Abstract
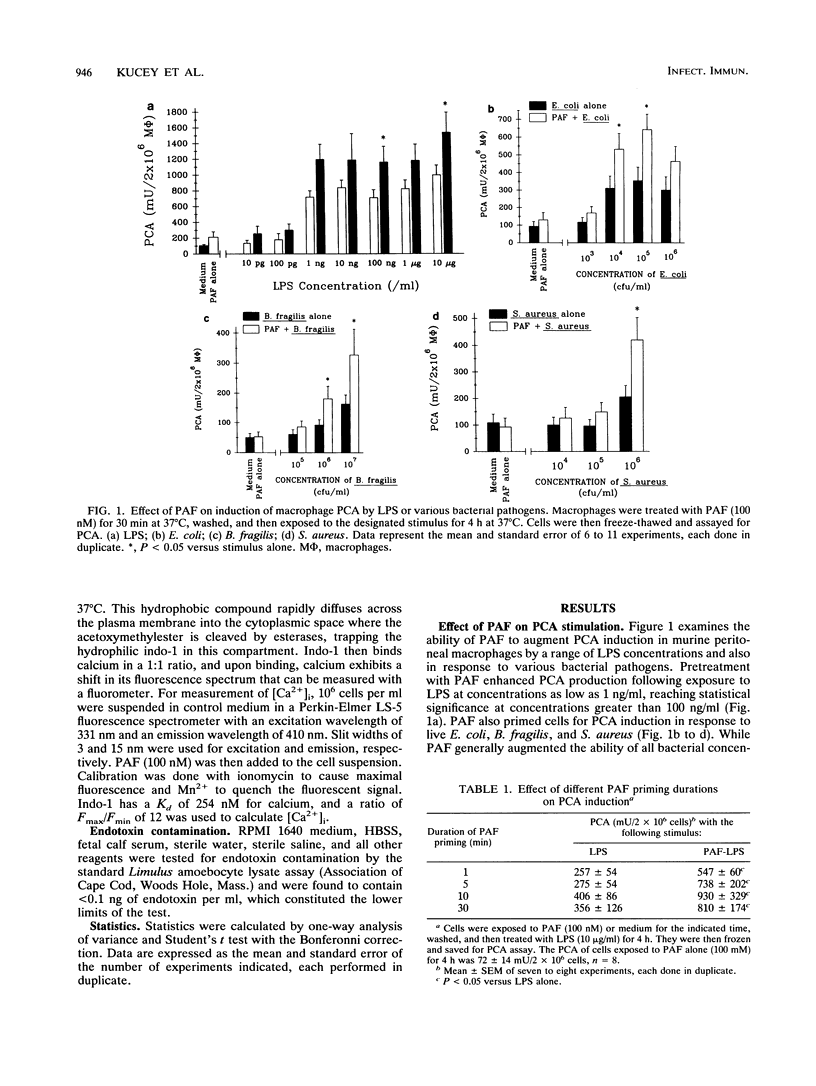
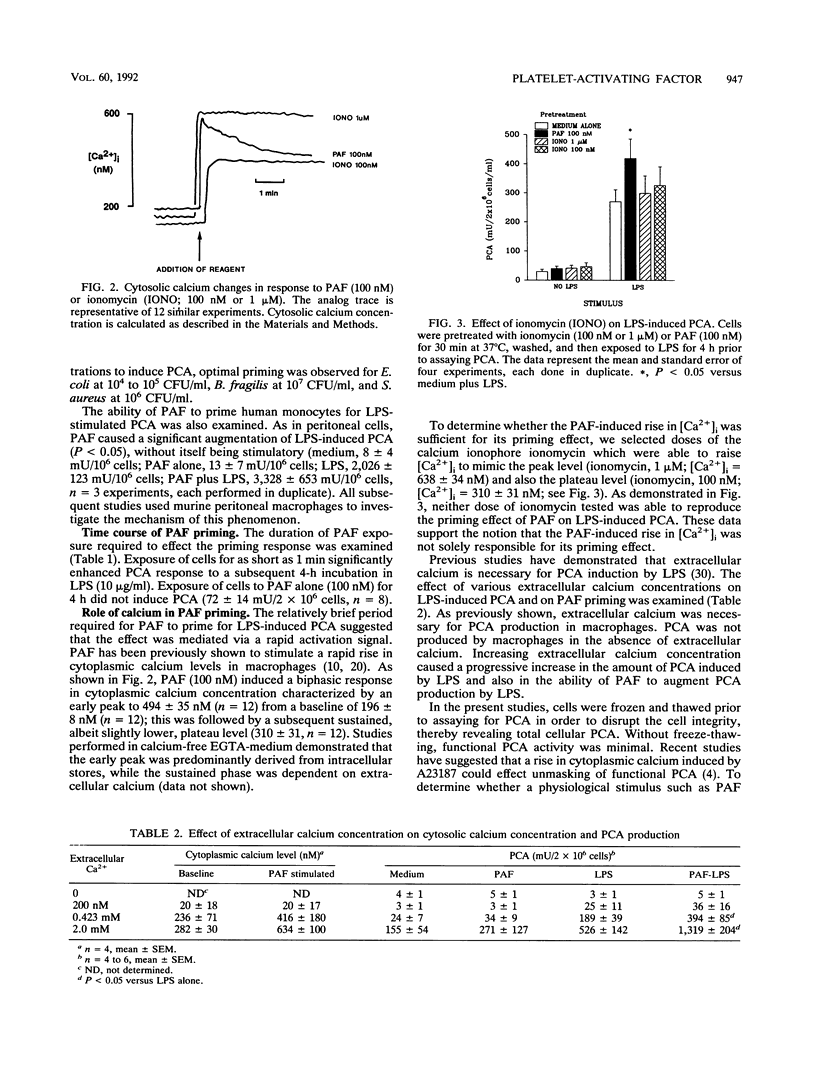
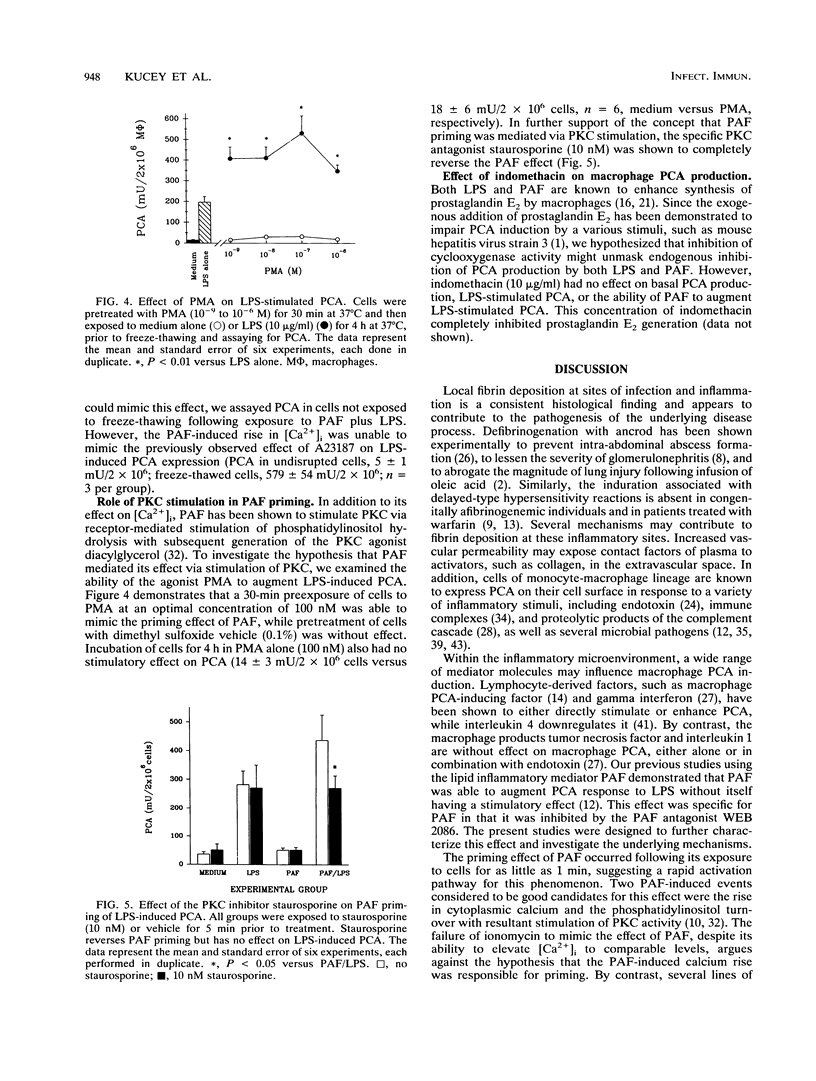
Macrophage procoagulant activity is an important mediator of extravascular fibrin deposition at sites of infection and appears to contribute to the pathogenesis of several infectious disease processes. Previous studies have shown that the inflammatory mediator platelet-activating factor was able to prime macrophages for induction of procoagulant activity by bacterial lipopolysaccharide. The present studies were designed to examine the mechanism of this priming effect. Platelet-activating factor (100 nM) primed macrophages for procoagulant activity generation in response to endotoxin at concentrations as low as 100 ng/ml and also following exposure to Escherichia coli, Bacteroides fragilis, and Staphylococcus aureus. The priming effect occurred following a pretreatment with platelet-activating factor for as short as 1 min, suggesting a rapid activation event. Two different doses of the calcium ionophore ionomycin were used to mimic the peak and sustained effects of platelet-activating factor on cytoplasmic calcium levels (1 microM and 100 nM, respectively). Neither dose was able to mimic the priming effect. However, extracellular calcium was necessary for induction of procoagulant activity and the priming effect. By contrast, the protein kinase C agonist phorbol myristate acetate reproduced the priming phenomenon observed for platelet-activating factor. In further support of the concept that protein kinase C activation mediated the effect of platelet-activating factor, the specific protein kinase C inhibitor staurosporine reversed the ability of platelet-activating factor to augment induction of macrophage procoagulant activity by endotoxin. These data suggest mechanisms by which inflammatory mediators within the microenvironment of infection might modulate the host response to bacterial pathogens.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abecassis M., Falk J. A., Makowka L., Dindzans V. J., Falk R. E., Levy G. A. 16, 16 Dimethyl prostaglandin E2 prevents the development of fulminant hepatitis and blocks the induction of monocyte/macrophage procoagulant activity after murine hepatitis virus strain 3 infection. J Clin Invest. 1987 Sep;80(3):881–889. doi: 10.1172/JCI113147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allard M. F., Doerschuk C. M., Brumwell M. L., Belzberg A., Hogg J. C. Oleic acid-induced lung injury in rabbits: effect of fibrinogen depletion with Arvin. J Appl Physiol (1985) 1988 Mar;64(3):920–928. doi: 10.1152/jappl.1988.64.3.920. [DOI] [PubMed] [Google Scholar]
