Abstract
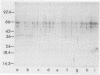
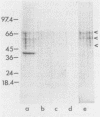
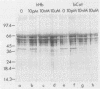
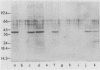
Haemophilus influenzae is a heme-dependent bacterium. However, little is known of the heme-iron uptake mechanism in this organism. By using a batch ligand affinity chromatography method, a hemin-binding protein of 39,500 molecular weight was isolated from total membranes derived from H. influenzae type b grown under iron-depleted but not under iron-sufficient conditions. Detection of the hemin-binding protein in a whole-cell binding assay demonstrated a surface-exposed location. Competition binding experiments indicated that this hemin-protein interaction was specific, since only hemin or heme-containing proteins, such as human hemoglobin and bovine catalase, but not protoporphyrin IX, iron-loaded human lactoferrin, or transferrin, could abrogate binding. In a limited survey of other H. influenzae strains, an identical hemin-binding protein was isolated, implying that this polypeptide may be structurally and functionally conserved among strains.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arcoleo J. P., Greer J. Hemoglobin binding site and its relationship to the serine protease-like active site of haptoglobin. J Biol Chem. 1982 Sep 10;257(17):10063–10068. [PubMed] [Google Scholar]
- BIBERSTEIN E. L., MINI P. D., GILLS M. G. ACTION OF HAEMOPHILUS CULTURES ON DELTA-AMINOLEVULINIC ACID. J Bacteriol. 1963 Oct;86:814–819. doi: 10.1128/jb.86.4.814-819.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaven G. H., Chen S. H., d' Albis A., Gratzer W. B. A spectroscopic study of the haemin--human-serum-albumin system. Eur J Biochem. 1974 Feb 1;41(3):539–546. doi: 10.1111/j.1432-1033.1974.tb03295.x. [DOI] [PubMed] [Google Scholar]
- Brown S. B., Shillcock M., Jones P. Equilibrium and kinetic studies of the aggregation of porphyrins in aqueous solution. Biochem J. 1976 Feb 1;153(2):279–285. doi: 10.1042/bj1530279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullen J. J. The significance of iron in infection. Rev Infect Dis. 1981 Nov-Dec;3(6):1127–1138. doi: 10.1093/clinids/3.6.1127. [DOI] [PubMed] [Google Scholar]
- Crosa J. H. Genetics and molecular biology of siderophore-mediated iron transport in bacteria. Microbiol Rev. 1989 Dec;53(4):517–530. doi: 10.1128/mr.53.4.517-530.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson R. E., Takano T., Eisenberg D., Kallai O. B., Samson L., Cooper A., Margoliash E. Ferricytochrome c. I. General features of the horse and bonito proteins at 2.8 A resolution. J Biol Chem. 1971 Mar 10;246(5):1511–1535. [PubMed] [Google Scholar]
- Evans N. M., Smith D. D., Wicken A. J. Haemin and nicotinamide adenine dinucleotide requirements of Haemophilus influenzae and Haemophilus parainfluenzae. J Med Microbiol. 1974 Aug;7(3):359–365. doi: 10.1099/00222615-7-3-359. [DOI] [PubMed] [Google Scholar]
- Galbraith R. A., McElrath M. J. Heme binding to Leishmania mexicana amazonensis. Mol Biochem Parasitol. 1988 May;29(1):47–53. doi: 10.1016/0166-6851(88)90118-1. [DOI] [PubMed] [Google Scholar]
- Galbraith R. A., Sassa S., Kappas A. Heme binding to murine erythroleukemia cells. Evidence for a heme receptor. J Biol Chem. 1985 Oct 5;260(22):12198–12202. [PubMed] [Google Scholar]
- Gräsbeck R., Kouvonen I., Lundberg M., Tenhunen R. An intestinal receptor for heme. Scand J Haematol. 1979 Jul;23(1):5–9. doi: 10.1111/j.1600-0609.1979.tb02845.x. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M., Smith A. Antioxidant protection by haemopexin of haem-stimulated lipid peroxidation. Biochem J. 1988 Dec 15;256(3):861–865. doi: 10.1042/bj2560861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn C. R. Membrane receptors for hormones and neurotransmitters. J Cell Biol. 1976 Aug;70(2 Pt 1):261–286. doi: 10.1083/jcb.70.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian M. A taxonomic study of the genus Haemophilus, with the proposal of a new species. J Gen Microbiol. 1976 Mar;93(1):9–62. doi: 10.1099/00221287-93-1-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee B. C. Iron sources for Haemophilus ducreyi. J Med Microbiol. 1991 Jun;34(6):317–322. doi: 10.1099/00222615-34-6-317. [DOI] [PubMed] [Google Scholar]
