Abstract
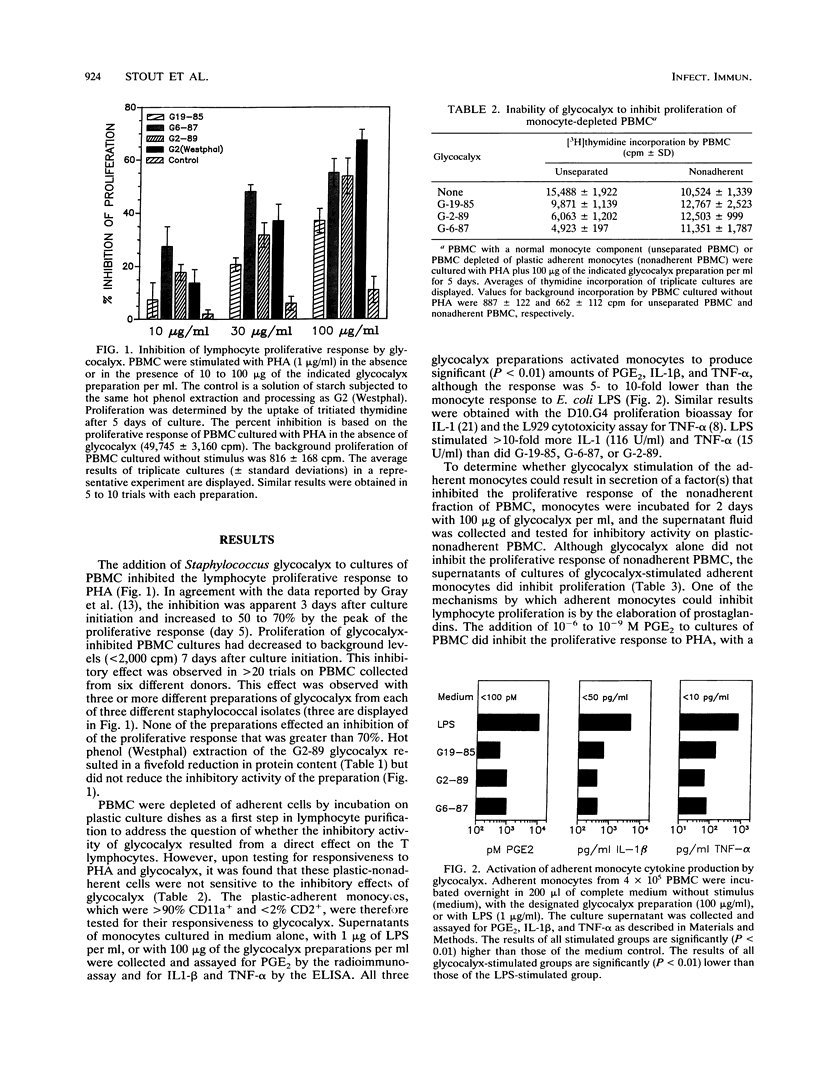
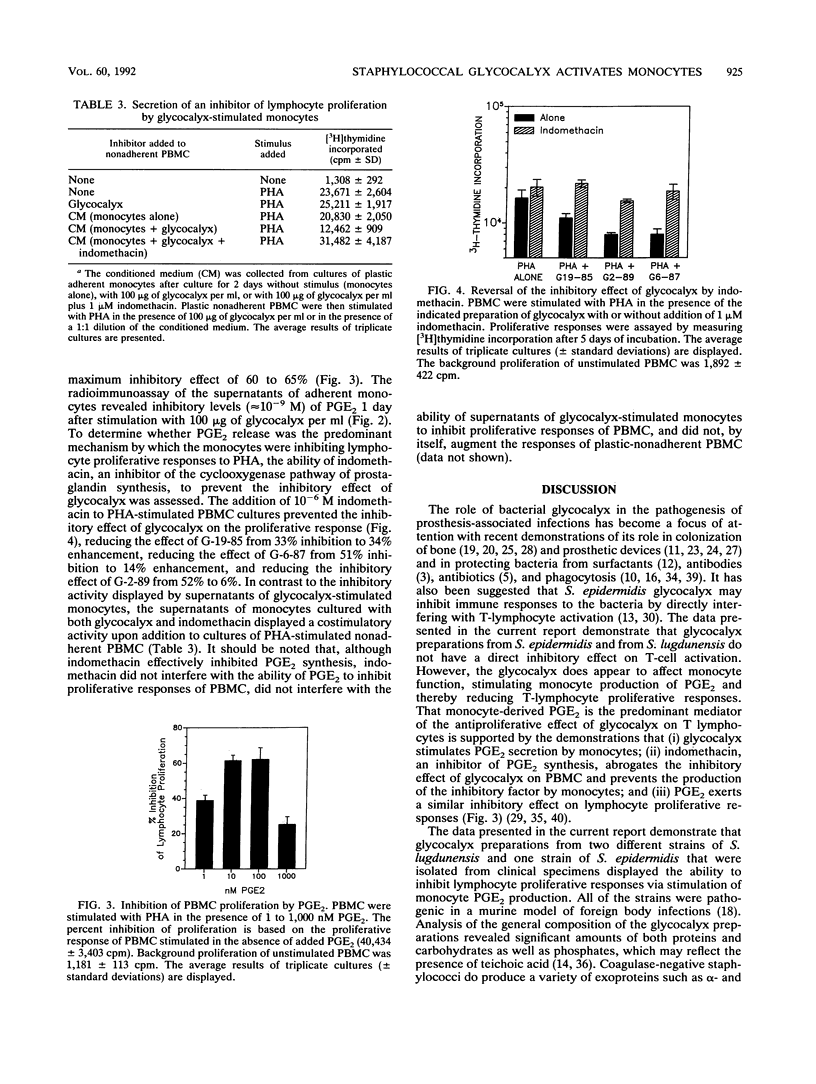
The glycocalyx (exopolysaccharides) of Staphylococcus epidermidis has been reported to inhibit a variety of host defense mechanisms. We have examined the inhibitory effects of glycocalyx on the proliferation of human peripheral blood mononuclear cells (PBMC) and the mechanism of this inhibition. Glycocalyx isolated and partially purified under endotoxin-free conditions from defined liquid medium cultures of S. epidermidis and Staphylococcus lugdunensis inhibited the proliferative response of PBMC when added to cultures at 10 to 100 micrograms/ml. Glycocalyx-mediated inhibition of phytohemagglutinin-stimulated proliferation of PBMC required the presence of plastic-adherent peripheral blood monocytes. Culture supernatants of monocytes stimulated with glycocalyx contained a soluble factor that inhibited the proliferation of monocyte-depleted PBMC. This soluble inhibitory factor was not produced in the absence of glycocalyx or in the presence of both glycocalyx and indomethacin. Analysis of the supernatants of cultures of adherent monocytes revealed that glycocalyx from S. epidermidis and from S. lugdunensis could activate monocyte production of prostaglandin E2 (PGE2), human interleukin-1, and tumor necrosis factor alpha. The addition of purified PGE2, at the same levels of PGE2 (greater than or equal to 10(-9) M) generated in the monocyte cultures, to PBMC cultures resulted in a similar inhibition of proliferative responses. It is concluded that, contrary to previous suggestions, the bacterial glycocalyx does not have a direct inhibitory effect on T lymphocytes. However, it does appear that glycocalyx from coagulase-negative staphylococci can activate monocyte PGE2 production and that it is this activity that in turn contributes to the inhibition of T-cell proliferation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baddour L. M., Christensen G. D., Hester M. G., Bisno A. L. Production of experimental endocarditis by coagulase-negative staphylococci: variability in species virulence. J Infect Dis. 1984 Nov;150(5):721–727. doi: 10.1093/infdis/150.5.721. [DOI] [PubMed] [Google Scholar]
- Baltimore R. S., Mitchell M. Immunologic investigations of mucoid strains of Pseudomonas aeruginosa: comparison of susceptibility to opsonic antibody in mucoid and nonmucoid strains. J Infect Dis. 1980 Feb;141(2):238–247. doi: 10.1093/infdis/141.2.238. [DOI] [PubMed] [Google Scholar]
