Abstract
Background
Archived formalin-fixed, paraffin-embedded specimens represent an important resource for pharmacogenomic analysis in retrospective clinical studies but the quality of results from formalin-fixed, paraffin-embedded samples is of concern due to the fact of the degradation of DNAs and RNAs from formalin-fixed, paraffin-embedded tissues.
Methods
In the present study, we used DNA from fresh frozen as well as formalin-fixed, paraffin-embedded tumor to detect copy-number changes in colorectal cancer, and our data shows that formalin-fixed, paraffin-embedded DNAs were able to deliver reliable copy-number data, and that quantitative PCR had the ability to detect copy-number changes from deletion to amplification, with high concordance of copy-number calls among formalin-fixed, paraffin-embedded and frozen DNAs.
Results
The amplification of topoisomerase I and deletion of thymidylate synthase were found in 23% (12/52) and 27% (14/52) of colorectal cancers, but EGF receptor amplification was not common (5/52, < 10%). Among 52 colorectal cancers, 31 tumors were both topoisomerase I and thymidylate synthase diploid, which may have a worse outcome for tumor chemotherapy; and there were five tumors with favorable genomics (topoisomerase I amplification and thymidylate synthase deletion). Furthermore, topoisomerase I-amplified tumors had a two-times higher RNA level and a nearly twofold higher protein expression level than did the diploid tumors (p < 0.001 and 0.01, respectively), but there were no correlations between copy-number status and RNA or protein level for thymidylate synthase.
Conclusions
Our study suggests a potential pharmacogenomic influence of topoisomerase I copy-number alteration on its RNA/protein expressions, which could be reflected on tumor response to chemotherapy in human colorectal cancer.
Keywords: colorectal cancer, DNA copy-number, formalin-fixed paraffin-embedded tissue, quantitative PCR, thymidylate synthase, topoisomerase I
In the post-genomic era, the study of human genetic variation at the DNA level constitutes a major challenge and holds promises to benefit patients through identification of those significant variants in clinical settings [1,2]. Recent molecular cytogenetic analysis suggests that alteration in DNA copy number can be a mechanism for variability in tumor sensitivity to anticancer therapy [3,4], and most studies on DNA copy-number changes have been using unfixed fresh-frozen specimens that give high quality DNA samples and deliver reliable copy- number data for downstream analysis. Such tumor sets are often small and lack clinical annotation, whereas formalin-fixed, paraffin-embedded (FFPE) materials are abundant [5,6]. FFPE specimens represent a source of archived and morphologically defined disease-specific biological material for which extensive clinical data are often available. Extraction of DNA and RNA from these specimens provides an opportunity for retrospective analysis using molecular techniques such as microarrays, cytogenetic hybridizations, or real-time quantitative PCR (qPCR) that may accelerate the discovery of associations among genomic changes or gene-expression signatures and treatment outcomes.
A barrier to the analysis of FFPE samples is that DNA and RNA extracted from FFPE tissues is often significantly degraded to an average fragment length around 300−400 bp for DNA and 200 bp for RNA [7,8]. While researchers using PCR-based approaches to analyze gene copy-number changes and gene-expression levels in FFPE have been able to successfully obtain data [9,10], the reliability and accuracy of results from FFPE DNAs/RNAs would become the major concern. In the present study, we have used the fresh frozen as well as FFPE DNAs to detect the copy-number changes of two key genes (see below) in colorectal cancer. By comparing the results from matched fresh-frozen and FFPE DNAs, we will be able to provide evidence for the accuracy and reliability of the qPCR in detection of DNA copy-number alterations in the FFPE DNAs.
In human colorectal cancer, chromosome 20 gain and 18 loss are common, including the TOP1 and TYMS genes [11], the key target genes for irinotecan- and 5-fluorouracil-based chemo-therapy, respectively. The present study assessed DNA copy-number changes and alterations in RNA and protein expressions for TOP1 and TYMS in order to establish potential associations between genomic DNA copy-number changes and phenotypic RNA/protein profiles as a preliminary pharmacogenomic assessment.
