Abstract
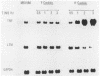
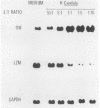
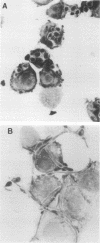
The dimorphic transition of Candida albicans from the yeast (Y-Candida) to the hyphal (H-Candida) form is a complex event; the relevance of this transition in fungal pathogenicity is still poorly understood. By using a cloned macrophage cell line (ANA-1), we questioned whether the interaction between macrophages and Y-Candida or H-Candida could affect specific cell functions, i.e., tumor necrosis factor and lysozyme production. We found that ANA-1 macrophages selectively responded to H-Candida with increased tumor necrosis factor and downregulated lysozyme, as assessed by measurement of relative mRNA levels and secreted biological activities. The H-Candida-mediated effects were (i) dependent upon the ratio between ANA-1 macrophages and H-Candida, (ii) detectable after 1 h of coincubation, and (iii) accomplished without fungal ingestion. Conversely, Y-Candida, which was found inside the ANA-1 macrophages, did not affect tumor necrosis factor and lysozyme production, nor did it prevent the macrophage response to other stimuli. Overall, these results indicate that a macrophage can distinguish between Y-Candida and H-Candida and that only the latter is able to modulate specific functions. H-Candida is recognized and probably processed as an extracellular target. The possible implication of macrophages as autocrine and paracrine regulatory cells during Candida infections is discussed.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai T., Mikami Y., Yokoyama K. Phagocytosis of Candida albicans by rabbit alveolar macrophages and guinea pig neutrophils. Sabouraudia. 1977 Jul;15(2):171–177. [PubMed] [Google Scholar]
- Baccarini M., Bistoni F., Lohmann-Matthes M. L. In vitro natural cell-mediated cytotoxicity against Candida albicans: macrophage precursors as effector cells. J Immunol. 1985 Apr;134(4):2658–2665. [PubMed] [Google Scholar]
- Baccarini M., Bistoni F., Lohmann-Matthes M. L. Organ-associated macrophage precursor activity: isolation of candidacidal and tumoricidal effectors from the spleens of cyclophosphamide-treated mice. J Immunol. 1986 Feb 1;136(3):837–843. [PubMed] [Google Scholar]
- Beno D. W., Mathews H. L. Growth inhibition of Candida albicans by interleukin-2-induced lymph node cells. Cell Immunol. 1990 Jun;128(1):89–100. doi: 10.1016/0008-8749(90)90009-g. [DOI] [PubMed] [Google Scholar]
- Bistoni F., Vecchiarelli A., Cenci E., Puccetti P., Marconi P., Cassone A. Evidence for macrophage-mediated protection against lethal Candida albicans infection. Infect Immun. 1986 Feb;51(2):668–674. doi: 10.1128/iai.51.2.668-674.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bistoni F., Verducci G., Perito S., Vecchiarelli A., Puccetti P., Marconi P., Cassone A. Immunomodulation by a low-virulence, agerminative variant of Candida albicans. Further evidence for macrophage activation as one of the effector mechanisms of nonspecific anti-infectious protection. J Med Vet Mycol. 1988;26(5):285–299. doi: 10.1080/02681218880000401. [DOI] [PubMed] [Google Scholar]
- Blasi E., Barluzzi R., Bocchini V., Mazzolla R., Bistoni F. Immortalization of murine microglial cells by a v-raf/v-myc carrying retrovirus. J Neuroimmunol. 1990 May;27(2-3):229–237. doi: 10.1016/0165-5728(90)90073-v. [DOI] [PubMed] [Google Scholar]
- Blasi E., Farinelli S., Varesio L., Bistoni F. Augmentation of GG2EE macrophage cell line-mediated anti-Candida activity by gamma interferon, tumor necrosis factor, and interleukin-1. Infect Immun. 1990 Apr;58(4):1073–1077. doi: 10.1128/iai.58.4.1073-1077.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasi E., Mathieson B. J., Varesio L., Cleveland J. L., Borchert P. A., Rapp U. R. Selective immortalization of murine macrophages from fresh bone marrow by a raf/myc recombinant murine retrovirus. Nature. 1985 Dec 19;318(6047):667–670. doi: 10.1038/318667a0. [DOI] [PubMed] [Google Scholar]
- Blasi E., Mazzolla R., Barluzzi R., Bistoni F. Microglial cell-mediated anti-Candida activity: temperature, ions, protein kinase C as crucial elements. J Neuroimmunol. 1991 Oct;34(1):53–60. doi: 10.1016/0165-5728(91)90098-r. [DOI] [PubMed] [Google Scholar]
- Blasi E., Mazzolla R., Barluzzi R., Mosci P., Bartoli A., Bistoni F. Intracerebral transfer of an in vitro established microglial cell line: local induction of a protective state against lethal challenge with Candida albicans. J Neuroimmunol. 1991 Jun;32(3):249–257. doi: 10.1016/0165-5728(91)90195-d. [DOI] [PubMed] [Google Scholar]