- Almdahl S. M., Osterud B. Experimental gram-negative peritonitis: decreased thromboplastin activity in organs with a simultaneous rise of thromboplastin in blood monocytes and peritoneal macrophages. Res Exp Med (Berl) 1986;186(5):317–324. doi: 10.1007/BF01852097. [DOI] [PubMed] [Google Scholar]
- Bach R., Rifkin D. B. Expression of tissue factor procoagulant activity: regulation by cytosolic calcium. Proc Natl Acad Sci U S A. 1990 Sep;87(18):6995–6999. doi: 10.1073/pnas.87.18.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankey P., Carlson A., Ortiz M., Singh R., Cerra F. Tumor necrosis factor production by Kupffer cells requires protein kinase C activation. J Surg Res. 1990 Sep;49(3):256–261. doi: 10.1016/0022-4804(90)90130-t. [DOI] [PubMed] [Google Scholar]
- Chang S. W., Fernyak S., Voelkel N. F. Beneficial effect of a platelet-activating factor antagonist, WEB 2086, on endotoxin-induced lung injury. Am J Physiol. 1990 Jan;258(1 Pt 2):H153–H158. doi: 10.1152/ajpheart.1990.258.1.H153. [DOI] [PubMed] [Google Scholar]
- Chao W., Liu H., Hanahan D. J., Olson M. S. Regulation of platelet-activating factor receptor and PAF receptor-mediated arachidonic acid release by protein kinase C activation in rat Kupffer cells. Arch Biochem Biophys. 1990 Oct;282(1):188–197. doi: 10.1016/0003-9861(90)90103-6. [DOI] [PubMed] [Google Scholar]
- Cole E. H., Glynn M. F., Laskin C. A., Sweet J., Mason N., Levy G. A. Ancrod improves survival in murine systemic lupus erythematosus. Kidney Int. 1990 Jan;37(1):29–35. doi: 10.1038/ki.1990.4. [DOI] [PubMed] [Google Scholar]
- Colvin R. B., Mosesson M. W., Dvorak H. F. Delayed-type hypersensitivity skin reactions in congenital afibrinogenemia lack fibrin deposition and induration. J Clin Invest. 1979 Jun;63(6):1302–1306. doi: 10.1172/JCI109425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad G. W., Rink T. J. Platelet activating factor raises intracellular calcium ion concentration in macrophages. J Cell Biol. 1986 Aug;103(2):439–450. doi: 10.1083/jcb.103.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dindzans V. J., Skamene E., Levy G. A. Susceptibility/resistance to mouse hepatitis virus strain 3 and macrophage procoagulant activity are genetically linked and controlled by two non-H-2-linked genes. J Immunol. 1986 Oct 1;137(7):2355–2360. [PubMed] [Google Scholar]