Materials & methods
Patients & samples
Tumor specimens and paired normal colon tissues used in this study were from 52 Dukes’ C (stage III) colorectal cancer patients (29 male/23 female, age range: 32−96 [median: 69.5] years), who had not received any chemotherapeutic agents before surgery. Samples were snap frozen in liquid nitrogen immediately after surgery and stored at −80°C. At the same time, FFPE tissues from these patients and from 18 glioma patients were also included in this study. The tumor specimens selected for DNA and RNA isolation had high tumor cellularities. Written informed consent was obtained from all patients to bank tumor tissue and to perform genomic analysis. This study was approved by the Washington University Human Subjects Committee.
Extraction of DNA
All DNA was extracted at the Siteman Cancer Center Tissue Procurement Core in Barnes-Jewish Hospital (MO, USA). Archival tissue blocks included gliomas, colorectal tumors, and normal colon tissues; and fresh-frozen tissues were colorectal tumors and matched normal colons. Frozen tissues were first embedded in OCT blocks, and then one 50-μ section from the block was placed in ALT buffer (included in QIAamp® kit) and proteinase K was added in the buffer for an overnight digestion at 56°C. A 50-μ section was punched out from FFPE blocks and subjected to standard deparaffinization procedures and proteinase K digestion overnight. After tissue digestion, the fresh DNAs were isolated according to the protocol with QIAamp DNA Mini Kit from Qiagen (CA, USA); and the FFPE DNAs were extracted following the instructions for DNA isolation with the phenol:chloroform:isoamyl alcohol (25:24:1) solution from Ambion, Inc. (TX, USA). DNA quality was assessed based on the optical density (OD) 260/280 ratio and 1% agarose gel electrophoresis.
Quantitative PCR
Real-time quantitative PCR was employed to detect gene copy-number changes and RNA levels. Briefly, 10-μl reaction mixture for qPCR was composed of 5 μl of 2× TaqMan® universal PCR master mix (Applied Biosystems Inc. – ABI), 5 μl of primer and probe mix (600 nM each forward and reverse primers, 200 nM specific TaqMan probe at final concentration), and 10 ng of DNA/cDNA (dried in wells of PCR plate overnight before adding the reaction mixture). All real-time PCR assays were performed in triplicate on an ABI PRISM 7900HT Sequence Detector System (ABI) and followed the program: 50°C for 2 min, 95°C for 10 min, and 40 cycles of both 15 s at 95°C and 60 s at 60°C. Primers and TaqMan probes used in this study were designed using Primer Express version 1.5 (ABI) and are displayed in Table 1. Determination of RNA levels was performed according to our previous studies [12,13].
Table 1.
Primer and probe sequence for DNA copy-number analysis in qPCR assay.
| Gene symbol | Oligo | Sequence | Amplicon size (bp) |
|---|---|---|---|
| ACTB | Forward | CAGCCATGTACGTTGCTATCCA | 82 |
| Reverse | TCACCGGAGTCCATCACGAT | ||
| TaqMan® probe | TGTGCTATCCCTGTACGCCTCTGGCC | ||
| LINE1 | Forward | TTGCGGCATTATTCACAATAGC | 83 |
| Reverse | GTGCCACATTTTCTTAATCCAGTCT | ||
| TaqMan probe | TGGAACCAACCCAAATGTCCAACAATGA | ||
| TOP1 | Forward | GGCGAGTGAATCTAAGGATAATGAA | 97 |
| Reverse | TGGATATCTTAAAGGGTACAGCGAA | ||
| TaqMan probe | TCAGTGACTGAAACCATTTTCCCATCATCC | ||
| TYMS | Forward | GCCTCGGTGTGCCTTTCA | 65 |
| Reverse | CGTGATGTGCGCAATCATG | ||
| TaqMan probe | CATCGCCAGCTACGCCCTGCTC | ||
| EGFR | Forward | GAATTCGGATGCAGAGCTTC | 150 |
| Reverse | GACATGCTGCGGTGTTTTC | ||
| TaqMan probe | TCCCATGATGATCTGTCCCTCACA |
qPCR: Qualitative PCR.
