombinant factor VIIa; PCO2 = partial carbon dioxide tension; PFC = perfluorocarbon; PO2 = partial oxygen tension; TNF = tumor necrosis factor.
Competing interests
The authors declare that they have no competing interests.
Acknowledgments
Acknowledgements
The authors wish to express their sincere thanks to Bobbi Smith for her superb help in editing this manuscript. This work was supported by USPHS grants R37 GM39519 and RO1 GM37127.
References
- Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma. 2006;60:S3–S11. doi: 10.1097/01.ta.0000199961.02677.19. [DOI] [PubMed] [Google Scholar]
- Kauvar DS, Wade CE. The epidemiology and modern management of traumatic hemorrhage: US and international perspectives. Crit Care. 2005;9:S1–S9. doi: 10.1186/cc3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossaint R, Cerny V, Coats TJ, Duranteau J, Fernandez-Mondejar E, Gordini G, Stahel PF, Hunt BJ, Neugebauer E, Spahn DR. Key issues in advanced bleeding care in trauma. Shock. 2006;26:322–331. doi: 10.1097/01.shk.0000225403.15722.e9. [DOI] [PubMed] [Google Scholar]
- Durham RM, Moran JJ, Mazuski JE, Shapiro MJ, Baue AE, Flint LM. Multiple organ failure in trauma patients. J Trauma. 2003;55:608–616. doi: 10.1097/01.TA.0000092378.10660.D1. [DOI] [PubMed] [Google Scholar]
- Franklin GA, Boaz PW, Spain DA, Lukan JK, Carrillo EH, Richardson JD. Prehospital hypotension as a valid indicator of trauma team activation. J Trauma. 2000;48:1034–1037. doi: 10.1097/00005373-200006000-00006. [DOI] [PubMed] [Google Scholar]
- Heckbert SR, Vedder NB, Hoffman W, Winn RK, Hudson LD, Jurkovich GJ, Copass MK, Harlan JM, Rice CL, Maier RV. Outcome after hemorrhagic shock in trauma patients. J Trauma. 1998;45:545–549. doi: 10.1097/00005373-199809000-00022. [DOI] [PubMed] [Google Scholar]
- Peitzman AB, Billiar TR, Harbrecht BG, Kelly E, Udekwu AO, Simmons RL. Hemorrhagic shock. Curr Probl Surg. 1995;32:925–1002. doi: 10.1016/S0011-3840(05)80008-5. [DOI] [PubMed] [Google Scholar]
- Cairns CB. Rude unhinging of the machinery of life: metabolic approaches to hemorrhagic shock. Curr Opin Crit Care. 2001;7:437–443. doi: 10.1097/00075198-200112000-00011. [DOI] [PubMed] [Google Scholar]
- Jarrar D, Chaudry IH, Wang P. Organ dysfunction following hemorrhage and sepsis: mechanisms and therapeutic approaches [review] Int J Mol Med. 1999;4:575–583. doi: 10.3892/ijmm.4.6.575. [DOI] [PubMed] [Google Scholar]
- Ayala A, Ertel W, Chaudry IH. Trauma-induced suppression of antigen presentation and expression of major histocompatibility class II antigen complex in leukocytes. Shock. 1996;5:79–90. doi: 10.1097/00024382-199602000-00001. [DOI] [PubMed] [Google Scholar]
- Chaudry IH, Zellweger R, Ayala A. The role of bacterial translocation on Kupffer cell immune function following hemorrhage. Prog Clin Biol Res. 1995;392:209–218. [PubMed] [Google Scholar]
- Harbrecht BG, Billiar TR. The role of nitric oxide in Kupffer cell-hepatocyte interactions. Shock. 1995;3:79–87. [PubMed] [Google Scholar]
- Szabó C, Billiar TR. Novel roles of nitric oxide in hemorrhagic shock. Shock. 1999;12:1–9. doi: 10.1097/00024382-199907000-00001. [DOI] [PubMed] [Google Scholar]
- Hierholzer C, Billiar TR. Molecular mechanisms in the early phase of hemorrhagic shock. Langenbecks Arch Surg. 2001;386:302–308. doi: 10.1007/s004230100242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MA, Shapiro MB, Nathens AB, Johnson JL, Moore EE, Minei JP, Bankey PE, Freeman B, Harbrecht BG, McKinley BA, Moore FA, Maier RV. Inflammation and the host response to injury, a large-scale collaborative project: patient-oriented research core-standard operating procedures for clinical care. IV. Guidelines for transfusion in the trauma patient. J Trauma. 2006;61:436–439. doi: 10.1097/01.ta.0000232517.83039.c4. [DOI] [PubMed] [Google Scholar]
- Moore FA, McKinley BA, Moore EE, Nathens AB, West M, Shapiro MB, Bankey P, Freeman B, Harbrecht BG, Johnson JL, Minei JP, Maier RV. Inflammation and the host response to injury, a large-scale collaborative project: patient-oriented research core: standard operating procedures for clinical care. III. Guidelines for shock resuscitation. J Trauma. 2006;61:82–89. doi: 10.1097/01.ta.0000225933.08478.65. [DOI] [PubMed] [Google Scholar]
- Spahn DR, Cerny V, Coats TJ, Duranteau J, Fernandez-Mondejar E, Gordini G, Stahel PF, Hunt BJ, Komadina R, Neugebauer E, Ozier Y, Riddez L, Schultz A, Vincent JL, Rossaint R, Task Force for Advanced Bleeding Care in Trauma Management of bleeding following major trauma: a European guideline. Crit Care. 2007;11:R17. doi: 10.1186/cc5686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone DL, Dunne J, Tracy JK, Putnam AT, Scalea TM, Napolitano LM. Blood transfusion, independent of shock severity, is associated with worse outcome in trauma. J Trauma. 2003;54:898–905. doi: 10.1097/01.TA.0000060261.10597.5C. [DOI] [PubMed] [Google Scholar]
- Eastridge BJ, Malone D, Holcomb JB. Early predictors of transfusion and mortality after injury: a review of the data-based literature. J Trauma. 2006;60:S20–S25. doi: 10.1097/01.ta.0000199544.63879.5d. [DOI] [PubMed] [Google Scholar]
- Napolitano L. Cumulative risks of early red blood cell transfusion. J Trauma. 2006;60:S26–S34. doi: 10.1097/01.ta.0000199979.95789.17. [DOI] [PubMed] [Google Scholar]
- Cotton BA, Guy JS, Morris JA, Jr, Abumrad NN. The cellular, metabolic, and systemic consequences of aggressive fluid resuscitation strategies. Shock. 2006;26:115–121. doi: 10.1097/01.shk.0000209564.84822.f2. [DOI] [PubMed] [Google Scholar]
