Abstract
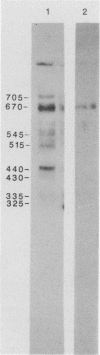
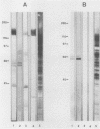
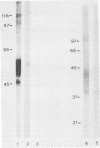
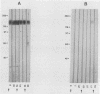
Pneumocystis carinii contains a major group of antigens which migrates as a broad band of 45 to 55 kDa and 35 to 45 kDa in organisms derived from rats and humans, respectively. This complex is among the most common P. carinii antigens found in the respiratory tract and is recognized by serum antibodies of infected individuals. We have isolated a cDNA clone encoding the 3' portion of a 45- to 55-kDa antigen of rat-derived P. carinii. The predicted protein encoded by this cDNA contains a distinctive domain composed of 10 copies of a 7-amino-acid sequence motif rich in glutamic acid residues. Affinity-purified antibodies to this peptide reacted with the 45- to 55-kDa band of rat-derived P. carinii and with the 35- to 45-kDa band of human-derived P. carinii, indicating shared epitopes. The fusion protein was recognized by serum antibodies from rats and humans with natural exposure to P. carinii and by human immunodeficiency virus patients with P. carinii pneumonia. The production of this recombinant protein should allow more detailed studies of the host-parasite relationship of this important opportunistic infection.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison L. A., Wong J. K., Fitzpatrick V. D., Moyle M., Ingles C. J. The C-terminal domain of the largest subunit of RNA polymerase II of Saccharomyces cerevisiae, Drosophila melanogaster, and mammals: a conserved structure with an essential function. Mol Cell Biol. 1988 Jan;8(1):321–329. doi: 10.1128/mcb.8.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beall J. A., Mitchell G. F. Identification of a particular antigen from a parasite cDNA library using antibodies affinity purified from selected portions of Western blots. J Immunol Methods. 1986 Feb 12;86(2):217–223. doi: 10.1016/0022-1759(86)90456-4. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- De Stefano J. A., Cushion M. T., Sleight R. G., Walzer P. D. Analysis of Pneumocystis carinii cyst wall. I. Evidence for an outer surface membrane. J Protozool. 1990 Sep-Oct;37(5):428–435. doi: 10.1111/j.1550-7408.1990.tb01167.x. [DOI] [PubMed] [Google Scholar]
- Dziegiel M., Borre M. B., Jepsen S., Hogh B., Petersen E., Vuust J. Recombinant Plasmodium falciparum glutamate rich protein; purification and use in enzyme-linked immunosorbent assay. Am J Trop Med Hyg. 1991 Mar;44(3):306–313. doi: 10.4269/ajtmh.1991.44.306. [DOI] [PubMed] [Google Scholar]
- Edman J. C., Edman U., Cao M., Lundgren B., Kovacs J. A., Santi D. V. Isolation and expression of the Pneumocystis carinii dihydrofolate reductase gene. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8625–8629. doi: 10.1073/pnas.86.22.8625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman J. C., Kovacs J. A., Masur H., Santi D. V., Elwood H. J., Sogin M. L. Ribosomal RNA sequence shows Pneumocystis carinii to be a member of the fungi. Nature. 1988 Aug 11;334(6182):519–522. doi: 10.1038/334519a0. [DOI] [PubMed] [Google Scholar]
- Edman U., Edman J. C., Lundgren B., Santi D. V. Isolation and expression of the Pneumocystis carinii thymidylate synthase gene. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6503–6507. doi: 10.1073/pnas.86.17.6503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichinger D. J., Arnot D. E., Tam J. P., Nussenzweig V., Enea V. Circumsporozoite protein of Plasmodium berghei: gene cloning and identification of the immunodominant epitopes. Mol Cell Biol. 1986 Nov;6(11):3965–3972. doi: 10.1128/mcb.6.11.3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezekowitz R. A., Williams D. J., Koziel H., Armstrong M. Y., Warner A., Richards F. F., Rose R. M. Uptake of Pneumocystis carinii mediated by the macrophage mannose receptor. Nature. 1991 May 9;351(6322):155–158. doi: 10.1038/351155a0. [DOI] [PubMed] [Google Scholar]
- Gigliotti F., Stokes D. C., Cheatham A. B., Davis D. S., Hughes W. T. Development of murine monoclonal antibodies to Pneumocystis carinii. J Infect Dis. 1986 Aug;154(2):315–322. doi: 10.1093/infdis/154.2.315. [DOI] [PubMed] [Google Scholar]
- Graves D. C., McNabb S. J., Worley M. A., Downs T. D., Ivey M. H. Analyses of rat Pneumocystis carinii antigens recognized by human and rat antibodies by using western immunoblotting. Infect Immun. 1986 Oct;54(1):96–103. doi: 10.1128/iai.54.1.96-103.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hagler D. N., Deepe G. S., Pogue C. L., Walzer P. D. Blastogenic responses to Pneumocystis carinii among patients with human immunodeficiency (HIV) infection. Clin Exp Immunol. 1988 Oct;74(1):7–13. [PMC free article] [PubMed] [Google Scholar]