Determination of DNA copy-number changes
In this study, we detected copy-number changes for three genes: TOP1, TYMS and EGFR. Two internal reference genes (a house keeping gene: ACTB and a genomic repeat: LINE1 [4]) were used to normalize the DNA input between individual DNA samples, and the normal DNA samples were used to scale the copy number by diploid genome. For each gene (both reference and target genes), a standard curve was established to calculate the raw copy value (RCV) for all DNA samples based on computed tomography (CT) values generated by the 7900HT. The ratio between target and reference genes for each DNA sample (including tumor and normal) was resolved by two RCVs: one for target gene and the other for reference gene in a same DNA sample. Finally, the copy number for a specific gene in a specific DNA sample was the product of the overall ratio between two target-reference ratios from tumor and normal DNAs multiplied by 2, and then was presented in integer by rounding up the product.
Immunohistochemical staining
The protein expression of TOP1 and TYMS was determined on customized tissue microarray by immunohistochemical staining as previously described [14], and the score of 0, 1, 2 or 3 was graded to each sample based on their intensity and number of positive cells. The final score for a sample was average of replicate spots on the arrays.
Statistical analysis
Statistical analyses were performed with the software STATISTICA (StatSoft, Inc., OK, USA). Significance of difference in RNA and protein levels between copy-number statuses was evaluated by the Mann–Whitney U test. The significance level was set at p < 0.05.
Results & discussion
Most of FFPE DNAs were able to deliver reliable copy-number data
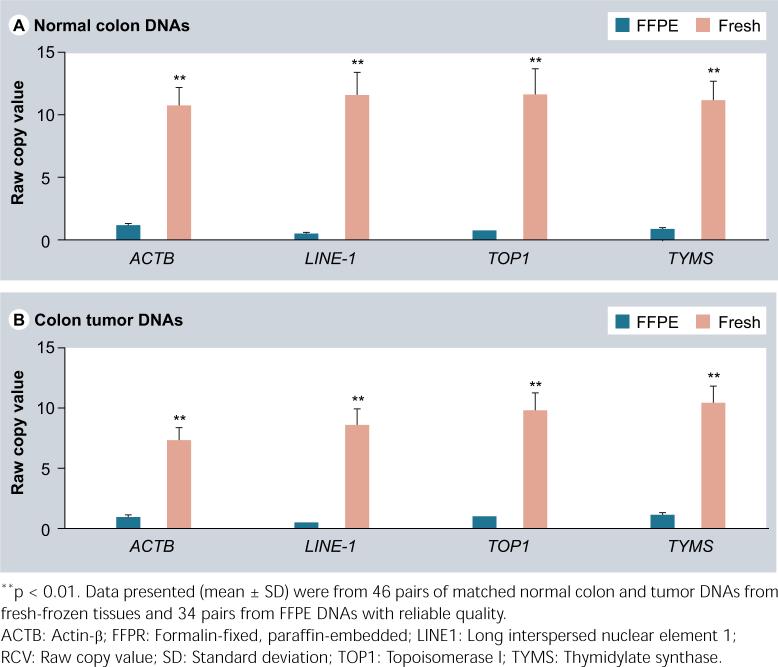
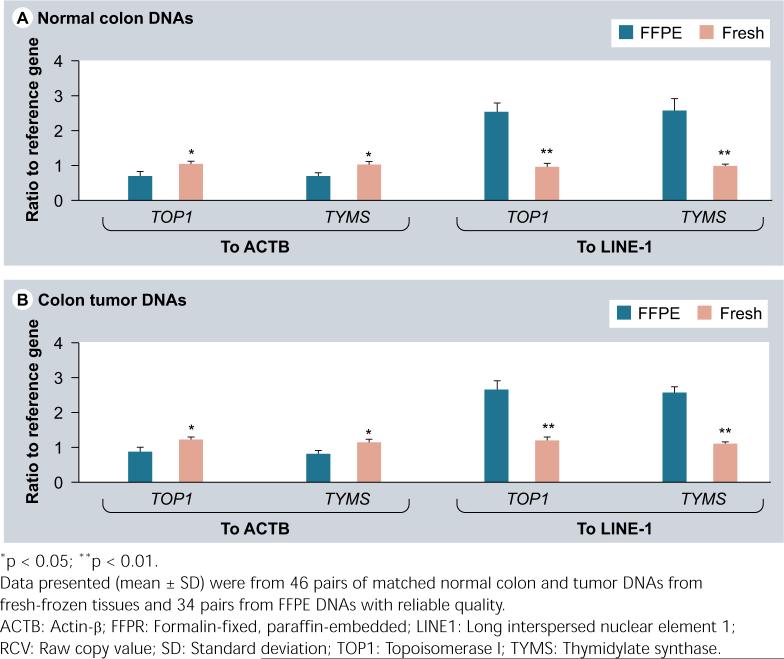
In this study, 46 of 52 tumors had matched frozen and FFPE DNAs. All 52 frozen DNAs and 34 of 46 FFPE DNAs were able to deliver reliable copy-number data with qPCR assays. A total of 26% (12/46) of the FFPE DNAs had the CT values greater than 35 even after 40 cycles, which was excluded for further analysis. In addition, using the same amount of DNA (10 ng), the tumor FFPE DNAs were tenfold lower than the tumor fresh DNAs in RCVs for ACTB, TOP1 and TYMS, and over 20-fold lower in RCV for LINE1 (Figure 1). The normal tissue FFPE DNAs had a similar decline in RCV. This reflects the fact that the FFPE DNAs have been heavily fragmented (the size around 300 bp) and much less fragments