- Choudhry MA, Bland KI, Chaudry IH. Trauma and immune response: effect of gender differences. Injury. 2007;38:1382–1391. doi: 10.1016/j.injury.2007.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bone RC. Toward an epidemiology and natural history of SIRS (systemic inflammatory response syndrome) JAMA. 1992;268:3452–3455. doi: 10.1001/jama.268.24.3452. [DOI] [PubMed] [Google Scholar]
- Center for Disease Control Mortality patterns: United States, 1989. MMWR Morb Mortal Wkly Rep. 1992;41:121–125. [PubMed] [Google Scholar]
- McGowan JE, Barnes MW, Finland N. Bacteremia at Boston City Hospital: occurrence and mortality during 12 selected years (1935–1972) with special reference to hospital-acquired cases. J Infect Dis. 1975;132:316–335. doi: 10.1093/infdis/132.3.316. [DOI] [PubMed] [Google Scholar]
- Schroder J, Kahlke V, Staubach KH, Zabel P, Stuber F. Gender differences in human sepsis. Arch Surg. 1998;133:1200–1205. doi: 10.1001/archsurg.133.11.1200. [DOI] [PubMed] [Google Scholar]
- Angele MK, Schwacha MG, Ayala A. Effect of gender and sex hormones on immune responses following shock. Shock. 2000;14:81–90. doi: 10.1097/00024382-200014020-00001. [DOI] [PubMed] [Google Scholar]
- Angele MK, Knöferl MW, Ayala A, Cioffi WG, Bland KI, Chaudry IH. Male and female sex steroids: do they produce deleterious or beneficial effects on immune responses following trauma-hemorrhage? Surg Forum. 1998;49:43–45. [Google Scholar]
- Knoferl MW, Diodato MD, Angele MK, Ayala A, Cioffi WG, Bland KI, Chaudry IH. Do female sex steroids adversely or beneficially affect the depressed immune responses in males after trauma-hemorrhage? Arch Surg. 2000;135:425–433. doi: 10.1001/archsurg.135.4.425. [DOI] [PubMed] [Google Scholar]
- Deitch EA, Livingston DH, Lavery RF. Hormonally active women tolerate shock-trauma better than do men: a prospective study of over 4000 trauma patients. Ann Surg. 2007;246:447–453. doi: 10.1097/SLA.0b013e318148566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catania RA, Angele MK, Ayala A, Cioffi WG, Bland KI, Chaudry IH. Dehydroepiandrosterone restores immune function following trauma-hemorrhage by a direct effect on T-lymphocytes. Cytokine. 1998;11:443–50. doi: 10.1006/cyto.1998.0458. [DOI] [PubMed] [Google Scholar]
- Angele MK, Catania RA, Ayala A, Cioffi WG, Bland KI, Chaudry IH. Dehydroepiandrosterone (DHEA): an inexpensive steroid hormone which decreases the mortality from sepsis. Arch Surg. 1998;133:1281–1288. doi: 10.1001/archsurg.133.12.1281. [DOI] [PubMed] [Google Scholar]
- Frantz MC, Prix NJ, Wichmann MW, Engel NK van den, Hernandez-Richter T, Faist E, Chaudry IH, Jauch KW, Angele MK. Dehydroepiandrosterone restores depressed peripheral blood mononuclear cell function following major abdominal surgery via the estrogen receptors. Crit Care Med. 2005;33:1779–1786. doi: 10.1097/01.CCM.0000172278.91959.38. [DOI] [PubMed] [Google Scholar]
- Schneider CP, Schwacha MG, Chaudry IH. Influence of gender and age on T-cell responses in a murine model of trauma-hemorrhage: differences between circulating and tissue-fixed cells. J Appl Physiol. 2006;100:826–833. doi: 10.1152/japplphysiol.00898.2005. [DOI] [PubMed] [Google Scholar]
- Schneider CP, Schwacha MG, Chaudry IH. Impact of sex and age on bone marrow immune responses in a murine model of trauma-hemorrhage. J Appl Physiol. 2007;102:113–121. doi: 10.1152/japplphysiol.00848.2006. [DOI] [PubMed] [Google Scholar]
- Hildebrand F, Pape HC, van GM, Meier S, Hasenkamp S, Krettek C, Stuhrmann M. Genetic predisposition for a compromised immune system after multiple trauma. Shock. 2005;24:518–522. doi: 10.1097/01.shk.0000184212.97488.4e. [DOI] [PubMed] [Google Scholar]
- Giannoudis PV, van GM, Tsiridis E, Pape HC. The genetic predisposition to adverse outcome after trauma. J Bone Joint Surg Br. 2007;89:1273–1279. doi: 10.1302/0301-620X.89B10.19022. [DOI] [PubMed] [Google Scholar]
- Schroeder O, Schulte KM, Schroeder J, Ekkernkamp A, Laun RA. The -1082 interleukin-10 polymorphism is associated with acute respiratory failure after major trauma: a prospective cohort study. Surgery. 2008;143:233–242. doi: 10.1016/j.surg.2007.07.040. [DOI] [PubMed] [Google Scholar]
- Canter JA, Norris PR, Moore JH, Jenkins JM, Morris JA. Specific polymorphic variation in the mitochondrial genome and increased in-hospital mortality after severe trauma. Ann Surg. 2007;246:406–411. doi: 10.1097/SLA.0b013e3181469955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catania RA, Chaudry IH. Immunological consequences of trauma and shock. Ann Acad Med Singapore. 1999;28:120–132. [PubMed] [Google Scholar]
- Moore FA, McKinley BA, Moore EE. The next generation in shock resuscitation. Lancet. 2004;363:1988–1996. doi: 10.1016/S0140-6736(04)16415-5. [DOI] [PubMed] [Google Scholar]
- Revell M, Greaves I, Porter K. Endpoints for fluid resuscitation in hemorrhagic shock. J Trauma. 2003;54:S63–S67. doi: 10.1097/01.TA.0000056157.94970.FA. [DOI] [PubMed] [Google Scholar]
- Rixen D, Siegel JH. Bench-to-bedside review: oxygen debt and its metabolic correlates as quantifiers of the severity of hemorrhagic and post-traumatic shock. Crit Care. 2005;9:441–453. doi: 10.1186/cc3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rixen D, Raum M, Holzgraefe B, Schafer U, Hess S, Tenhunen J, Tuomisto L, Neugebauer EA, Shock and Trauma Study Group Local lactate and histamine changes in small bowel circulation measured by microdialysis in pig hemorrhagic shock. Shock. 2002;18:355–359. doi: 10.1097/00024382-200210000-00011. [DOI] [PubMed] [Google Scholar]
- Beerthuizen GIJM, Goris RJA, Kreuzer FJA. Early detection of shock in critically ill patients by skeletal muscle PO2 assessment. Arch Surg. 1989;124:853–855. doi: 10.1001/archsurg.1989.01410070113022. [DOI] [PubMed] [Google Scholar]
