Abstract
Decline in muscle mass, protein synthesis, and mitochondrial function occurs with age, and amino acids are reported to enhance both muscle protein synthesis and mitochondrial function. It is unclear whether increasing dietary protein intake corrects postabsorptive muscle changes in aging. We determined whether a 10-day diet of high [HP; 3.0 g protein·kg fat-free mass (FFM)−1·day−1] vs. usual protein intake (UP; 1.5 g protein·kg FFM−1·day−1) favorably affects mitochondrial function, protein metabolism, and nitrogen balance or adversely affects insulin sensitivity and glomerular filtration rate (GFR) in 10 healthy younger (24 ± 1 yr) and 9 older (70 ± 2 yr) participants in a randomized crossover study. Net daily nitrogen balance increased equally in young and older participants, but postabsorptive catabolic state also increased, as indicated by higher whole body protein turnover and leucine oxidation with no change in protein synthesis. Maximal muscle mitochondrial ATP production rate was lower in older people, with no change occurring in diet. GFR was lower in older people, and response to HP was significantly different between the two groups, with a significant increase occurring only in younger people, thus widening the differences in GFR between the young and older participants. In conclusion, a short-term high-protein diet increased net daily nitrogen balance but increased the postabsorptive use of protein as a fuel. HP did not enhance protein synthesis or muscle mitochondrial function in either young or older participants. Additionally, widening differences in GFR between young and older patients is a potential cause of concern in using HP diet in older people.
Keywords: dietary protein, protein metabolism, mitochondrial function, kidney function, aging
age is associated with a progressive decline in body protein content, as reflected by declining fat-free mass (FFM) (33). The reduction in FFM is attributed mainly to loss of skeletal muscle, i.e., sarcopenia, and is associated with reduced muscle strength and exercise endurance as well as predisposition to many metabolic disorders (33). The disabilities related to sarcopenia and associated disorders are not fully assessed but result in substantial health care costs (25).
One of the potential mechanisms contributing to sarcopenia is reduced muscle protein synthesis, which was shown to decline by 3.5% per decade (49). Aging is also associated with reductions in the skeletal muscle mitochondrial ATP production and the abundance of mitochondrial DNA and transcripts of genes that encode mitochondrial proteins (44, 47). Reduced ability to produce ATP, the key chemical form of energy for cellular functions, could potentially contribute to the vitality of the aging population and may contribute to the overall reduction in many muscle functions (33). The reduced ability to maintain muscle proteins may result from decreased sensitivity of muscle to efficiently use exogenous amino acids for protein anabolism. It has been reported (56) that skeletal muscle protein synthesis increases in elderly people following administration of amino acids by either intravenous or oral routes (55). Moreover, amino acids play a key role in translational regulation of protein synthesis (54). Administration of insulin and amino acids can enhance mitochondrial biogenesis and ATP production (51). Collectively, these data demonstrate that acute administration of amino acids increases net protein balance in skeletal muscle of older people by enhancing protein synthesis and may improve mitochondrial function. However, it remains to be determined whether increasing dietary protein over a longer period improves postabsorptive muscle protein synthesis and muscle mitochondrial function, in which case such an approach has potential in treating/preventing age-related muscle changes.
We addressed the hypothesis that increased protein intake improves whole body and muscle protein synthesis and enhances muscle mitochondrial function in healthy younger and older people. To test this hypothesis, we examined the effect of 10-day “usual” or “high” protein diets on whole body and skeletal muscle protein metabolism and muscle mitochondrial function. We also measured insulin sensitivity and glomerular filtration rate (GFR) to address concerns that a high-protein diet may adversely affect insulin action and kidney function.
MATERIALS AND METHODS
Subjects.
Ten younger and 10 older participants were enrolled in the study between November 2003 and September 2004. Nineteen participants completed the study, and one older person withdrew for unknown reasons. Data and analyses presented are for the 10 younger and nine older people who completed the study (Table 1). Participants were recruited from the local community through advertisements and were paid for their participation. All were healthy based on history, physical examination, and laboratory tests. Exclusion criteria included body mass index ≥32 kg/m2, smoking, pregnancy, regular exercise for more than 30 min twice/wk during the past 3 mo that could impact the study outcomes, and medications including β-blockers, steroids, and any medication affecting metabolism or muscle, endocrine, cardiovascular, or digestive function.
Table 1.
Subject characteristics
| Younger (n = 5 Women/5 Men) | Older (n = 4 Women/5 Men) | |
|---|---|---|
| Age, yr | 24.3±1.2 | 70.0±1.8* |
| Body mass, kg | 69.5±5.3 | 77.8±2.8 |
| BMI, kg/m2 | 23.3±1.0 | 27.2±0.9* |
| FFM, kg | 48.5±4.1 | 45.7±4.1 |
| Body fat, %mass | 17.4±0.5 | 27.1±0.8* |
Values are means ± SE. BMI, body mass index; FFM, fat-free mass.
P < 0.05 vs. younger participants.
Study protocol.
