Abstract
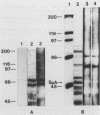
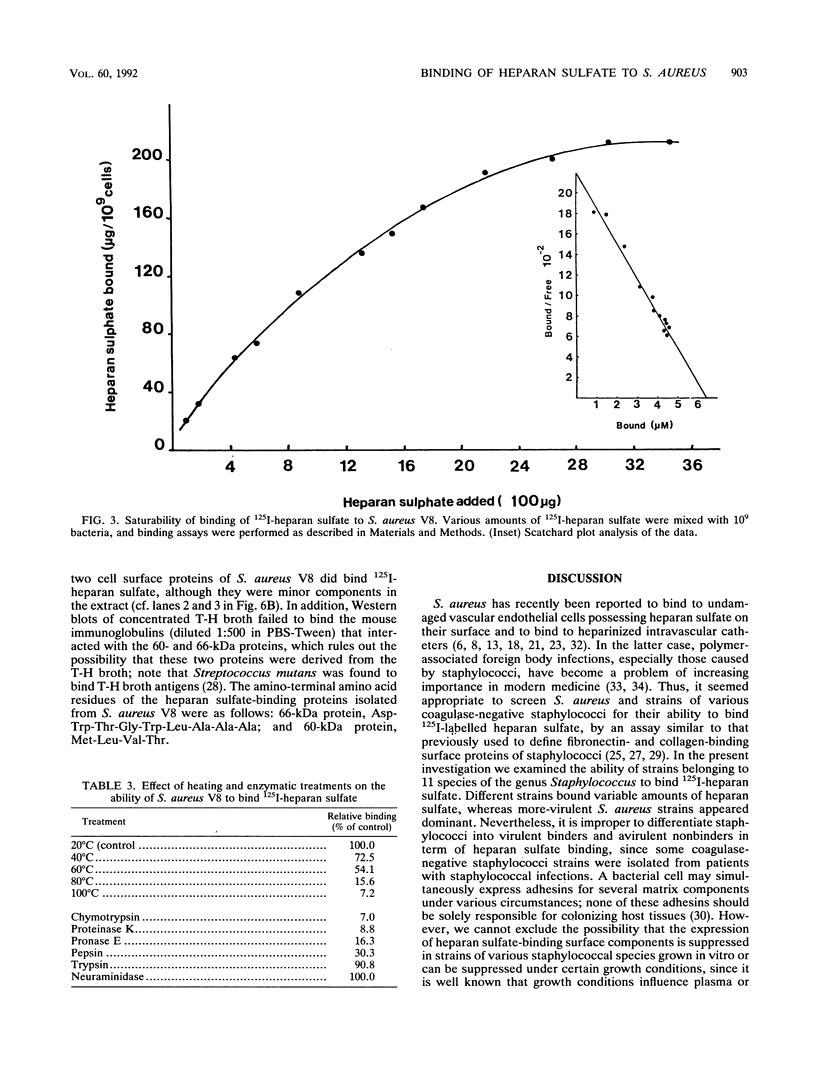
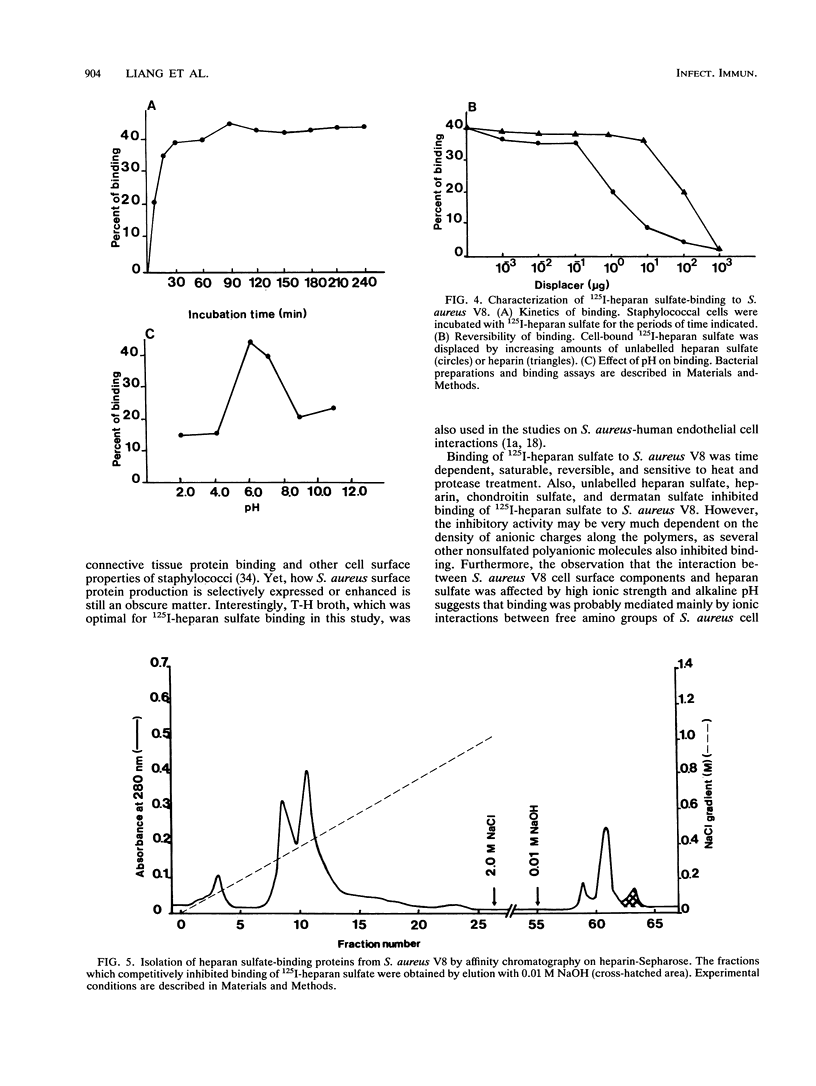
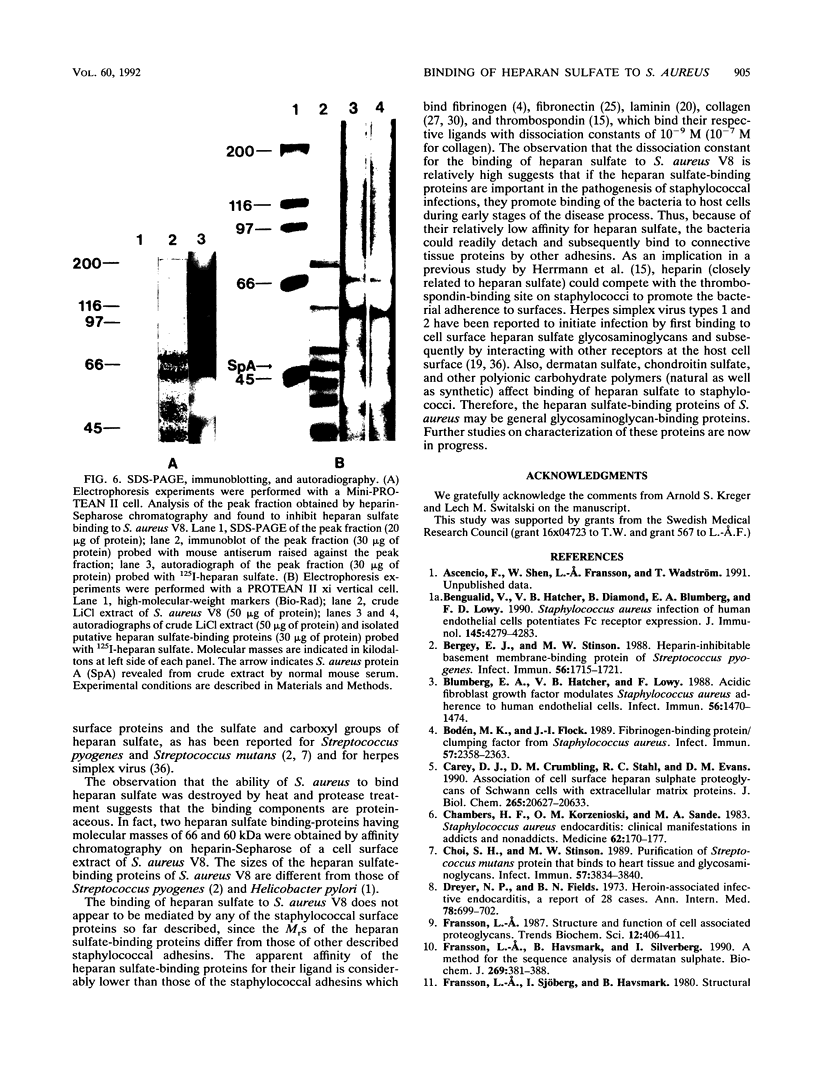
Heparan sulfate binds to proteins present on the surface of Staphylococcus aureus cells. Binding of 125I-heparan sulfate to S. aureus was time dependent, saturable, and influenced by pH and ionic strength, and cell-bound 125I-heparan sulfate was displaced by unlabelled heparan sulfate or heparin. Other glycosaminoglycans of comparable size (chondroitin sulfate and dermatan sulfate), highly glycosylated glycoprotein (hog gastric mucin), and some anionic polysaccharides (dextran sulfate and RNA) inhibited heparan sulfate binding to various extents. Heat treatment (80 degrees C for 10 min) and treatment of the bacteria with pronase E, proteinase K, pepsin, and chymotrypsin considerably reduced their ability to bind 125I-heparan sulfate, but treatment with trypsin and neuraminidase did not affect binding. Scatchard plot analysis indicated the presence of cell surface components with low affinity (Kd = 3 x 10(-5) M) for heparan sulfate. Cell surface components were released by stirring bacteria with 1 M LiCl at 37 degrees C for 2 h. Proteins of this extract that competitively inhibited binding of 125I-heparan sulfate to S. aureus were isolated by affinity chromatography on heparin-Sepharose. Two proteins having molecular masses of approximately 66 and 60 kDa and the ability to bind 125I-heparan sulfate were obtained. The first 9 amino-terminal amino acid residues of the 66-kDa protein are Asp-Trp-Thr-Gly-Trp-Leu-Ala-Ala-Ala, and the first 4 amino-terminal amino acid residues of the 60-kDa protein are Met-Leu-Val-Thr.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bengualid V., Hatcher V. B., Diamond B., Blumberg E. A., Lowy F. D. Staphylococcus aureus infection of human endothelial cells potentiates Fc receptor expression. J Immunol. 1990 Dec 15;145(12):4279–4283. [PubMed] [Google Scholar]
- Bergey E. J., Stinson M. W. Heparin-inhibitable basement membrane-binding protein of Streptococcus pyogenes. Infect Immun. 1988 Jul;56(7):1715–1721. doi: 10.1128/iai.56.7.1715-1721.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg E. A., Hatcher V. B., Lowy F. D. Acidic fibroblast growth factor modulates Staphylococcus aureus adherence to human endothelial cells. Infect Immun. 