- Hopf HW, Glass-Heidenreich L, Silva J, Pearce F, Ochsner MG, Rozycki G, et al. Subcutaneous tissue oxygen tension in 'well resuscitated' trauma patients. Crit Care Med. 1994;22:A60. doi: 10.1097/00003246-199401000-00109. [DOI] [Google Scholar]
- McKinley BA, Parmley CL, Butler BD. Skeletal muscle PO2, PCO2, and pH in hemorrhage, shock, and resuscitation in dogs. J Trauma. 1997;44:119–27. doi: 10.1097/00005373-199801000-00015. [DOI] [PubMed] [Google Scholar]
- Knudson MM, Lee S, Erickson V, Morabito D, Derugin N, Manley GT. Tissue oxygen monitoring during hemorrhagic shock and resuscitation: a comparison of lactated Ringer's solution, hypertonic saline dextran, and HBOC-201. J Trauma. 2003;54:242–252. doi: 10.1097/01.TA.0000037776.28201.75. [DOI] [PubMed] [Google Scholar]
- Cairns CB, Moore FA, Haenel JB. Evidence for early supply independent mitochondrial dysfunction in patients developing multiple organ failure after trauma. J Trauma. 1997;42:532–536. doi: 10.1097/00005373-199703000-00023. [DOI] [PubMed] [Google Scholar]
- Clavijo-Alvarez JA, Sims CA, Menconi M, Shim I, Ochoa C, Puyana JC. Bladder mucosa pH and Pco2 as a minimally invasive monitor of hemorrhagic shock and resuscitation. J Trauma. 2004;57:1199–1209. doi: 10.1097/01.ta.0000145484.40534.3b. [DOI] [PubMed] [Google Scholar]
- Clavijo-Alvarez JA, Sims CA, Pinsky MR, Puyana JC. Monitoring skeletal muscle and subcutaneous tissue acid-base status and oxygenation during hemorrhagic shock and resuscitation. Shock. 2005;24:270–275. doi: 10.1097/01.shk.0000172364.89128.28. [DOI] [PubMed] [Google Scholar]
- Velmahos GC, Demetriades D, Shoemaker WC, Chan LS, Tatevossian R, Wo CC, Vassiliu P, Cornwell EE, 3rd, Murray JA, Roth B, Belzberg H, Asensio JA, Berne TV. Endpoints of resuscitation of critically injured patients: normal or supranormal? A prospective randomized trial. Ann Surg. 2000;232:409–418. doi: 10.1097/00000658-200009000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristagno G, Tang W, Sun S, Weil MH. Role of buccal PCO2 in the management of fluid resuscitation during hemorrhagic shock. Crit Care Med. 2006;34:S442–S446. doi: 10.1097/01.CCM.0000247722.24781.D0. [DOI] [PubMed] [Google Scholar]
- Vaezy S, Zderic V. Hemorrhage control using high intensity focused ultrasound. Int J Hyperthermia. 2007;23:203–211. doi: 10.1080/02656730601169779. [DOI] [PubMed] [Google Scholar]
- Chaudry IH, Ayala A. Immunological Aspects of Hemorrhage. Austin, TX: Medical Intelligence Unit, R.G. Landes Company; 1992. [Google Scholar]
- Ayala A, Perrin MM, Meldrum DR, Ertel W, Chaudry IH. Hemorrhage induces an increase in serum TNF which is not associated with elevated levels of endotoxin. Cytokine. 1990;2:170–174. doi: 10.1016/1043-4666(90)90012-I. [DOI] [PubMed] [Google Scholar]
- Ayala A, Wang P, Ba ZF, Perrin MM, Ertel W, Chaudry IH. Differential alterations in plasma IL-6 and TNF levels following trauma and hemorrhage. Am J Physiol. 1991;260:R167–R171. doi: 10.1152/ajpregu.1991.260.1.R167. [DOI] [PubMed] [Google Scholar]
- Roumen RM, Hendriks T, van der Ven-Jongekrijg J, Nieuwenhuijzen GA, Sauerwein RW, van der Meer JW, Goris RJ. Cytokine patterns in patients after major vascular surgery, hemorrhagic shock, and severe blunt trauma. Relation with subsequent adult respiratory distress syndrome and multiple organ failure. Ann Surg. 1993;218:769–76. doi: 10.1097/00000658-199312000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagnani S. Th1 and Th2 in human diseases. Clin Immunol Immunopathol. 1996;80:225–235. doi: 10.1006/clin.1996.0118. [DOI] [PubMed] [Google Scholar]
- Martin C, Boisson C, Haccoun M, Thomachot L, Mege JL. Patterns of cytokine evolution (tumor necrosis factor-α and inter-leukin-6) after septic shock, hemorrhagic shock, and severe trauma. Crit Care Med. 1997;25:1813–1819. doi: 10.1097/00003246-199711000-00018. [DOI] [PubMed] [Google Scholar]
- Angele MK, Chaudry IH. Surgical trauma and immunosuppression: pathophysiology and potential immunomodulatory approaches. Langenbecks Arch Surg. 2005;390:333–341. doi: 10.1007/s00423-005-0557-4. [DOI] [PubMed] [Google Scholar]
- Menger MD, Vollmar B. Surgical trauma: hyperinflammation versus immunosuppression? Langenbecks Arch Surg. 2004;389:475–484. doi: 10.1007/s00423-004-0472-0. [DOI] [PubMed] [Google Scholar]
- Zedler S, Faist E. The impact of endogenous triggers on trauma-associated inflammation. Curr Opin Crit Care. 2006;12:595–601. doi: 10.1097/MCC.0b013e3280106806. [DOI] [PubMed] [Google Scholar]
- Cobb JP, O'Keefe GE. Injury research in the genomic era. Lancet. 2004;363:2076–2083. doi: 10.1016/S0140-6736(04)16460-X. [DOI] [PubMed] [Google Scholar]
- Tschoeke SK, Ertel W. Immunoparalysis after multiple trauma. Injury. 2007;38:1346–1357. doi: 10.1016/j.injury.2007.08.041. [DOI] [PubMed] [Google Scholar]
- Shires T, Coln D, Carrico J, Lightfoot S. Fluid therapy in hemorrhagic shock. Arch Surg. 1964;88:688–693. doi: 10.1001/archsurg.1964.01310220178027. [DOI] [PubMed] [Google Scholar]
- Healey MA, Davis RE, Liu FC, Loomis WH, Hoyt DB. Lactated ringer's is superior to normal saline in a model of massive hemorrhage and resuscitation. J Trauma. 1998;45:894–899. doi: 10.1097/00005373-199811000-00010. [DOI] [PubMed] [Google Scholar]
- Todd SR, Malinoski D, Muller PJ, Schreiber MA. Lactated Ringer's is superior to normal saline in the resuscitation of uncontrolled hemorrhagic shock. J Trauma. 2007;62:636–639. doi: 10.1097/TA.0b013e31802ee521. [DOI] [PubMed] [Google Scholar]
- Savage SA, Fitzpatrick CM, Kashyap VS, Clouse WD, Kerby JD. Endothelial dysfunction after lactated Ringer's solution resuscitation for hemorrhagic shock. J Trauma. 2005;59:284–290. doi: 10.1097/01.ta.0000179453.89769.1c. [DOI] [PubMed] [Google Scholar]