The study was approved by the Institutional Review Board of the Mayo Clinic, and written, informed consent was obtained before participation. A randomized crossover design was used to compare the effects of 10 days of usual protein (UP) to 10 days of high dietary protein (HP). Participants received both diets in a random order. The same measurements were performed at the end of both dietary periods, and the two study phases were separated by 2–8 wk. A single-blind design was used; i.e., participants were not told whether they were in the UP or HP phases. Since protein turnover and energy expenditure are more closely related to FFM than body mass, the diets were prescribed on the basis of FFM, with 1.5 g·kg FFM−1·day−1 for the UP diet and 3.0 g·kg FFM−1·day−1 for the HP diet. The UP diet was based on the average protein intake of the US population (http://www.cdc.gov/nchs/nhanes.htm) (57), whereas the HP diet had twice the protein content of UP (Table 2). Dietary energy from protein as a percentage of calories was 11 (younger) and 12% (older) in the UP diets and 21 (younger) and 24% (older) of calories in the HP diets, respectively. Carbohydrate content of both diets was kept constant at 50% of calories, whereas the fat content was adjusted to keep the diets isocaloric. All food was prepared in the Metabolic Kitchen at the Clinical Research Unit of the Mayo Clinic Center for Translational Science Activities (CTSA) during the 10-day dietary periods. Calorie (energy) intake was based on resting metabolic rate and physical activity levels to maintain body weight. Participants were weighed daily, and total dietary energy intake was adjusted to ensure weight maintenance. Compliance to the diet was checked by measuring 24-h urinary nitrogen and protein oxidation rate at the end of each 10-day dietary period. Body composition was measured at beginning of the study using dual-energy X-ray absorptiometry (DPX-IQ; Lunar) (26). This measurement was not repeated after each test since it was considered unlikely that a significant change in body composition would be detected with only 10 days of dietary intervention. Subjects were admitted on the evening of day 8, and testing including collection of 24-h urine was performed on days 9–11; other tests were performed following an overnight fast.
Table 2.
Energy and protein intake and substrate oxidation rate
|
Younger |
Older
|
|||
|---|---|---|---|---|
| UP | HP | UP | HP | |
| Energy intake | ||||
| kcal/day | 2,615±155 | 2,626±148 | 2,314±105 | 2,296±102 |
| kcal·kg BW−1·day−1 | 38.4±1.7 | 38.2±1.2 | 29.8±1.0† | 29.7±1.0† |
| kcal·kg FFM−1·day−1 | 55.2±1.8 | 55.4±2.1 | 51.2±1.7 | 50.7±1.6 |
| Protein intake | ||||
| g/day | 72.7±6.1 | 146.5±12.9* | 68.6±4.6 | 137.1±9.5* |
| g·kg BW−1·day−1 | 1.04±0.03 | 2.08±0.07* | 0.89±0.05 | 1.79±0.10* |
| g·kg FFM−1·day−1 | 1.5±0.0 | 3.0±0.0* | 1.5±0.0 | 3.0±0.0* |
| %Total energy | 11.1±0.4 | 21.8±0.8* | 11.8±0.4 | 23.6±0.9* |
| Substrate oxidation, mg·kg FFM−1·min−1 | ||||
| Protein | 1.03±0.07 | 1.44±0.08* | 1.17±0.06 | 1.41±0.05* |
| Carbohydrate | 2.04±0.23 | 1.76±0.46 | 1.57±0.38 | 1.30±0.30 |
| Lipid | 1.29±0.12 | 1.24±0.13 | 1.41±0.20 | 1.28±0.16 |
Data are means ± SE. UP, usual-protein diet; HP, high-protein diet; BW, body weight.
P < 0.05 vs. UP;
P < 0.05 vs. younger participants (same diet).
Methods.
GFR was measured on day 9 of each experimental period using nonradiolabeled iothalamate meglumine (Conray 60%), as previously reported (3), and was standardized for a body surface area of 1.73 m2.
Insulin sensitivity was measured on day 10 using an intravenous glucose tolerance test after an overnight fast, as described previously (4, 53). The glucose dose was 0.3 g/kg body wt, and the insulin dose was 0.03 IU/kg body wt. The minimal model was used to calculate insulin sensitivity (4). Glucose was measured with a Beckman Glucose Analyzer (Beckman Instruments, Porterville, CA). Insulin and human growth hormone were measured with two-site immunoenzymatic assays (Access System; Beckman Instruments, Chaska, MN).
Amino acid kinetics were measured on day 11. Primed, continuous infusions of l-[1-13C]leucine (7.5 μmol·min−1·kg FFM−1) and l-[15N]lysine (15.5 μmol·min−1·kg FFM−1) were administered as described previously (36, 40, 49). Blood samples and breath were drawn before tracer infusion and at hourly intervals for the next 8 h. Indirect calorimetry was performed for 45 min at ∼6 h of isotope infusion (midpoint of steady-state period used for calculations) to measure total CO2 production. At 3 and 8 h during isotope infusion, percutaneous muscle biopsies of the vastus lateralis were performed under local anesthesia (35, 39). Part of the muscle samples was used for mitochondrial measurements, described below, while the rest was frozen in liquid nitrogen for the remaining measurements.