with the whole range of qPCR amplicons are available in the reactions even though the input amount is same. Particularly, because of the large copy number of LINE1 (over 50,000 copies per diploid cell) in the genome, which makes LINE1 having more chances to be fragmented, it is not surprising that LINE1 had a bigger RCV drop than diploid genes in the FFPE DNAs. On the other hand, the target-reference RCV ratio in tumor FFPE DNAs was shifted down by 37% than that in tumor fresh DNAs for both TOP1 and TYMS when normalizing to ACTB, but it was shifted up by approximately 130% over tumor fresh DNAs for these two genes when normalizing to LINE1 (Figure 2). The normal FFPE DNAs had a similar shift in target-reference RCV ratio. This phenomenon warns the requirement of using normal FFPE DNAs to scale tumor FFPE DNAs but not using fresh normal tissue DNAs in order to calculate the final copy-number value per diploid genome [15,16].
Figure 1.
The same 10-ng DNAs from the FFPE tissues had much lower RCV values in both normal colon (A) and tumor (B) samples than that from the fresh tissues.
Figure 2.
The target-reference RCV ratios from the FFPE DNAs were shifted down and up in both normal colon (A) and tumor (B) samples when compared with that from the fresh DNAs and normalized to ACTB and LINE1, respectively.
The qPCR gave good prediction for EGFR copy-number with wide-range increases
A previous study from our collaborate group has identified EGFR copy-number changes by fluorescence in situ hybridization (FISH) on slides from FFPE blocks of glioma patients [17]. We selected 18 tumors from that study, of which six tumors from each of three categories in EGFR copy-number status (amplification, polysomy, normal) were included. Using LINE1 as reference gene and normal colon FFPE DNAs as diploid control, the qPCR reliably detected EGFR amplification and normal copy-number in the FFPE DNAs of gliomas, with a wide range of copy-number increases from 20 to 113 copies per diploid cell, which perfectly falls into EGFR-amplified category of gliomas identified by FISH (Figure 3). Detection of EGFR-polysomy tumors seemed a bit underestimated by the qPCR. Four of the six polysomy tumors were shown 3−4 copies of EGFR and another two polysomy tumors were identified as ‘normal copy-number’. The reason for this underestimation could be dilution of the EGFR copy number in the polysomy tumor cells by coexist diploid normal cells. This is a merit of FISH over qPCR in detection of polysomy tumors [18].