- Beutler B., Cerami A. The biology of cachectin/TNF--a primary mediator of the host response. Annu Rev Immunol. 1989;7:625–655. doi: 10.1146/annurev.iy.07.040189.003205. [DOI] [PubMed] [Google Scholar]
- Costerton J. W., Irvin R. T., Cheng K. J. The role of bacterial surface structures in pathogenesis. Crit Rev Microbiol. 1981;8(4):303–338. doi: 10.3109/10408418109085082. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1 and its biologically related cytokines. Adv Immunol. 1989;44:153–205. doi: 10.1016/s0065-2776(08)60642-2. [DOI] [PubMed] [Google Scholar]
- Drysdale B. E., Zacharchuk C. M., Shin H. S. Mechanism of macrophage-mediated cytotoxicity: production of a soluble cytotoxic factor. J Immunol. 1983 Nov;131(5):2362–2367. [PubMed] [Google Scholar]
- Falcieri E., Vaudaux P., Huggler E., Lew D., Waldvogel F. Role of bacterial exopolymers and host factors on adherence and phagocytosis of Staphylococcus aureus in foreign body infection. J Infect Dis. 1987 Mar;155(3):524–531. doi: 10.1093/infdis/155.3.524. [DOI] [PubMed] [Google Scholar]
- Franson T. R., Sheth N. K., Rose H. D., Sohnle P. G. Scanning electron microscopy of bacteria adherent to intravascular catheters. J Clin Microbiol. 1984 Sep;20(3):500–505. doi: 10.1128/jcm.20.3.500-505.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govan J. R. Mucoid strains of Pseudomonas aeruginosa: the influence of culture medium on the stability of mucus production. J Med Microbiol. 1975 Nov;8(4):513–522. doi: 10.1099/00222615-8-4-513. [DOI] [PubMed] [Google Scholar]
- Gray E. D., Peters G., Verstegen M., Regelmann W. E. Effect of extracellular slime substance from Staphylococcus epidermidis on the human cellular immune response. Lancet. 1984 Feb 18;1(8373):365–367. doi: 10.1016/s0140-6736(84)90413-6. [DOI] [PubMed] [Google Scholar]
- Hussain M., Hastings J. G., White P. J. Isolation and composition of the extracellular slime made by coagulase-negative staphylococci in a chemically defined medium. J Infect Dis. 1991 Mar;163(3):534–541. doi: 10.1093/infdis/163.3.534. [DOI] [PubMed] [Google Scholar]
- Ishak M. A., Gröschel D. H., Mandell G. L., Wenzel R. P. Association of slime with pathogenicity of coagulase-negative staphylococci causing nosocomial septicemia. J Clin Microbiol. 1985 Dec;22(6):1025–1029. doi: 10.1128/jcm.22.6.1025-1029.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G. M., Lee D. A., Regelmann W. E., Gray E. D., Peters G., Quie P. G. Interference with granulocyte function by Staphylococcus epidermidis slime. Infect Immun. 1986 Oct;54(1):13–20. doi: 10.1128/iai.54.1.13-20.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lambe D. W., Jr, Ferguson K. P., Ferguson D. A., Jr The Bacteroides glycocalyx as visualized by differential interference contrast microscopy. Can J Microbiol. 1988 Nov;34(11):1189–1195. doi: 10.1139/m88-209. [DOI] [PubMed] [Google Scholar]