- Drake T. A., Rodgers G. M., Sande M. A. Tissue factor is a major stimulus for vegetation formation in enterococcal endocarditis in rabbits. J Clin Invest. 1984 Jun;73(6):1750–1753. doi: 10.1172/JCI111383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory S. A., Kornbluth R. S., Helin H., Remold H. G., Edgington T. S. Monocyte procoagulant inducing factor: a lymphokine involved in the T cell-instructed monocyte procoagulant response to antigen. J Immunol. 1986 Nov 15;137(10):3231–3239. [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hartung H. P. Acetyl glyceryl ether phosphorylcholine (platelet-activating factor) mediates heightened metabolic activity in macrophages. Studies on PGE, TXB2 and O2- production, spreading, and the influence of calmodulin-inhibitor W-7. FEBS Lett. 1983 Aug 22;160(1-2):209–212. doi: 10.1016/0014-5793(83)80968-5. [DOI] [PubMed] [Google Scholar]
- Hsueh W., González-Crussi F., Arroyave J. L. Platelet-activating factor: an endogenous mediator for bowel necrosis in endotoxemia. FASEB J. 1987 Nov;1(5):403–405. doi: 10.1096/fasebj.1.5.3678700. [DOI] [PubMed] [Google Scholar]
- Janco R. L., Morris P. J. Regulation of monocyte procoagulant by chemoattractants. Blood. 1985 Mar;65(3):545–552. [PubMed] [Google Scholar]
- Kovacs E. J., Radzioch D., Young H. A., Varesio L. Differential inhibition of IL-1 and TNF-alpha mRNA expression by agents which block second messenger pathways in murine macrophages. J Immunol. 1988 Nov 1;141(9):3101–3105. [PubMed] [Google Scholar]
- Kucey D. S., Kubicki E. I., Rotstein O. D. Platelet-activating factor primes endotoxin-stimulated macrophage procoagulant activity. J Surg Res. 1991 May;50(5):436–441. doi: 10.1016/0022-4804(91)90021-d. [DOI] [PubMed] [Google Scholar]
- Kurland J. I., Bockman R. Prostaglandin E production by human blood monocytes and mouse peritoneal macrophages. J Exp Med. 1978 Mar 1;147(3):952–957. doi: 10.1084/jem.147.3.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy G. A., Edgington T. S. Lymphocyte cooperation is required for amplification of macrophage procoagulant activity. J Exp Med. 1980 May 1;151(5):1232–1244. doi: 10.1084/jem.151.5.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy G. A., Leibowitz J. L., Edgington T. S. Induction of monocyte procoagulant activity by murine hepatitis virus type 3 parallels disease susceptibility in mice. J Exp Med. 1981 Oct 1;154(4):1150–1163. doi: 10.1084/jem.154.4.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy G. A., Schwartz B. S., Edgington T. S. The kinetics and metabolic requirements for direct lymphocyte induction of human procoagulant monokines by bacterial lipopolysaccharide. J Immunol. 1981 Jul;127(1):357–363. [PubMed] [Google Scholar]