- Sims CA, Wattanasirichaigoon S, Menconi MJ, Ajami AM, Fink MP. Ringer's ethyl pyruvate solution ameliorates ischemia/reperfusion-induced intestinal mucosal injury in rats. Crit Care Med. 2001;29:1513–1518. doi: 10.1097/00003246-200108000-00003. [DOI] [PubMed] [Google Scholar]
- Mulier KE, Beilman GJ, Conroy MJ, Taylor JH, Skarda DE, Hammer BE. Ringer's ethyl pyruvate in hemorrhagic shock and resuscitation does not improve early hemodynamics or tissue energetics. Shock. 2005;23:248–252. [PubMed] [Google Scholar]
- Holcroft JW, Trunkey DD. Extravascular lung water following hemorrhagic shock in the baboon: comparison between resuscitation with Ringer's lactate and Plasmanate. Ann Surg. 1974;180:408–417. doi: 10.1097/00000658-197410000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schierhout G, Roberts I. Fluid resuscitation with colloid or crystalloid solutions in critically ill patients: a systematic review of randomised trials. BMJ. 1998;316:961–964. doi: 10.1136/bmj.316.7136.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi PT, Yip G, Quinonez LG, Cook DJ. Crystalloids vs. colloids in fluid resuscitation: a systematic review. Crit Care Med. 1999;27:200–210. doi: 10.1097/00003246-199901000-00053. [DOI] [PubMed] [Google Scholar]
- Rizoli SB. Crystalloids and colloids in trauma resuscitation: a brief overview of the current debate. J Trauma. 2003;54:S82–S88. doi: 10.1097/01.TA.0000064525.03761.0C. [DOI] [PubMed] [Google Scholar]
- Watters JM, Tieu BH, Todd SR, Jackson T, Muller PJ, Malinoski D, Schreiber MA. Fluid resuscitation increases inflammatory gene transcription after traumatic injury. J Trauma. 2006;61:300–308. doi: 10.1097/01.ta.0000224211.36154.44. [DOI] [PubMed] [Google Scholar]
- Roberts I, Alderson P, Bunn F. Colloids versus crystalloids for fluid resuscitation in critically ill patients. Cochrane Database Syst Rev. 2004:CD000567. doi: 10.1002/14651858.CD000567.pub2. [DOI] [PubMed] [Google Scholar]
- Vercueil A, Grocott MP, Mythen MG. Physiology, pharmacology, and rationale for colloid administration for the maintenance of effective hemodynamic stability in critically ill patients. Transfus Med Rev. 2005;19:93–109. doi: 10.1016/j.tmrv.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Lee CC, Chang IJ, Yen ZS. Effect of different resuscitation fluids on cytokine response in a rat model of hemorrhagic shock. Shock. 2005;24:177–181. doi: 10.1097/01.shk.0000171870.42900.15. [DOI] [PubMed] [Google Scholar]
- Cochrane Injuries Group Human albumin administration in critically ill patients: systematic review of randomised controlled trials. Cochrane Injuries Group Albumin Reviewers. BMJ. 1998;317:235–240. doi: 10.1136/bmj.317.7153.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita S, Chuang VT, Kanazawa M, Tanase S, Kawai K, Maruyama T, Suenaga A, Otagiri M. Recombinant human serum albumin dimer has high blood circulation activity and low vascular permeability in comparison with native human serum albumin. Pharm Res. 2006;23:882–891. doi: 10.1007/s11095-006-9933-1. [DOI] [PubMed] [Google Scholar]
- Finfer S, Bellomo R, Boyce N, French J, Myburgh J, Norton R. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004;350:2247–2256. doi: 10.1056/NEJMoa040232. [DOI] [PubMed] [Google Scholar]
- de Felippe J, Jr, Timoner J, Velasco IT, Lopes OU, Rochae-Silva M. Treatment of refractory hypovolaemic shock by 7.5% sodium chloride injections. Lancet. 1980;2:1002–1004. doi: 10.1016/S0140-6736(80)92157-1. [DOI] [PubMed] [Google Scholar]
- Bagshaw SM, Bellomo R. The influence of volume management on outcome. Curr Opin Crit Care. 2007;13:541–548. doi: 10.1097/MCC.0b013e3282e2a978. [DOI] [PubMed] [Google Scholar]
- Nascimento P, Jr, de Paiva FO, de Carvalho LR, Braz JR. Early hemodynamic and renal effects of hemorrhagic shock resuscitation with lactated Ringer's solution, hydroxyethyl starch, and hypertonic saline with or without 6% dextran-70. J Surg Res. 2006;136:98–105. doi: 10.1016/j.jss.2006.04.021. [DOI] [PubMed] [Google Scholar]
- Smith GJ, Kramer GC, Perron P, Nakayama S, Gunther RA, Holcroft JW. A comparison of several hypertonic solutions for resuscitation of bled sheep. J Surg Res. 1985;39:517–528. doi: 10.1016/0022-4804(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Wade CE, Kramer GC, Grady JJ, Fabian TC, Younes RN. Efficacy of hypertonic 7.5% saline and 6% dextran-70 in treating trauma: a meta-analysis of controlled clinical studies. Surgery. 1997;122:609–616. doi: 10.1016/S0039-6060(97)90135-5. [DOI] [PubMed] [Google Scholar]
- Himmelseher S. Hypertonic saline solutions for treatment of intracranial hypertension. Curr Opin Anaesthesiol. 2007;20:414–426. doi: 10.1097/ACO.0b013e3282eff9ea. [DOI] [PubMed] [Google Scholar]
- Cooper DJ, Myles PS, McDermott FT, Murray LJ, Laidlaw J, Cooper G, Tremayne AB, Bernard SS, Ponsford J, HTS Study Investigators Prehospital hypertonic saline resuscitation of patients with hypotension and severe traumatic brain injury: a randomized controlled trial. JAMA. 2004;291:1350–1357. doi: 10.1001/jama.291.11.1350. [DOI] [PubMed] [Google Scholar]
- Kentner R, Safar P, Prueckner S, Behringer W, Wu X, Henchir J, Ruemelin A, Tisherman SA. Titrated hypertonic/hyperoncotic solution for hypotensive fluid resuscitation during uncontrolled hemorrhagic shock in rats. Resuscitation. 2005;65:87–95. doi: 10.1016/j.resuscitation.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Watters JM, Tieu BH, Differding JA, Muller PJ, Schreiber MA. A single bolus of 3% hypertonic saline with 6% dextran provides optimal initial resuscitation after uncontrolled hemorrhagic shock. J Trauma. 2006;61:75–81. doi: 10.1097/01.ta.0000222723.54559.47. [DOI] [PubMed] [Google Scholar]