- Lambe D. W., Jr, Ferguson K. P., Keplinger J. L., Gemmell C. G., Kalbfleisch J. H. Pathogenicity of Staphylococcus lugdunensis, Staphylococcus schleiferi, and three other coagulase-negative staphylococci in a mouse model and possible virulence factors. Can J Microbiol. 1990 Jul;36(7):455–463. doi: 10.1139/m90-080. [DOI] [PubMed] [Google Scholar]
- Lambe D. W., Jr, Ferguson K. P., Mayberry-Carson K. J., Tober-Meyer B., Costerton J. W. Foreign-body-associated experimental osteomyelitis induced with Bacteroides fragilis and Staphylococcus epidermidis in rabbits. Clin Orthop Relat Res. 1991 May;(266):285–294. [PubMed] [Google Scholar]
- Lichtman A. H., Kurt-Jones E. A., Abbas A. K. B-cell stimulatory factor 1 and not interleukin 2 is the autocrine growth factor for some helper T lymphocytes. Proc Natl Acad Sci U S A. 1987 Feb;84(3):824–827. doi: 10.1073/pnas.84.3.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrie T. J., Costerton J. W. A scanning and transmission electron microscopic study of the surfaces of intrauterine contraceptive devices. Am J Obstet Gynecol. 1983 Jun 15;146(4):384–394. doi: 10.1016/0002-9378(83)90818-9. [DOI] [PubMed] [Google Scholar]
- Marrie T. J., Costerton J. W. Mode of growth of bacterial pathogens in chronic polymicrobial human osteomyelitis. J Clin Microbiol. 1985 Dec;22(6):924–933. doi: 10.1128/jcm.22.6.924-933.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrie T. J., Costerton J. W. Scanning electron microscopic study of uropathogen adherence to a plastic surface. Appl Environ Microbiol. 1983 Mar;45(3):1018–1024. doi: 10.1128/aem.45.3.1018-1024.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrie T. J., Harding G. K., Ronald A. R., Dikkema J., Lam J., Hoban S., Costerton J. W. Influence of mucoidy on antibody coating of Pseudomonas aeruginosa. J Infect Dis. 1979 Mar;139(3):357–361. doi: 10.1093/infdis/139.3.357. [DOI] [PubMed] [Google Scholar]
- Marrie T. J., Nelligan J., Costerton J. W. A scanning and transmission electron microscopic study of an infected endocardial pacemaker lead. Circulation. 1982 Dec;66(6):1339–1341. doi: 10.1161/01.cir.66.6.1339. [DOI] [PubMed] [Google Scholar]