Figure 3.
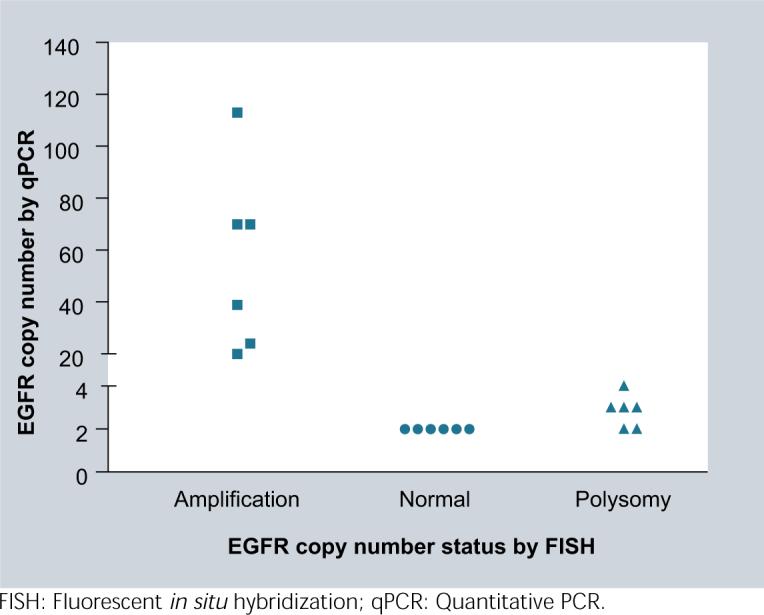
The qPCR reliably detected wide-ranged EGFR amplification defined by FISH in 18 human gliomas, and compromised a bit in detection of EGFR polysomy tumors likely due to dilution of the copy-number in polysomy tumor cells by co-existing diploid normal cells.
qPCR had good prediction for TOP1 & TYMS copy number with small-range changes
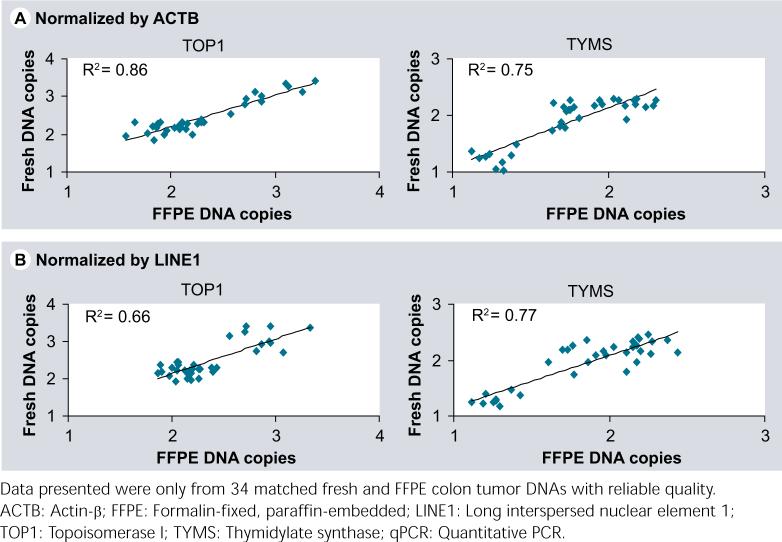
The qPCR had been done for detection of TOP1 and TYMS copy-number changes both in fresh and FFPE DNAs from the colorectal cancer patients. With one discrepancy (2 copies in FFPE but 1 copy in fresh DNAs [copy-number product 1.59 vs 1.33] for a tumor referenced by ACTB), the copy-number calls for TOP1 and TYMS in the FFPE DNAs were highly concordant to the changes in the fresh DNAs for the same individuals (Figure 4), in both cases where either LINE1 or ACTB was used as internal reference. These copy-number changes were small, with a range from 1 to 2 copies for TYMS, and 2−4 copies for TOP1. This indicates that the qPCR has the ability to detect low copy-number changes in FFPE DNAs as well as fresh DNAs, and the FFPE DNA can be a reliable resource for copy-number study, especially for association studies where the treatment and patient outcome data as well as archived pre- and post-therapy specimens are available.