- Hong S. T., Steele P. E., Cushion M. T., Walzer P. D., Stringer S. L., Stringer J. R. Pneumocystis carinii karyotypes. J Clin Microbiol. 1990 Aug;28(8):1785–1795. doi: 10.1128/jcm.28.8.1785-1795.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahlon J., Whitley R. J. Antibody response of the newborn after herpes simplex virus infection. J Infect Dis. 1988 Nov;158(5):925–933. doi: 10.1093/infdis/158.5.925. [DOI] [PubMed] [Google Scholar]
- Kovacs J. A., Halpern J. L., Lundgren B., Swan J. C., Parrillo J. E., Masur H. Monoclonal antibodies to Pneumocystis carinii: identification of specific antigens and characterization of antigenic differences between rat and human isolates. J Infect Dis. 1989 Jan;159(1):60–70. doi: 10.1093/infdis/159.1.60. [DOI] [PubMed] [Google Scholar]
- Li W. B., Bzik D. J., Gu H. M., Tanaka M., Fox B. A., Inselburg J. An enlarged largest subunit of Plasmodium falciparum RNA polymerase II defines conserved and variable RNA polymerase domains. Nucleic Acids Res. 1989 Dec 11;17(23):9621–9636. doi: 10.1093/nar/17.23.9621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linke M. J., Cushion M. T., Walzer P. D. Properties of the major antigens of rat and human Pneumocystis carinii. Infect Immun. 1989 May;57(5):1547–1555. doi: 10.1128/iai.57.5.1547-1555.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgren B., Lipschik G. Y., Kovacs J. A. Purification and characterization of a major human Pneumocystis carinii surface antigen. J Clin Invest. 1991 Jan;87(1):163–170. doi: 10.1172/JCI114966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peglow S. L., Smulian A. G., Linke M. J., Pogue C. L., Nurre S., Crisler J., Phair J., Gold J. W., Armstrong D., Walzer P. D. Serologic responses to Pneumocystis carinii antigens in health and disease. J Infect Dis. 1990 Feb;161(2):296–306. doi: 10.1093/infdis/161.2.296. [DOI] [PubMed] [Google Scholar]
- Pisetsky D. S., Grudier J. P. Polyspecific binding of Escherichia coli beta-galactosidase by murine antibodies to DNA. J Immunol. 1989 Dec 1;143(11):3609–3613. [PubMed] [Google Scholar]
- Pottratz S. T., Martin W. J., 2nd Mechanism of Pneumocystis carinii attachment to cultured rat alveolar macrophages. J Clin Invest. 1990 Nov;86(5):1678–1683. doi: 10.1172/JCI114891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pottratz S. T., Martin W. J., 2nd Role of fibronectin in Pneumocystis carinii attachment to cultured lung cells. J Clin Invest. 1990 Feb;85(2):351–356. doi: 10.1172/JCI114445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radding J. A., Armstrong M. Y., Ullu E., Richards F. F. Identification and isolation of a major cell surface glycoprotein of Pneumocystis carinii. Infect Immun. 1989 Jul;57(7):2149–2157. doi: 10.1128/iai.57.7.2149-2157.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smythe J. A., Coppel R. L., Brown G. V., Ramasamy R., Kemp D. J., Anders R. F. Identification of two integral membrane proteins of Plasmodium falciparum. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5195–5199. doi: 10.1073/pnas.85.14.5195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringer S. L., Hong S. T., Giuntoli D., Stringer J. R. Repeated DNA in Pneumocystis carinii. J Clin Microbiol. 1991 Jun;29(6):1194–1201. doi: 10.1128/jcm.29.6.1194-1201.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringer S. L., Stringer J. R., Blase M. A., Walzer P. D., Cushion M. T. Pneumocystis carinii: sequence from ribosomal RNA implies a close relationship with fungi. Exp Parasitol. 1989 May;68(4):450–461. doi: 10.1016/0014-4894(89)90130-6. [DOI] [PubMed] [Google Scholar]
- Walzer P. D., Linke M. J. A comparison of the antigenic characteristics of rat and human Pneumocystis carinii by immunoblotting. J Immunol. 1987 Apr 1;138(7):2257–2265. [PubMed] [Google Scholar]
- Walzer P. D., Rutledge M. E. Comparison of rat, mouse, and human Pneumocystis carinii by immunofluorescence. J Infect Dis. 1980 Sep;142(3):449–449. doi: 10.1093/infdis/142.3.449. [DOI] [PubMed] [Google Scholar]
- Walzer P. D., Rutledge M. E. Humoral immunity in experimental Pneumocystis carinii infection. I. Serum and bronchial lavage fluid antibody responses in rats. J Lab Clin Med. 1981 Jun;97(6):820–833. [PubMed] [Google Scholar]
- Walzer P. D., Stanforth D., Linke M. J., Cushion M. T. Pneumocystis carinii: immunoblotting and immunofluorescent analyses of serum antibodies during experimental rat infection and recovery. Exp Parasitol. 1987 Jun;63(3):319–328. doi: 10.1016/0014-4894(87)90179-2. [DOI] [PubMed] [Google Scholar]
- Watanabe J., Hori H., Tanabe K., Nakamura Y. Phylogenetic association of Pneumocystis carinii with the 'Rhizopoda/Myxomycota/Zygomycota group' indicated by comparison of 5S ribosomal RNA sequences. Mol Biochem Parasitol. 1989 Jan 15;32(2-3):163–167. doi: 10.1016/0166-6851(89)90067-4. [DOI] [PubMed] [Google Scholar]