- Coimbra R, Melbostad H, Hoyt DB. Effects of phosphodiesterase inhibition on the inflammatory response after shock: role of pentoxifylline. J Trauma. 2004;56:442–449. doi: 10.1097/01.TA.0000096642.54111.E8. [DOI] [PubMed] [Google Scholar]
- Yada-Langui MM, Coimbra R, Lancellotti C, Mimica I, Garcia C, Correia N, Jr, Rocha e Silva M. Hypertonic saline and pentoxifylline prevent lung injury and bacterial translocation after hemorrhagic shock. Shock. 2000;14:594–598. doi: 10.1097/00024382-200014060-00004. [DOI] [PubMed] [Google Scholar]
- Coimbra R, Porcides R, Loomis W, Melbostad H, Lall R, Deree J, Wolf P, Hoyt DB. HSPTX protects against hemorrhagic shock resuscitation-induced tissue injury: an attractive alternative to Ringer's lactate. J Trauma. 2006;60:41–51. doi: 10.1097/01.ta.0000197417.03460.0a. [DOI] [PubMed] [Google Scholar]
- Deree J, de CT, Shenvi E, Loomis WH, Hoyt DB, Coimbra R. Hypertonic saline and pentoxifylline attenuates gut injury after hemorrhagic shock: the kinder, gentler resuscitation. J Trauma. 2007;62:818–827. doi: 10.1097/TA.0b013e31802d9745. [DOI] [PubMed] [Google Scholar]
- Brasel KJ, Bulger E, Cook AJ, Morrison LJ, Newgard CD, Tisherman SA, Kerby JD, Coimbra R, Hata JS, Hoyt DB, Resuscitation Outcomes Consortium Investigators Hypertonic resuscitation: design and implementation of a prehospital intervention trial. J Am Coll Surg. 2008;206:220–232. doi: 10.1016/j.jamcollsurg.2007.07.020. [DOI] [PubMed] [Google Scholar]
- Moore FA, Moore EE, Sauaia A. Blood transfusion. An independent risk factor for postinjury multiple organ failure. Arch Surg. 1997;132:620–624. [PubMed] [Google Scholar]
- Gong MN, Thompson BT, Williams P, Pothier L, Boyce PD, Christiani DC. Clinical predictors of and mortality in acute respiratory distress syndrome: potential role of red cell transfusion. Crit Care Med. 2005;33:1191–1198. doi: 10.1097/01.CCM.0000165566.82925.14. [DOI] [PubMed] [Google Scholar]
- Shorr AF, Jackson WL, Kelly KM, Fu M, Kollef MH. Transfusion practice and blood stream infections in critically ill patients. Chest. 2005;127:1722–1728. doi: 10.1378/chest.127.5.1722. [DOI] [PubMed] [Google Scholar]
- Klein HG, Spahn DR, Carson JL. Red blood cell transfusion in clinical practice. Lancet. 2007;370:415–426. doi: 10.1016/S0140-6736(07)61197-0. [DOI] [PubMed] [Google Scholar]
- Spahn DR, Kocian R. Artificial O2 carriers: status in 2005. Curr Pharm Des. 2005;11:4099–4114. doi: 10.2174/138161205774913354. [DOI] [PubMed] [Google Scholar]
- Riess JG. Understanding the fundamentals of perfluorocarbons and perfluorocarbon emulsions relevant to in vivo oxygen delivery. Artif Cells Blood Substit Immobil Biotechnol. 2005;33:47–63. doi: 10.1081/BIO-200046659. [DOI] [PubMed] [Google Scholar]
- Habler OP, Kleen MS, Hutter JW, Podtschaske AH, Tiede M, Kemming GI, Welte MV, Corso CO, Batra S, Keipert PE, Faithfull NS, Messmer KF. Hemodilution and intravenous perflubron emulsion as an alternative to blood transfusion: effects on tissue oxygenation during profound hemodilution in anesthetized dogs. Transfusion. 1998;38:145–155. doi: 10.1046/j.1537-2995.1998.38298193096.x. [DOI] [PubMed] [Google Scholar]
- Habler O, Kleen M, Hutter J, Podtschaske A, Tiede M, Kemming G, Welte M, Corso C, Batra S, Keipert P, Faithfull S, Messmer K. IV perflubron emulsion versus autologous transfusion in severe normovolemic anemia: effects on left ventricular perfusion and function. Res Exp Med (Berl) 1998;197:301–318. doi: 10.1007/s004330050079. [DOI] [PubMed] [Google Scholar]
- Spahn DR, van Brempt R, Theilmeier G, Reibold JP, Welte M, Heinzerling H, Birck KM, Keipert PE, Messmer K, Heinzerling H, Birck KM, Keipert PE, Messmer K. Perflubron emulsion delays blood transfusions in orthopedic surgery. European Perflubron Emulsion Study Group. Anesthesiology. 1999;91:1195–1208. doi: 10.1097/00000542-199911000-00009. [DOI] [PubMed] [Google Scholar]
- Frumento RJ, Mongero L, Naka Y, nett-Guerrero E. Preserved gastric tonometric variables in cardiac surgical patients administered intravenous perflubron emulsion. Anesth Analg. 2002;94:809–814. doi: 10.1097/00000539-200204000-00007. [DOI] [PubMed] [Google Scholar]
- Spahn DR, Waschke KF, Standl T, Motsch J, Van HL, Welte M, Gombotz H, Coriat P, Verkh L, Faithfull S, Kepert P, Eurpoean Perflubron Emulsion in Non-Cardiac Surgery Study Group Use of perflubron emulsion to decrease allogeneic blood transfusion in high-blood-loss non-cardiac surgery: results of a European phase 3 study. Anesthesiology. 2002;97:1338–1349. doi: 10.1097/00000542-200212000-00004. [DOI] [PubMed] [Google Scholar]
- Habler O, Kleen M, Pape A, Meisner F, Kemming G, Messmer K. Diaspirin-crosslinked hemoglobin reduces mortality of severe hemorrhagic shock in pigs with critical coronary stenosis. Crit Care Med. 2000;28:1889–1898. doi: 10.1097/00003246-200006000-00034. [DOI] [PubMed] [Google Scholar]
- Nolte D, Steinhauser P, Pickelmann S, Berger S, Hartl R, Messmer K. Effects of diaspirin-cross-linked hemoglobin (DCLHb) on local tissue oxygen tension in striated skin muscle: an efficacy study in the hamster. J Lab Clin Med. 1997;130:328–338. doi: 10.1016/S0022-2143(97)90028-7. [DOI] [PubMed] [Google Scholar]
- Schultz SC, Hamilton IN, Jr, Malcolm DS. Use of base deficit to compare resuscitation with lactated Ringer's solution, Haemaccel, whole blood, and diaspirin cross-linked hemoglobin following hemorrhage in rats. J Trauma. 1993;35:619–625. doi: 10.1097/00005373-199310000-00019. [DOI] [PubMed] [Google Scholar]