Figure 4.
Data plots demonstrated good prediction by qPCR for TOP1 and TYMS copy-number changes between the FFPE and fresh DNAs either normalized by ACTB (A) or LINE1 (B).
Amplification of TOP1 & deletion of TYMS were found in 23 and 27% of untreated colorectal cancers, but EGFR amplification was not common
TOP1 is on chromosome 20q and TYMS on chromosome 18p, which are hot spots for genetic aberrations in colorectal cancers. Rooney et al. have reported that ZNF217 is amplified by 3−13 copies in 61% of Dukes’ A-C colorectal cancers and this gene is within the chromosome 20q13.2, a region which contains also TOP1 and has been previously shown to be highly amplified in colorectal cancer by comparative genomic hybridization (CGH) [19]. Our results in fresh DNAs showed that 23% (12/52) of the Dukes’ C-only colorectal tumors were amplified for TOP1 with 3 or 4 copies (either LINE1 or ACTB used as internal reference) and these tumor specimens were taken before chemotherapy. The differential rate of TOP1 amplification between the two studies could be due to tumor stage, treatment, and/or different qPCR approaches (selection of reference gene and, simplex or multiplex reaction). On the other hand, Wang et al. [4] have shown that TYMS can acquire resistance to 5-fluorouracil treatment due to gene amplification after chemotherapy. Our data from fresh DNAs indicated that there was deletion of TYMS in 27% (14/52) of untreated colorectal cancers and no tumors in our study showed any amplification for TYMS (either LINE1 or ACTB as internal reference). A previous study using traditional CGH method [11] showed that the chromosome 18p was frequently lost in colorectal tumors (20.7%). In addition, 31 tumors were both TOP1 and TYMS diploid, which may have a worse outcome for tumor chemotherapy; and there were five tumors with favorable genomics (TOP1 amplification/TYMS deletion). The EGFR amplification was not common in colorectal cancers, and only less than 10% (5/52) of tumors had EGFR-amplified with a copy number of 3 in this study.
There were positive associations between copy number & RNA or protein level for TOP1 but not for TYMS
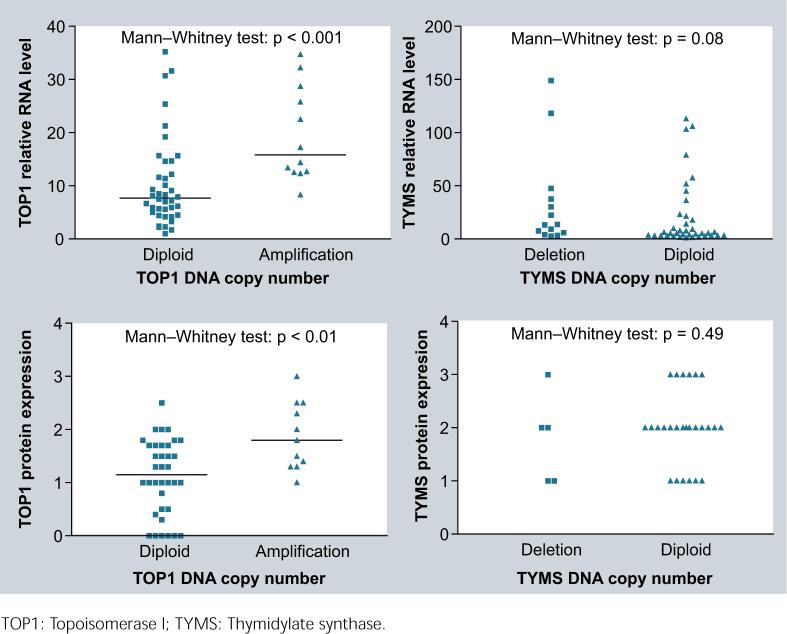
The RNA data for TOP1 and TYMS were published in our previous studies [12,13]. The median TOP1 RNA level in amplified tumors was two-times higher than that in diploid tumors (p < 0.001). But there was no significant difference in the RNA level between TYMS diploid and deleted tumors (Figure 5A). Again, TOP1-amplified tumors had a higher protein expression than did diploid tumors (median score 1.8 versus 1.0; p < 0.01). However, there was no significant difference in the protein expression between TYMS deleted and diploid tumors (Figure 5B). These results implicate that the TOP1 amplification has functional significance that may contribute to variable responses of cancer patients to TOP1-targeting drugs. However, the significance of TYMS deletion in naive tumors remains unclear and needs further study.