- Lee B. C., Schryvers A. B. Specificity of the lactoferrin and transferrin receptors in Neisseria gonorrhoeae. Mol Microbiol. 1988 Nov;2(6):827–829. doi: 10.1111/j.1365-2958.1988.tb00095.x. [DOI] [PubMed] [Google Scholar]
- Meyer T. E., Cusanovich M. A. Structure, function and distribution of soluble bacterial redox proteins. Biochim Biophys Acta. 1989 Jun 23;975(1):1–28. doi: 10.1016/s0005-2728(89)80196-3. [DOI] [PubMed] [Google Scholar]
- Muller-Eberhard U., Nikkilä H. Transport of tetrapyrroles by proteins. Semin Hematol. 1989 Apr;26(2):86–104. [PubMed] [Google Scholar]
- Neilands J. B. Microbial envelope proteins related to iron. Annu Rev Microbiol. 1982;36:285–309. doi: 10.1146/annurev.mi.36.100182.001441. [DOI] [PubMed] [Google Scholar]
- O'Callaghan C. H., Morris A., Kirby S. M., Shingler A. H. Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972 Apr;1(4):283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Pidcock K. A., Wooten J. A., Daley B. A., Stull T. L. Iron acquisition by Haemophilus influenzae. Infect Immun. 1988 Apr;56(4):721–725. doi: 10.1128/iai.56.4.721-725.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Solubilization of the cytoplasmic membrane of Escherichia coli by Triton X-100. J Bacteriol. 1971 Oct;108(1):545–552. doi: 10.1128/jb.108.1.545-552.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schryvers A. B. Identification of the transferrin- and lactoferrin-binding proteins in Haemophilus influenzae. J Med Microbiol. 1989 Jun;29(2):121–130. doi: 10.1099/00222615-29-2-121. [DOI] [PubMed] [Google Scholar]
- Seery V. L., Muller-Eberhard U. Binding of porphyrins to rabbit hemopexin and albumin. J Biol Chem. 1973 Jun 10;248(11):3796–3800. [PubMed] [Google Scholar]
- Shurin S. B., Anderson P., Zollinger J., Rathbun R. K. Pathophysiology of hemolysis in infections with Hemophilus influenzae type b. J Clin Invest. 1986 Apr;77(4):1340–1348. doi: 10.1172/JCI112439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A., Ledford B. E. Expression of the haemopexin-transport system in cultured mouse hepatoma cells. Links between haemopexin and iron metabolism. Biochem J. 1988 Dec 15;256(3):941–950. doi: 10.1042/bj2560941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A., Morgan W. T. Hemopexin-mediated heme uptake by liver. Characterization of the interaction of heme-hemopexin with isolated rabbit liver plasma membranes. J Biol Chem. 1984 Oct 10;259(19):12049–12053. [PubMed] [Google Scholar]
- Smith M. L., Paul J., Ohlsson P. I., Hjortsberg K., Paul K. G. Heme-protein fission under nondenaturing conditions. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):882–886. doi: 10.1073/pnas.88.3.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stull T. L. Protein sources of heme for Haemophilus influenzae. Infect Immun. 1987 Jan;55(1):148–153. doi: 10.1128/iai.55.1.148-153.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui K., Mueller G. C. Affinity chromatography of heme-binding proteins: an improved method for the synthesis of hemin-agarose. Anal Biochem. 1982 Apr;121(2):244–250. doi: 10.1016/0003-2697(82)90475-4. [DOI] [PubMed] [Google Scholar]
- Vincent S. H. Oxidative effects of heme and porphyrins on proteins and lipids. Semin Hematol. 1989 Apr;26(2):105–113. [PubMed] [Google Scholar]
- WHITE D. C., GRANICK S. HEMIN BIOSYNTHESIS IN HAEMOPHILUS. J Bacteriol. 1963 Apr;85:842–850. doi: 10.1128/jb.85.4.842-850.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE D. C. Respiratory systems in the hemin-requiring Haemophilus species. J Bacteriol. 1963 Jan;85:84–96. doi: 10.1128/jb.85.1.84-96.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg E. D. Iron withholding: a defense against infection and neoplasia. Physiol Rev. 1984 Jan;64(1):65–102. doi: 10.1152/physrev.1984.64.1.65. [DOI] [PubMed] [Google Scholar]
- Zimmermann W., Rosselet A. Function of the outer membrane of Escherichia coli as a permeability barrier to beta-lactam antibiotics. Antimicrob Agents Chemother. 1977 Sep;12(3):368–372. doi: 10.1128/aac.12.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]