- Lyberg T., Prydz H. Phorbol esters induce synthesis of thromboplastin activity in human monocytes. Biochem J. 1981 Mar 15;194(3):699–706. doi: 10.1042/bj1940699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRitchie D. I., Girotti M. J., Glynn M. F., Goldberg J. M., Rotstein O. D. Effect of systemic fibrinogen depletion on intraabdominal abscess formation. J Lab Clin Med. 1991 Jul;118(1):48–55. [PubMed] [Google Scholar]
- Moon D. K., Geczy C. L. Recombinant IFN-gamma synergizes with lipopolysaccharide to induce macrophage membrane procoagulants. J Immunol. 1988 Sep 1;141(5):1536–1542. [PubMed] [Google Scholar]
- Muhlfelder T. W., Niemetz J., Kreutzer D., Beebe D., Ward P. A., Rosenfeld S. I. C5 chemotactic fragment induces leukocyte production of tissue factor activity: a link between complement and coagulation. J Clin Invest. 1979 Jan;63(1):147–150. doi: 10.1172/JCI109269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F. Secretory products of macrophages. J Clin Invest. 1987 Feb;79(2):319–326. doi: 10.1172/JCI112815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterud B., Eskeland T. The mandatory role of complement in the endotoxin-induced synthesis of tissue thromboplastin in blood monocytes. FEBS Lett. 1982 Nov 22;149(1):75–79. doi: 10.1016/0014-5793(82)81075-2. [DOI] [PubMed] [Google Scholar]
- Prpic V., Uhing R. J., Weiel J. E., Jakoi L., Gawdi G., Herman B., Adams D. O. Biochemical and functional responses stimulated by platelet-activating factor in murine peritoneal macrophages. J Cell Biol. 1988 Jul;107(1):363–372. doi: 10.1083/jcb.107.1.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prydz H., Lyberg T., Deteix P., Allison A. C. In vitro stimulation of tissue thromboplastin (factor III) activity in human monocytes by immune complexes and lectins. Thromb Res. 1979;15(3-4):465–474. doi: 10.1016/0049-3848(79)90152-x. [DOI] [PubMed] [Google Scholar]
- Prydz H., Lyberg T. Effect of some drugs on thromboplastin (factor III) activity of human monocytes in vitro. Biochem Pharmacol. 1980 Jan 1;29(1):9–14. doi: 10.1016/0006-2952(80)90236-1. [DOI] [PubMed] [Google Scholar]
- Rosenthal G. A., Levy G., Rotstein O. D. Induction of macrophage procoagulant activity by Bacteroides fragilis. Infect Immun. 1989 Feb;57(2):338–343. doi: 10.1128/iai.57.2.338-343.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotstein O. D., Kao J. Prevention of intra-abdominal abscesses by fibrinolysis using recombinant tissue plasminogen activator. J Infect Dis. 1988 Oct;158(4):766–772. doi: 10.1093/infdis/158.4.766. [DOI] [PubMed] [Google Scholar]