1988 Jun;56(6):1470–1474. doi: 10.1128/iai.56.6.1470-1474.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodén M. K., Flock J. I. Fibrinogen-binding protein/clumping factor from Staphylococcus aureus. Infect Immun. 1989 Aug;57(8):2358–2363. doi: 10.1128/iai.57.8.2358-2363.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey D. J., Crumbling D. M., Stahl R. C., Evans D. M. Association of cell surface heparan sulfate proteoglycans of Schwann cells with extracellular matrix proteins. J Biol Chem. 1990 Nov 25;265(33):20627–20633. [PubMed] [Google Scholar]
- Chambers H. F., Korzeniowski O. M., Sande M. A. Staphylococcus aureus endocarditis: clinical manifestations in addicts and nonaddicts. Medicine (Baltimore) 1983 May;62(3):170–177. [PubMed] [Google Scholar]
- Choi S. H., Stinson M. W. Purification of a Streptococcus mutans protein that binds to heart tissue and glycosaminoglycans. Infect Immun. 1989 Dec;57(12):3834–3840. doi: 10.1128/iai.57.12.3834-3840.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer N. P., Fields B. N. Heroin-associated infective endocarditis. A report of 28 cases. Ann Intern Med. 1973 May;78(5):699–702. doi: 10.7326/0003-4819-78-5-699. [DOI] [PubMed] [Google Scholar]
- Fransson L. A., Havsmark B., Silverberg I. A method for the sequence analysis of dermatan sulphate. Biochem J. 1990 Jul 15;269(2):381–388. doi: 10.1042/bj2690381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher J. T., Lyon M., Steward W. P. Structure and function of heparan sulphate proteoglycans. Biochem J. 1986 Jun 1;236(2):313–325. doi: 10.1042/bj2360313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould K., Ramirez-Ronda C. H., Holmes R. K., Sanford J. P. Adherence of bacteria to heart valves in vitro. J Clin Invest. 1975 Dec;56(6):1364–1370. doi: 10.1172/JCI108216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heremans A., De Cock B., Cassiman J. J., Van den Berghe H., David G. The core protein of the matrix-associated heparan sulfate proteoglycan binds to fibronectin. J Biol Chem. 1990 May 25;265(15):8716–8724. [PubMed] [Google Scholar]