- Sprung J, Mackenzie CF, Barnas GM, Williams JE, Parr M, Christenson RH, Hoff GH, Sakamoto R, Kramer A, Lottes M. Oxygen transport and cardiovascular effects of resuscitation from severe hemorrhagic shock using hemoglobin solutions. Crit Care Med. 1995;23:1540–1553. doi: 10.1097/00003246-199509000-00015. [DOI] [PubMed] [Google Scholar]
- Johnson JL, Moore EE, Gonzalez RJ, Fedel N, Partrick DA, Silliman CC. Alteration of the postinjury hyperinflammatory response by means of resuscitation with a red cell substitute. J Trauma. 2003;54:133–139. doi: 10.1097/00005373-200301000-00016. [DOI] [PubMed] [Google Scholar]
- Pickelmann S, Nolte D, Leiderer R, Schutze E, Messmer K. Attenuation of postischemic reperfusion injury in striated skin muscle by diaspirin-cross-linked Hb. Am J Physiol. 1998;275:H361–H368. doi: 10.1152/ajpheart.1998.275.2.H361. [DOI] [PubMed] [Google Scholar]
- Botzlar A, Nolte D, Messmer K. Effects of ultra-purified polymerized bovine hemoglobin on the microcirculation of striated skin muscle in the hamster. Eur J Med Res. 1996;1:471–478. [PubMed] [Google Scholar]
- Sloan EP, Koenigsberg M, Gens D, Cipolle M, Runge J, Mallory MN, Rodman G., Jr Diaspirin cross-linked hemoglobin (DCLHb) in the treatment of severe traumatic hemorrhagic shock: a randomized controlled efficacy trial. JAMA. 1999;282:1857–1864. doi: 10.1001/jama.282.19.1857. [DOI] [PubMed] [Google Scholar]
- Gould SA, Moore EE, Hoyt DB, Ness PM, Norris EJ, Carson JL, Hides GA, Freeman IH, DeWoskin R, Moss GS. The life-sustaining capacity of human polymerized hemoglobin when red cells might be unavailable. J Am Coll Surg. 2002;195:445–452. doi: 10.1016/S1072-7515(02)01335-2. [DOI] [PubMed] [Google Scholar]
- Alayash AI. Hemoglobin-based blood substitutes: oxygen carriers, pressor agents, or oxidants? Nat Biotechnol. 1999;17:545–549. doi: 10.1038/9849. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick CM, Biggs KL, Atkins BZ, Quance-Fitch FJ, Dixon PS, Savage SA, Jenkins DH, Kerby JD. Prolonged low-volume resuscitation with HBOC-201 in a large-animal survival model of controlled hemorrhage. J Trauma. 2005;59:273–281. doi: 10.1097/01.ta.0000174730.62338.88. [DOI] [PubMed] [Google Scholar]
- Arnaud F, Hammett M, Asher L, Philbin N, Rice J, Dong F, Pearce B, Flournoy WS, Nicholson C, McCarron R, Freilich D. Effects of bovine polymerized hemoglobin on coagulation in controlled hemorrhagic shock in swine. Shock. 2005;24:145–152. doi: 10.1097/01.shk.0000170354.18437.2f. [DOI] [PubMed] [Google Scholar]
- Rice J, Philbin N, Handrigan M, Hall C, McGwin G, Ahlers S, Pearce LB, Arnaud F, McCarron R, Freilich D. Vasoactivity of bovine polymerized hemoglobin (HBOC-201) in swine with traumatic hemorrhagic shock with and without brain injury. J Trauma. 2006;61:1085–1099. doi: 10.1097/01.ta.0000236640.62893.fa. [DOI] [PubMed] [Google Scholar]
- Wettstein R, Tsai AG, Erni D, Winslow RM, Intaglietta M. Resuscitation with polyethylene glycol-modified human hemoglobin improves microcirculatory blood flow and tissue oxygenation after hemorrhagic shock in awake hamsters. Crit Care Med. 2003;31:1824–1830. doi: 10.1097/01.CCM.0000069340.16319.F2. [DOI] [PubMed] [Google Scholar]
- Winslow RM. Current status of oxygen carriers ('blood substitutes'): 2006. Vox Sang. 2006;91:102–110. doi: 10.1111/j.1423-0410.2006.00789.x. [DOI] [PubMed] [Google Scholar]
- Goto Y, Terajima K, Tsueshita T, Miyashita M, Horinouchi H, Sakai H, Tsuchida E, Sakamoto A. Fluid resuscitation with hemoglobin-vesicle solution does not increase hypoxia or inflammatory responses in moderate hemorrhagic shock. Biomed Res. 2006;27:283–288. doi: 10.2220/biomedres.27.283. [DOI] [PubMed] [Google Scholar]
- Terajima K, Tsueshita T, Sakamoto A, Ogawa R. Fluid resuscitation with hemoglobin vesicles in a rabbit model of acute hemorrhagic shock. Shock. 2006;25:184–189. doi: 10.1097/01.shk.0000192118.68295.5d. [DOI] [PubMed] [Google Scholar]
- Voelckel WG, Wenzel V. Managing hemorrhagic shock: fluids on the way out: drugs on the way in? Crit Care Med. 2003;31:2552–2553. doi: 10.1097/01.CCM.0000089944.54391.D6. [DOI] [PubMed] [Google Scholar]
- Chaudry IH, Baue AE. Overview of hemorrhagic shock. In: Cowley RA, Trump BF, editor. Pathophysiology of Shock, Anoxia, and Ischemia. Baltimore: Williams and Wilkins; 1982. pp. 203–219. [Google Scholar]
- Daut J, Maier-Rudolph W, von BN, Mehrke G, Gunther K, Goedel-Meinen L. Hypoxic dilation of coronary arteries is mediated by ATP-sensitive potassium channels. Science. 1990;247:1341–1344. doi: 10.1126/science.2107575. [DOI] [PubMed] [Google Scholar]
- Rushing GD, Britt RC, Collins JN, Cole FJ, Weireter LJ, Britt LD. Adrenal insufficiency in hemorrhagic shock. Am Surg. 2006;72:552–554. [PubMed] [Google Scholar]
- Holmes CL, Landry DW, Granton JT. Science review: vasopressin and the cardiovascular system part 1 – receptor physiology. Crit Care. 2003;7:427–434. doi: 10.1186/cc2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel V, Krismer AC, Arntz HR, Sitter H, Stadlbauer KH, Lindner KH. A comparison of vasopressin and epinephrine for out-of-hospital cardiopulmonary resuscitation. N Engl J Med. 2004;350:105–113. doi: 10.1056/NEJMoa025431. [DOI] [PubMed] [Google Scholar]
- Dunser MW, Mayr AJ, Ulmer H, Knotzer H, Sumann G, Pajk W, et al. Arginine vasopressin in advanced vasodilatory shock: a prospective, randomized, controlled study. Circulation. 2003;107:2313–2319. doi: 10.1161/01.CIR.0000066692.71008.BB. [DOI] [PubMed] [Google Scholar]
- Voelckel WG, Raedler C, Wenzel V, Lindner KH, Krismer AC, Schmittinger CA, Herff H, Rheinberger K, Königsrainer A. Arginine vasopressin, but not epinephrine, improves survival in uncontrolled hemorrhagic shock after liver trauma in pigs. Crit Care Med. 2003;31:1160–1165. doi: 10.1097/01.CCM.0000060014.75282.69. [DOI] [PubMed] [Google Scholar]