- Mayberry-Carson K. J., Tober-Meyer B., Smith J. K., Lambe D. W., Jr, Costerton J. W. Bacterial adherence and glycocalyx formation in osteomyelitis experimentally induced with Staphylococcus aureus. Infect Immun. 1984 Mar;43(3):825–833. doi: 10.1128/iai.43.3.825-833.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger Z., Hoffeld J. T., Oppenheim J. J. Macrophage-mediated suppression. I. Evidence for participation of both hdyrogen peroxide and prostaglandins in suppression of murine lymphocyte proliferation. J Immunol. 1980 Feb;124(2):983–988. [PubMed] [Google Scholar]
- Podzorski R. P., Gray G. R., Nelson R. D. Different effects of native Candida albicans mannan and mannan-derived oligosaccharides on antigen-stimulated lymphoproliferation in vitro. J Immunol. 1990 Jan 15;144(2):707–716. [PubMed] [Google Scholar]
- Regan D. R., Cohen P. L., Cromartie W. J., Schwab J. H. Immunosuppressive macrophages induced by arthropathic peptidoglycan-polysaccharide polymers from bacterial cell walls. Clin Exp Immunol. 1988 Dec;74(3):365–370. [PMC free article] [PubMed] [Google Scholar]
- Schwarzmann S., Boring J. R. Antiphagocytic Effect of Slime from a Mucoid Strain of Pseudomonas aeruginosa. Infect Immun. 1971 Jun;3(6):762–767. doi: 10.1128/iai.3.6.762-767.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stobo J. D., Kennedy M. S., Goldyne M. E. Prostaglandin E modulation of the mitogenic response of human T cells. Differential response of T-cell subpopulations. J Clin Invest. 1979 Nov;64(5):1188–1203. doi: 10.1172/JCI109572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tojo M., Yamashita N., Goldmann D. A., Pier G. B. Isolation and characterization of a capsular polysaccharide adhesin from Staphylococcus epidermidis. J Infect Dis. 1988 Apr;157(4):713–722. doi: 10.1093/infdis/157.4.713. [DOI] [PubMed] [Google Scholar]
- Tomai M. A., Elmquist B. J., Warmka S. M., Fitzgerald T. J. Macrophage-mediated suppression of con A-induced IL-2 production in spleen cells from syphilitic rabbits. J Immunol. 1989 Jul 1;143(1):309–314. [PubMed] [Google Scholar]
- Veringa E. M., Ferguson D. A., Jr, Lambe D. W., Jr, Verhoef J. Trospectomycin enhances surface phagocytosis of Bacteroides and Staphylococcus by altering the bacterial glycocalyx. Zentralbl Bakteriol. 1989 Sep;271(3):311–320. doi: 10.1016/s0934-8840(89)80029-5. [DOI] [PubMed] [Google Scholar]
- Walker C., Kristensen F., Bettens F., deWeck A. L. Lymphokine regulation of activated (G1) lymphocytes. I. Prostaglandin E2-induced inhibition of interleukin 2 production. J Immunol. 1983 Apr;130(4):1770–1773. [PubMed] [Google Scholar]
- van de Rijn I., Kessler R. E. Growth characteristics of group A streptococci in a new chemically defined medium. Infect Immun. 1980 Feb;27(2):444–448. doi: 10.1128/iai.27.2.444-448.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]