- Herrmann M., Suchard S. J., Boxer L. A., Waldvogel F. A., Lew P. D. Thrombospondin binds to Staphylococcus aureus and promotes staphylococcal adherence to surfaces. Infect Immun. 1991 Jan;59(1):279–288. doi: 10.1128/iai.59.1.279-288.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koliakos G. G., Kouzi-Koliakos K., Furcht L. T., Reger L. A., Tsilibary E. C. The binding of heparin to type IV collagen: domain specificity with identification of peptide sequences from the alpha 1(IV) and alpha 2(IV) which preferentially bind heparin. J Biol Chem. 1989 Feb 5;264(4):2313–2323. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lowy F. D., Fant J., Higgins L. L., Ogawa S. K., Hatcher V. B. Staphylococcus aureus--human endothelial cell interactions. J Ultrastruct Mol Struct Res. 1988 Feb;98(2):137–146. doi: 10.1016/s0889-1605(88)80906-6. [DOI] [PubMed] [Google Scholar]
- Lycke E., Johansson M., Svennerholm B., Lindahl U. Binding of herpes simplex virus to cellular heparan sulphate, an initial step in the adsorption process. J Gen Virol. 1991 May;72(Pt 5):1131–1137. doi: 10.1099/0022-1317-72-5-1131. [DOI] [PubMed] [Google Scholar]
- Mota G. F., Carneiro C. R., Gomes L., Lopes J. D. Monoclonal antibodies to Staphylococcus aureus laminin-binding proteins cross-react with mammalian cells. Infect Immun. 1988 Jun;56(6):1580–1584. doi: 10.1128/iai.56.6.1580-1584.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S. K., Yurberg E. R., Hatcher V. B., Levitt M. A., Lowy F. D. Bacterial adherence to human endothelial cells in vitro. Infect Immun. 1985 Oct;50(1):218–224. doi: 10.1128/iai.50.1.218-224.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsson M., Wadström T. Vitronectin and type-I collagen binding by Staphylococcus aureus and coagulase-negative staphylococci. FEMS Microbiol Immunol. 1990 May;2(1):55–62. doi: 10.1111/j.1574-6968.1990.tb03479.x. [DOI] [PubMed] [Google Scholar]
- Reisberg B. E. Infective endocarditis in the narcotic addict. Prog Cardiovasc Dis. 1979 Nov-Dec;22(3):193–204. doi: 10.1016/0033-0620(79)90023-9. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. Proteoglycans in cell regulation. J Biol Chem. 1989 Aug 15;264(23):13369–13372. [PubMed] [Google Scholar]
- Rydén C., Rubin K., Speziale P., Hök M., Lindberg M., Wadström T. Fibronectin receptors from Staphylococcus aureus. J Biol Chem. 1983 Mar 10;258(5):3396–3401. [PubMed] [Google Scholar]