- Feinstein AJ, Cohn SM, King DR, Sanui M, Proctor KG. Early vasopressin improves short-term survival after pulmonary contusion. J Trauma. 2005;59:876–882. doi: 10.1097/01.ta.0000187654.24146.22. [DOI] [PubMed] [Google Scholar]
- Sanui M, King DR, Feinstein AJ, Varon AJ, Cohn SM, Proctor KG. Effects of arginine vasopressin during resuscitation from hemorrhagic hypotension after traumatic brain injury. Crit Care Med. 2006;34:433–438. doi: 10.1097/01.CCM.0000196206.83534.39. [DOI] [PubMed] [Google Scholar]
- Lienhart HG, Wenzel V, Braun J, Dorges V, Dunser M, Gries A, Hasibeder WR, Helm M, Lefering R, Schlechtriemen T, Trimmel H, Ulmer H, Ummenhofer W, Voelckel WG, Waydhas C, Lindner K. Vasopressin for therapy of persistent traumatic hemorrhagic shock: the VITRIS.at study. Anaesthesist. 2007;56:145–148. doi: 10.1007/s00101-006-1114-4. [DOI] [PubMed] [Google Scholar]
- Sperry JL, Minei JP, Frankel HL, West MA, Harbrecht BG, Moore EE, Maier RV, Nirula R. Early use of vasopressors after injury: caution before constriction. J Trauma. 2008;64:9–14. doi: 10.1097/TA.0b013e31815dd029. [DOI] [PubMed] [Google Scholar]
- Wu R, Wang P. Preclinical studies with adrenomedullin and its binding protein as cardiovascular protective agents for hemorrhagic shock. Cardiovasc Drug Rev. 2006;24:204–213. doi: 10.1111/j.1527-3466.2006.00204.x. [DOI] [PubMed] [Google Scholar]
- Song GY, Chung CS, Schwacha MG, Jarrar D, Chaudry IH, Ayala A. Splenic immune suppression in sepsis: A role for IL-10-induced changes in P38 MAPK signaling. J Surg Res. 1999;83:36–43. doi: 10.1006/jsre.1998.5556. [DOI] [PubMed] [Google Scholar]
- Yang S, Zhou M, Chaudry IH, Wang P. Novel approach to prevent the transition from the hyperdynamic phase to the hypodynamic phase of sepsis: role of adrenomedullin and adrenomedullin binding protein-1. Ann Surg. 2002;236:625–633. doi: 10.1097/00000658-200211000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R, Dong W, Zhou M, Simms HH, Marini CP, Ravikumar TS, Wang P. Adrenomedullin and adrenomedullin binding protein-1 prevent metabolic acidosis after uncontrolled hemorrhage in rats. Crit Care Med. 2007;35:912–918. doi: 10.1097/01.CCM.0000257327.61829.34. [DOI] [PubMed] [Google Scholar]
- Garrioch MA. The body's response to blood loss. Vox Sang. 2004;87:74–76. doi: 10.1111/j.1741-6892.2004.00435.x. [DOI] [PubMed] [Google Scholar]
- Cosgriff N, Moore EE, Sauaia A, Kenny-Moynihan M, Burch JM, Galloway B. Predicting life-threatening coagulopathy in the massively transfused trauma patient: hypothermia and acidosis revisited. J Trauma. 1997;42:857–861. doi: 10.1097/00005373-199705000-00016. [DOI] [PubMed] [Google Scholar]
- Erber WN, Perry DJ. Plasma and plasma products in the treatment of massive haemorrhage. Best Pract Res Clin Haematol. 2006;19:97–112. doi: 10.1016/j.beha.2005.01.026. [DOI] [PubMed] [Google Scholar]
- Goodnough LT, Shander AS. Recombinant factor VIIa: safety and efficacy. Curr Opin Hematol. 2007;14:504–509. doi: 10.1097/MOH.0b013e32826388c3. [DOI] [PubMed] [Google Scholar]
- Schreiber MA, Holcomb JB, Hedner U, Brundage SI, Macaitis JM, Hoots K. The effect of recombinant factor VIIa on coagulopathic pigs with grade V liver injuries. J Trauma. 2002;53:252–257. doi: 10.1097/00005373-200208000-00011. [DOI] [PubMed] [Google Scholar]
- Martinowitz U, Holcomb JB, Pusateri AE, Stein M, Onaca N, Freidman M, Macaitis JM, Castel D, Hedner U, Hess JR. Intravenous rFVIIa administered for hemorrhage control in hypothermic coagulopathic swine with grade V liver injuries. J Trauma. 2001;50:721–729. doi: 10.1097/00005373-200104000-00021. [DOI] [PubMed] [Google Scholar]
- Spinella PC, Perkins JG, McLaughlin DF, Niles SE, Grathwohl KW, Beekley AC, Salinas J, Mehta S, Wade CE, Holcomb JB. The effect of recombinant activated factor VII on mortality in combat-related casualties with severe trauma and massive transfusion. J Trauma. 2008;64:286–293. doi: 10.1097/TA.0b013e318162759f. [DOI] [PubMed] [Google Scholar]
- Boffard K, Riou B, Warren B, Choong PI, Rizoli S, Rossaint R, Axelsen M, Kluger Y, NovoSeven Trauma Study Group Recombinant factor VIIa as adjunctive therapy for bleeding control in severely injured trauma patients: two parallel randomized, placebo-controlled, double-blind clinical trials. J Trauma. 2005;59:8–15. doi: 10.1097/01.ta.0000171453.37949.b7. [DOI] [PubMed] [Google Scholar]
- Stanworth SJ, Birchall J, Doree CJ, Hyde C. Recombinant factor VIIa for the prevention and treatment of bleeding in patients without haemophilia. Cochrane Database Syst Rev. 2007:CD005011. doi: 10.1002/14651858.CD005011.pub2. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Rossaint R, Riou B, Ozier Y, Zideman D, Spahn DR. Recommendations on the use of recombinant activated factor VII as an adjunctive treatment for massive bleeding: a European perspective. Crit Care. 2006;10:R120. doi: 10.1186/cc5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, Manogue KR, Faist E, Abraham E, Andersson J, Andersson U, Molina PE, Abumrad NN, Sama A, Tracey KJ. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- Yang H, Ochani M, Li J. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc Natl Acad Sci USA. 2004;101:296–301. doi: 10.1073/pnas.2434651100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson U, Wang H, Palmblad K, Aveberger AC, Bloom O, Erlandsson-Harris H, Janson A, Kokkola R, Zhang M, Yang H, Tracey KJ. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med. 2000;192:565–570. doi: 10.1084/jem.192.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendon-Mitchell B, Ochani M, Li J, Han J, Wang H, Yang H, Susarla S, Czura C, Mitchell RA, Chen G, Sama AE, Tracey KJ, Wang H. IFN-gamma induces high mobility group box 1 protein release partly through a TNF-dependent mechanism. J Immunol. 2003;170:3890–3897. doi: 10.4049/jimmunol.170.7.3890. [DOI] [PubMed] [Google Scholar]