Figure 5.
Correlations were observed between DNA copy number and RNA (A) or protein (B) levels for TOP1, but not for TYMS in 52 colorectal cancer patients.
Our study suggests a potential pharmacogenomic influence of TOP1 copy-number alteration on its RNA/protein expressions, which could be reflected on tumor response to chemotherapy in human colorectal cancer.
Executive summary
Archived formalin-fixed, paraffin-embedded (FFPE) specimens represent an important resource for pharmacogenomic analysis in retrospective clinical studies but the quality of results from FFPE samples is of concern due to the fact of the degradation of DNAs and RNAs from FFPE tissues.
FFPE DNAs was able to deliver reliable copy-number data and quantitative PCR had the ability to detect copy-number changes from deletion to amplifications, with high concordance of copy-number calls among FFPE and frozen DNAs.
Amplification of topoisomerase I (TOP1) and deletion of thymidylate synthase (TYMS) were found in 23 and 27% of untreated colorectal cancers, and TOP1-amplified tumors had a twofold higher RNA level and a nearly twofold higher protein level than did the diploid tumors, but no any correlation was observed between copy-number status and RNA or protein level for TYMS in this study.
Our study suggests a potential pharmacogenomic influence of TOP1 copy-number alteration on its RNA/protein expressions, which could be reflected on tumor response to chemotherapy in human colorectal cancer.
Acknowledgement
This study was supported by grants from the National Institutes of Health (U01 GM63340, R21 CA102461, and P30 CA091842), Bethesda, MD, USA.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Bibliography
- 1.Ouahchi K, Lindeman N, Lee C. Copy number variants and pharmacogenomics. Pharmacogenomics. 2006;7:25–29. doi: 10.2217/14622416.7.1.25. [DOI] [PubMed] [Google Scholar]
- 2.Cheng Q, Yang W, Raimondi SC, Pui CH, Relling MV, Evans WE. Karyotypic abnormalities create discordance of germline genotype and cancer cell phenotypes. Nat. Genet. 2005;37:878–882. doi: 10.1038/ng1612. [DOI] [PubMed] [Google Scholar]
- 3.Brody JR, Hucl T, Gallmeier E, Winter JM, Kern SE, Murphy KM. Genomic copy number changes affecting the thymidylate synthase (TYMS) gene in cancer: a model for patient classification to aid fluoropyrimidine therapy. Cancer Res. 2006;66:9369–9373. doi: 10.1158/0008-5472.CAN-06-2165. [DOI] [PubMed] [Google Scholar]
- 4.Wang TL, Diaz LA, Jr, Romans K, et al. Digital karyotyping identifies thymidylate synthase amplification as a mechanism of resistance to 5-fluorouracil in metastatic colorectal cancer patients. Proc. Natl Acad. Sci. USA. 2004;101:3089–3094. doi: 10.1073/pnas.0308716101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moroni M, Veronese S, Benvenuti S, et al. Gene copy number for epidermal growth factor receptor (EGFR) and clinical response to anti-EGFR treatment in colorectal cancer: a cohort study. Lancet Oncol. 2005;6:279–286. doi: 10.1016/S1470-2045(05)70102-9. [DOI] [PubMed] [Google Scholar]