- Rotstein O. D., Pruett T. L., Simmons R. L. Fibrin in peritonitis. V. Fibrin inhibits phagocytic killing of Escherichia coli by human polymorphonuclear leukocytes. Ann Surg. 1986 Apr;203(4):413–419. doi: 10.1097/00000658-198604000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior R. M., Skogen W. F., Griffin G. L., Wilner G. D. Effects of fibrinogen derivatives upon the inflammatory response. Studies with human fibrinopeptide B. J Clin Invest. 1986 Mar;77(3):1014–1019. doi: 10.1172/JCI112353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair S. B., Rotstein O. D., Levy G. A. Disparate mechanisms of induction of procoagulant activity by live and inactivated bacteria and viruses. Infect Immun. 1990 Jun;58(6):1821–1827. doi: 10.1128/iai.58.6.1821-1827.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stecher V. J., Sorkin E. The chemotactic activity of fibrin lysis products. Int Arch Allergy Appl Immunol. 1972;43(6):879–886. doi: 10.1159/000230905. [DOI] [PubMed] [Google Scholar]
- Szabo G., Miller-Graziano C. L. Induction and regulation of monocyte procoagulant activity. Transplantation. 1990 Aug;50(2):301–309. doi: 10.1097/00007890-199008000-00026. [DOI] [PubMed] [Google Scholar]
- Tamaoki T., Nomoto H., Takahashi I., Kato Y., Morimoto M., Tomita F. Staurosporine, a potent inhibitor of phospholipid/Ca++dependent protein kinase. Biochem Biophys Res Commun. 1986 Mar 13;135(2):397–402. doi: 10.1016/0006-291x(86)90008-2. [DOI] [PubMed] [Google Scholar]
- Vercellotti G. M., Yin H. Q., Gustafson K. S., Nelson R. D., Jacob H. S. Platelet-activating factor primes neutrophil responses to agonists: role in promoting neutrophil-mediated endothelial damage. Blood. 1988 Apr;71(4):1100–1107. [PubMed] [Google Scholar]
- van Ginkel C. J., Thörig L., Thompson J., Oh J. I., van Aken W. G. Enhancement of generation of monocyte tissue thromboplastin by bacterial phagocytosis: possible pathway for fibrin formation on infected vegetations in bacterial endocarditis. Infect Immun. 1979 Jul;25(1):388–395. doi: 10.1128/iai.25.1.388-395.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ginkel C. J., van Aken W. G., Oh J. I., Vreeken J. Stimulation of monocyte procoagulant activity by adherence to different surfaces. Br J Haematol. 1977 Sep;37(1):35–45. [PubMed] [Google Scholar]