- Yang R, Harada T, Mollen KP, Prince JM, Levy RM, Englert JA, Gallowitsch-Puerta M, Yang L, Yang H, Tracey KJ, Harbrecht BG, Billiar TR, Fink MP. Anti-HMGB1 neutralizing antibody ameliorates gut barrier dysfunction and improves survival after hemorrhagic shock. Mol Med. 2006;12:105–114. doi: 10.2119/2006-00010.Yang. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensson J, Matejka GL, Ohlson M, Haraldsson B. Human endothelial cells produce orosomucoid, an important component of the capillary barrier. Am J Physiol. 1999;276:H530–H534. doi: 10.1152/ajpheart.1999.276.2.H530. [DOI] [PubMed] [Google Scholar]
- Vasson MP, Roch-Arveiller M, Couderc R, Baguet JC, Raichvarg D. Effects of alpha-1 acid glycoprotein on human polymor-phonuclear neutrophils: influence of glycan microheterogeneity. Clin Chim Acta. 1994;224:65–71. doi: 10.1016/0009-8981(94)90121-X. [DOI] [PubMed] [Google Scholar]
- Kuebler JF, Toth B, Yokoyama Y, Bland KI, Rue LW, III, Chaudry IH. Alpha1-acid-glycoprotein protects against trauma-hemorrhagic shock. J Surg Res. 2004;119:21–28. doi: 10.1016/j.jss.2003.07.007. [DOI] [PubMed] [Google Scholar]
- Mok YY, Atan MS, Yoke PC, Zhong JW, Bhatia M, Moochhala S, Moore PK. Role of hydrogen sulphide in haemorrhagic shock in the rat: protective effect of inhibitors of hydrogen sulphide biosynthesis. Br J Pharmacol. 2004;143:881–889. doi: 10.1038/sj.bjp.0706014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald MC, Mota-Filipe H, Wright JA, Abdelrahman M, Threadgill MD, Thompson AS, Thiemermann C. Effects of 5-aminoisoquinolinone, a water-soluble, potent inhibitor of the activity of poly (ADP-ribose) polymerase on the organ injury and dysfunction caused by haemorrhagic shock. Br J Pharmacol. 2000;130:843–850. doi: 10.1038/sj.bjp.0703391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink MP, Macias CA, Xiao J, Tyurina YY, Delude RL, Greenberger JS, Kagan VE, Wipf P. Hemigramicidin-TEMPO conjugates: novel mitochondria-targeted antioxidants. Crit Care Med. 2007;35:S461–S467. doi: 10.1097/01.CCM.0000279192.96303.E7. [DOI] [PubMed] [Google Scholar]
- Peckham RM, Handrigan MT, Bentley TB, Falabella MJ, Chrovian AD, Stahl GL, Tsokos GC. C5-blocking antibody reduces fluid requirements and improves responsiveness to fluid infusion in hemorrhagic shock managed with hypotensive resuscitation. J Appl Physiol. 2007;102:673–680. doi: 10.1152/japplphysiol.00917.2006. [DOI] [PubMed] [Google Scholar]
- Chaudry IH, Samy TS, Schwacha MG, Wang P, Rue LW, III, Bland KI. Endocrine targets in experimental shock. J Trauma. 2003;54:S118–S125. doi: 10.1097/01.TA.0000064511.14322.F1. [DOI] [PubMed] [Google Scholar]
- Choudhry MA, Schwacha MG, Hubbard WJ, Kerby JD, Rue LW, Bland KI, Chaudry IH. Gender differences in acute response to trauma-hemorrhage. Shock. 2005;24(suppl 1):101–106. doi: 10.1097/01.shk.0000191341.31530.5e. [DOI] [PubMed] [Google Scholar]
- Angele MK, Frantz MC, Chaudry IH. Gender and sex hormones influence the response to trauma and sepsis – potential therapeutic approaches. Clinics. 2006;61:479–488. doi: 10.1590/S1807-59322006000500017. [DOI] [PubMed] [Google Scholar]
- Choudhry MA, Bland KI, Chaudry IH. Trauma and immune response – effect of gender differences. Injury. 2007;38:1882–91. doi: 10.1016/j.injury.2007.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhry MA, Chaudry IH. 17β-estradiol: a novel hormone for improving immune and cardiovascular responses following trauma-hemorrhage. J Leukoc Biol. 2008;83:518–22. doi: 10.1189/jlb.0607369. [DOI] [PubMed] [Google Scholar]
- Wichmann MW, Angele MK, Ayala A, Cioffi WG, Chaudry IH. Flutamide: a novel agent for restoring the depressed cell-mediated immunity following soft-tissue trauma and hemorrhagic shock. Shock. 1997;8:242–248. doi: 10.1097/00024382-199710000-00002. [DOI] [PubMed] [Google Scholar]
- Angele MK, Wichmann MW, Ayala A, Cioffi WG, Chaudry IH. Testosterone receptor blockade after hemorrhage in males: Restoration of the depressed immune functions and improved survival following subsequent sepsis. Arch Surg. 1997;132:1207–1214. doi: 10.1001/archsurg.1997.01430350057010. [DOI] [PubMed] [Google Scholar]
- Jarrar D, Kuebler JF, Wang P, Bland KI, Chaudry IH. DHEA: a novel adjunct for the treatment of male trauma patients. Trends Mol Med. 2001;7:80–5. doi: 10.1016/S1471-4914(01)01917-7. [DOI] [PubMed] [Google Scholar]
- Jarrar D, Wang P, Song GY, Knoferl MW, Cioffi WG, Bland KI, Chaudry IH. Metoclopramide: a novel adjunct for improving cardiac and hepatocellular functions after trauma-hemorrhage. Am J Physiol Endocrinol Metab. 2000;278:E90–E95. doi: 10.1152/ajpendo.2000.278.1.E90. [DOI] [PubMed] [Google Scholar]
- Zellweger R, Zhu XH, Wichmann MW, Ayala A, DeMaso CM, Chaudry IH. Prolactin administration following hemorrhagic shock improves macrophage cytokine release capacity and decreases mortality from subsequent sepsis. J Immunol. 1996;157:5748–5754. [PubMed] [Google Scholar]
- Zellweger R, Wichmann MW, Ayala A, DeMaso CM, Chaudry IH. Prolactin: a novel and safe immuno-modulating hormone for the treatment of immunodepression following severe hemorrhage. J Surg Res. 1996;63:53–58. doi: 10.1006/jsre.1996.0222. [DOI] [PubMed] [Google Scholar]
- Kuebler JF, Jarrar D, Bland KI, Rue L, III, Wang P, Chaudry IH. Progesterone administration following trauma-hemorrhage in females with decreased sex steroids improves cardiovascular responses. Crit Care Med. 2003;31:1786–1793. doi: 10.1097/01.CCM.0000063441.41446.23. [DOI] [PubMed] [Google Scholar]