- 6.Devries S, Nyante S, Korkola J, et al. Array-based comparative genomic hybridization from formalin-fixed, paraffin-embedded breast tumors. J. Mol. Diagn. 2005;7:65–71. doi: 10.1016/S1525-1578(10)60010-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacobs S, Thompson ER, Nannya Y, et al. Genome-wide, high-resolution detection of copy number, loss of heterozygosity, and genotypes from formalin-fixed, paraffin-embedded tumor tissue using microarrays. Cancer Res. 2007;67:2544–2551. doi: 10.1158/0008-5472.CAN-06-3597. [DOI] [PubMed] [Google Scholar]
- 8.Antonov J, Goldstein DR, Oberli A, et al. Reliable gene expression measurements from degraded RNA by quantitative real-time PCR depend on short amplicons and a proper normalization. Lab. Invest. 2005;85:1040–1050. doi: 10.1038/labinvest.3700303. [DOI] [PubMed] [Google Scholar]
- 9.Lehmann U, Kreipe H. Real-time PCR analysis of DNA and RNA extracted from formalin-fixed and paraffin-embedded biopsies. Methods. 2001;25:409–418. doi: 10.1006/meth.2001.1263. [DOI] [PubMed] [Google Scholar]
- 10.Godfrey TE, Kim SH, Chavira M, et al. Quantitative mRNA expression analysis from formalin-fixed, paraffin-embedded tissues using 5′ nuclease quantitative reverse transcription-polymerase chain reaction. J. Mol. Diagn. 2000;2:84–91. doi: 10.1016/S1525-1578(10)60621-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rooney PH, Boonsong A, McKay JA, et al. Colorectal cancer genomics: evidence for multiple genotypes which influence survival. Br. J. Cancer. 2001;85:1492–1498. doi: 10.1054/bjoc.2001.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kidd EA, Yu J, Li X, Shannon WD, Watson MA, McLeod HL. Variance in the expression of 5-fluorouracil pathway genes in colorectal cancer. Clin. Cancer Res. 2005;11:2612–2619. doi: 10.1158/1078-0432.CCR-04-1258. [DOI] [PubMed] [Google Scholar]
- 13.Yu J, Shannon WD, Watson MA, McLeod HL. Gene expression profiling of the irinotecan pathway in colorectal cancer. Clin. Cancer Res. 2005;11:2053–2062. doi: 10.1158/1078-0432.CCR-04-1254. [DOI] [PubMed] [Google Scholar]
- 14.Zhang W, Shannon WD, Duncan J, Scheffer GL, Scheper RJ, McLeod HL. Expression of drug pathway proteins is independent of tumour type. J. Pathol. 2006;209:213–219. doi: 10.1002/path.1955. [DOI] [PubMed] [Google Scholar]
- 15.Konigshoff M, Wilhelm J, Bohle RM, Pingoud A, Hahn M. HER-2/neu gene copy number quantified by real-time PCR: comparison of gene amplification, heterozygosity, and immunohistochemical status in breast cancer tissue. Clin. Chem. 2003;49:219–229. doi: 10.1373/49.2.219. [DOI] [PubMed] [Google Scholar]
- 16.Weksberg R, Hughes S, Moldovan L, Bassett AS, Chow EW, Squire JA. A method for accurate detection of genomic microdeletions using real-time quantitative PCR. BMC Genomics. 2005;6:180. doi: 10.1186/1471-2164-6-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller CR, Dunham CP, Scheithauer BW, Perry A. Significance of necrosis in grading of oligodendroglial neoplasms: a clinicopathologic and genetic study of newly diagnosed high-grade gliomas. J. Clin. Oncol. 2006;24:5419–5426. doi: 10.1200/JCO.2006.08.1497. [DOI] [PubMed] [Google Scholar]
- 18.Halling KC, Kipp BR. Fluorescence in situ hybridization in diagnostic cytology. Hum. Pathol. 2007;38:1137–1144. doi: 10.1016/j.humpath.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 19.Rooney PH, Boonsong A, McFadyen MC, et al. The candidate oncogene ZNF217 is frequently amplified in colon cancer. J. Pathol. 2004;204:282–288. doi: 10.1002/path.1632. [DOI] [PubMed] [Google Scholar]