- Schmidtchen A., Carlstedt I., Malmström A., Fransson L. A. Inventory of human skin fibroblast proteoglycans. Identification of multiple heparan and chondroitin/dermatan sulphate proteoglycans. Biochem J. 1990 Jan 1;265(1):289–300. doi: 10.1042/bj2650289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speziale P., Raucci G., Visai L., Switalski L. M., Timpl R., Hök M. Binding of collagen to Staphylococcus aureus Cowan 1. J Bacteriol. 1986 Jul;167(1):77–81. doi: 10.1128/jb.167.1.77-81.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinson M. W., Jones C. A. Binding of Todd-Hewitt broth antigens by Streptococcus mutans. Infect Immun. 1983 Jun;40(3):1140–1145. doi: 10.1128/iai.40.3.1140-1145.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Switalski L. M., Rydén C., Rubin K., Ljungh A., Hök M., Wadström T. Binding of fibronectin to Staphylococcus strains. Infect Immun. 1983 Nov;42(2):628–633. doi: 10.1128/iai.42.2.628-633.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Switalski L. M., Speziale P., Hök M. Isolation and characterization of a putative collagen receptor from Staphylococcus aureus strain Cowan 1. J Biol Chem. 1989 Dec 15;264(35):21080–21086. [PubMed] [Google Scholar]
- Tompkins D. C., Hatcher V. B., Patel D., Orr G. A., Higgins L. L., Lowy F. D. A human endothelial cell membrane protein that binds Staphylococcus aureus in vitro. J Clin Invest. 1990 Apr;85(4):1248–1254. doi: 10.1172/JCI114560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercellotti G. M., Lussenhop D., Peterson P. K., Furcht L. T., McCarthy J. B., Jacob H. S., Moldow C. F. Bacterial adherence to fibronectin and endothelial cells: a possible mechanism for bacterial tissue tropism. J Lab Clin Med. 1984 Jan;103(1):34–43. [PubMed] [Google Scholar]
- Wadström T. Molecular aspects on pathogenesis of wound and foreign body infections due to staphylococci. Zentralbl Bakteriol Mikrobiol Hyg A. 1987 Aug;266(1-2):191–211. doi: 10.1016/s0176-6724(87)80032-9. [DOI] [PubMed] [Google Scholar]
- Westergren-Thorsson G., Onnervik P. O., Fransson L. A., Malmström A. Proliferation of cultured fibroblasts is inhibited by L-iduronate-containing glycosaminoglycans. J Cell Physiol. 1991 Jun;147(3):523–530. doi: 10.1002/jcp.1041470319. [DOI] [PubMed] [Google Scholar]
- WuDunn D., Spear P. G. Initial interaction of herpes simplex virus with cells is binding to heparan sulfate. J Virol. 1989 Jan;63(1):52–58. doi: 10.1128/jvi.63.1.52-58.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]