- Brawner D. L., Cutler J. E. Variability in expression of cell surface antigens of Candida albicans during morphogenesis. Infect Immun. 1986 Jan;51(1):337–343. doi: 10.1128/iai.51.1.337-343.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caput D., Beutler B., Hartog K., Thayer R., Brown-Shimer S., Cerami A. Identification of a common nucleotide sequence in the 3'-untranslated region of mRNA molecules specifying inflammatory mediators. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1670–1674. doi: 10.1073/pnas.83.6.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova M., Chaffin W. L. Phosphate-containing proteins and glycoproteins of the cell wall of Candida albicans. Infect Immun. 1991 Mar;59(3):808–813. doi: 10.1128/iai.59.3.808-813.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassone A. Cell wall of Candida albicans: its functions and its impact on the host. Curr Top Med Mycol. 1989;3:248–314. doi: 10.1007/978-1-4612-3624-5_10. [DOI] [PubMed] [Google Scholar]
- Cho S. Y., Choi H. Y. Opportunistic fungal infection among cancer patients. A ten-year autopsy study. Am J Clin Pathol. 1979 Oct;72(4):617–621. doi: 10.1093/ajcp/72.4.617. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Chung L. P., Keshav S., Gordon S. Cloning the human lysozyme cDNA: inverted Alu repeat in the mRNA and in situ hybridization for macrophages and Paneth cells. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6227–6231. doi: 10.1073/pnas.85.17.6227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. W., Mathieson B. J., Gandino L., Blasi E., Radzioch D., Varesio L. Heterogeneity of hematopoietic cells immortalized by v-myc/v-raf recombinant retrovirus infection of bone marrow or fetal liver. J Natl Cancer Inst. 1989 Oct 4;81(19):1492–1496. doi: 10.1093/jnci/81.19.1492. [DOI] [PubMed] [Google Scholar]
- Diamond R. D., Clark R. A., Haudenschild C. C. Damage to Candida albicans hyphae and pseudohyphae by the myeloperoxidase system and oxidative products of neutrophil metabolism in vitro. J Clin Invest. 1980 Nov;66(5):908–917. doi: 10.1172/JCI109958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond R. D., Krzesicki R., Jao W. Damage to pseudohyphal forms of Candida albicans by neutrophils in the absence of serum in vitro. J Clin Invest. 1978 Feb;61(2):349–359. doi: 10.1172/JCI108945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djeu J. Y., Blanchard D. K., Halkias D., Friedman H. Growth inhibition of Candida albicans by human polymorphonuclear neutrophils: activation by interferon-gamma and tumor necrosis factor. J Immunol. 1986 Nov 1;137(9):2980–2984. [PubMed] [Google Scholar]
- Djeu J. Y., Blanchard D. K., Richards A. L., Friedman H. Tumor necrosis factor induction by Candida albicans from human natural killer cells and monocytes. J Immunol. 1988 Dec 1;141(11):4047–4052. [PubMed] [Google Scholar]
- Ferrante A. Tumor necrosis factor alpha potentiates neutrophil antimicrobial activity: increased fungicidal activity against Torulopsis glabrata and Candida albicans and associated increases in oxygen radical production and lysosomal enzyme release. Infect Immun. 1989 Jul;57(7):2115–2122. doi: 10.1128/iai.57.7.2115-2122.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukazawa Y., Kagaya K. Host defence mechanisms against fungal infection. Microbiol Sci. 1988 Apr;5(4):124–127. [PubMed] [Google Scholar]
- Hasday J. D., Shah E. M., Lieberman A. P. Macrophage tumor necrosis factor-alpha release is induced by contact with some tumors. J Immunol. 1990 Jul 1;145(1):371–379. [PubMed] [Google Scholar]
- Jänicke R., Männel D. N. Distinct tumor cell membrane constituents activate human monocytes for tumor necrosis factor synthesis. J Immunol. 1990 Feb 1;144(3):1144–1150. [PubMed] [Google Scholar]
- Kindler V., Sappino A. P., Grau G. E., Piguet P. F., Vassalli P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell. 1989 Mar 10;56(5):731–740. doi: 10.1016/0092-8674(89)90676-4. [DOI] [PubMed] [Google Scholar]
- Klein R. S., Harris C. A., Small C. B., Moll B., Lesser M., Friedland G. H. Oral candidiasis in high-risk patients as the initial manifestation of the acquired immunodeficiency syndrome. N Engl J Med. 1984 Aug 9;311(6):354–358. doi: 10.1056/NEJM198408093110602. [DOI] [PubMed] [Google Scholar]
- Lehrer R. I., Szklarek D., Ganz T., Selsted M. E. Synergistic activity of rabbit granulocyte peptides against Candida albicans. Infect Immun. 1986 Jun;52(3):902–904. doi: 10.1128/iai.52.3.902-904.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitz S. M., Lyman C. A., Murata T., Sullivan J. A., Mandell G. L., Diamond R. D. Cytosolic calcium changes in individual neutrophils stimulated by opsonized and unopsonized Candida albicans hyphae. Infect Immun. 1987 Nov;55(11):2783–2788. doi: 10.1128/iai.55.11.2783-2788.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyman C. A., Simons E. R., Melnick D. A., Diamond R. D. Unopsonized Candida albicans hyphae stimulate a neutrophil respiratory burst and a cytosolic calcium flux without membrane depolarization. J Infect Dis. 1987 Nov;156(5):770–776. doi: 10.1093/infdis/156.5.770. [DOI] [PubMed] [Google Scholar]
- Marconi P., Bistoni F., Boncio L., Bersiani A., Bravi P., Pitzurra M. Utilizzazione di soluzione salina ipertonica di cloruro di potassio (3M KC1) per l'estrazione di antigeni solubili da Candia albicans. Ann Sclavo. 1976 Jan-Feb;18(1):61–66. [PubMed] [Google Scholar]
- Meshulam T., Diamond R. D., Lyman C. A., Wysong D. R., Melnick D. A. Temporal association of calcium mobilization, inositol trisphosphate generation, and superoxide anion release by human neutrophils activated by serum opsonized and nonopsonized particulate stimuli. Biochem Biophys Res Commun. 1988 Jan 29;150(2):532–539. doi: 10.1016/0006-291x(88)90426-3. [DOI] [PubMed] [Google Scholar]
- Mildvan D., Mathur U., Enlow R. W., Romain P. L., Winchester R. J., Colp C., Singman H., Adelsberg B. R., Spigland I. Opportunistic infections and immune deficiency in homosexual men. Ann Intern Med. 1982 Jun;96(6 Pt 1):700–704. doi: 10.7326/0003-4819-96-6-700. [DOI] [PubMed] [Google Scholar]
- Osserman E. F., Lawlor D. P. Serum and urinary lysozyme (muramidase) in monocytic and monomyelocytic leukemia. J Exp Med. 1966 Nov 1;124(5):921–952. doi: 10.1084/jem.124.5.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin B. Y., Anderson S. L., Sullivan S. A., Williamson B. D., Carswell E. A., Old L. J. Purification and characterization of a human tumor necrosis factor from the LuKII cell line. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6637–6641. doi: 10.1073/pnas.82.19.6637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena A., McElhaney-Feser G. E., Cihlar R. L. Mannan composition of the hyphal form of two relatively avirulent mutants of Candida albicans. Infect Immun. 1990 Jul;58(7):2061–2066. doi: 10.1128/iai.58.7.2061-2066.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaringi L., Blasi E., Cornacchione P., Bietta C., Bistoni F. A rapid Candida albicans hyphal-form growth inhibition assay: determination of myelomonocytic-mediated antifungal activity. Mycoses. 1991 Mar-Apr;34(3-4):119–123. doi: 10.1111/j.1439-0507.1991.tb00631.x. [DOI] [PubMed] [Google Scholar]
- Torosantucci A., Boccanera M., Casalinuovo I., Pellegrini G., Cassone A. Differences in the antigenic expression of immunomodulatory mannoprotein constituents on yeast and mycelial forms of Candida albicans. J Gen Microbiol. 1990 Jul;136(7):1421–1428. doi: 10.1099/00221287-136-7-1421. [DOI] [PubMed] [Google Scholar]
- Vecchiarelli A., Bistoni F., Cenci E., Perito S., Cassone A. In-vitro killing of Candida species by murine immunoeffectors and its relationship to the experimental pathogenicity. Sabouraudia. 1985 Oct;23(5):377–387. doi: 10.1080/00362178585380541. [DOI] [PubMed] [Google Scholar]
- Vecchiarelli A., Cenci E., Puliti M., Blasi E., Puccetti P., Cassone A., Bistoni F. Protective immunity induced by low-virulence Candida albicans: cytokine production in the development of the anti-infectious state. Cell Immunol. 1989 Dec;124(2):334–344. doi: 10.1016/0008-8749(89)90135-4. [DOI] [PubMed] [Google Scholar]
- Vecchiarelli A., Mazzolla R., Farinelli S., Cassone A., Bistoni F. Immunomodulation by Candida albicans: crucial role of organ colonization and chronic infection with an attenuated agerminative strain of C. albicans for establishment of anti-infectious protection. J Gen Microbiol. 1988 Sep;134(9):2583–2592. doi: 10.1099/00221287-134-9-2583. [DOI] [PubMed] [Google Scholar]
- Vecchiarelli A., Puliti M., Torosantucci A., Cassone A., Bistoni F. In vitro production of tumor necrosis factor by murine splenic macrophages stimulated with mannoprotein constituents of Candida albicans cell wall. Cell Immunol. 1991 Apr 15;134(1):65–76. doi: 10.1016/0008-8749(91)90331-5. [DOI] [PubMed] [Google Scholar]
- Vecchiarelli A., Todisco T., Puliti M., Dottorini M., Bistoni F. Modulation of anti-Candida activity of human alveolar macrophages by interferon-gamma or interleukin-1-alpha. Am J Respir Cell Mol Biol. 1989 Jul;1(1):49–55. doi: 10.1165/ajrcmb/1.1.49. [DOI] [PubMed] [Google Scholar]