Sample analyses and calculation of protein metabolism.
Urinary nitrogen was measured and nitrogen balance calculated by subtracting 24-h urinary nitrogen excretion from nitrogen intakes during the same period, as described previously (59).
Enrichment of plasma [13C]leucine, [13C]ketoisocaproate (KIC), and [15N]lysine and breath 13CO2, as well as plasma KIC concentration, was measured by mass spectrometry as described previously (2, 34). Plasma amino acid concentration was determined by HPLC with precolumn derivatization (20). Plasma leucine and lysine kinetics were calculated using the steady-state isotope dilution technique and the reciprocal pool model, using plasma KIC as the measurement of intracellular enrichment (28, 34, 45). Leucine oxidation is usually calculated (29, 39) assuming that only 81% of endogenously produced CO2 is expired due to CO2 fixation, based on recovery of 13CO2 following an infusion of [13C]sodium bicarbonate. Published estimates for the retained fraction of 13CO2 vary widely (16, 21, 23, 24, 58). It is unclear whether this variability is related to age. Therefore, we performed a secondary study to determine whether CO2 retention varies with age by performing a primed, continuous infusion of sodium [13C]bicarbonate (21) in a group of eight younger and eight older participants that had consumed the UP diet and measured 13CO2 in breath to calculate the actual fractional recovery of 13CO2 (52). The resulting recovery values were then used in subsequent calculations. Leucine oxidation was calculated as a percentage of endogenous leucine flux to normalize for potential changes in leucine flux with age or diet. Due to sample availability, mixed muscle protein fractional synthesis rate (FSR) was measured in a subset of 14 participants (9 younger and 5 older), as described previously (49), using the increment in [15N]lysine enrichment between 3 and 8 h. The use of either average plasma [15N]lysine enrichment between 3 and 8 h or the muscle tissue fluid [15N]lysine (3–8 h) as the precursor pool for those calculations gave similar outcomes. However, since the plasma-based approach gave less variable results, we report those data.
Substrate oxidation rate.
Carbohydrate and lipid oxidation rates were calculated by indirect calorimetry, as reported previously (17). Leucine oxidation rate was converted to the corresponding estimate of whole body protein oxidation using the conversion constant (24 h/day)/(590 μmol leucine/g protein) to yield grams of oxidized protein per day. These values matched closely with protein oxidation on the basis of urinary nitrogen (r = 0.92, P < 0.01) but were less variable.
Muscle biochemistry.
Skeletal muscle branched-chain aminotransferase (BCAT) and branched-chain α-keto acid dehydrogenase (BCKD) protein expression and activities were measured as described previously (1, 15). Skeletal mitochondrial ATP production rate was measured with a bioluminescent technique after mitochondrial isolation from fresh tissue, and activity of citrate synthase was measured as described previously (47).
Statistical analysis.
Data are presented as means ± SE, and statistical analysis was performed using StatView program 4.02 (Abacus Concepts, Berkley, CA). A repeated-measures analysis of variance was used to discriminate among the effects of age and diet. An a posteriori Fisher test was applied as appropriate to locate pairwise differences among means, i.e., categorical variables, after adjustments for multiple comparisons were made (Bonferroni adjustment). Student's t-test was also used to compare subject characteristics. P < 0.05 was considered as significant. Preliminary data on whole body Leu flux and oxidation, muscle protein FSR, and mitochondrial ATP production rate (47, 48, 51) were used to estimate sample size. Nine or 10 subjects per group were expected to provide 80% power to detect 20–30% differences in these variables between younger and older groups and between dietary phases.
RESULTS
Protein intake and substrate oxidation.
By design, dietary protein intake was doubled during the HP vs. UP diet (Table 2). The HP diet resulted in a similar increase in protein oxidation in both age groups, from 1.03 ± 0.07 to 1.44 ± 0.08 mg·kg FFM−1·min−1 in younger and from 1.17 ± 0.06 to 1.41 ± 0.05 mg·kg FFM−1·min−1 in older participants, but did not significantly change oxidation rates of either carbohydrate or fat. There were no differences in fuel oxidation with age on either diet.
Insulin sensitivity and hormone profile.
Insulin sensitivity and the acute insulin response to glucose did not vary with age or diet (Table 3). Likewise, fasting glucose and insulin and growth hormone concentrations did not vary with diet, although growth hormone changed with age (P < 0.05).
Table 3.
Insulin sensitivity and hormone profile
|
Younger |
Older
|
|||
|---|---|---|---|---|
| UP | HP | UP | HP | |
| SI, 10−5 ml·min−1·pmol−1·l−1 | 7.5±1.4 | 9.5±1.8 | 6.9±1.3 | 7.6±1.4 |
| AIRg, mU·l−1·min−1 | 445±103 | 418±62 | 330±44 | 371±60 |
| Fasting glucose, mmol/l | 5.1±0.2 | 5.1±0.2 | 5.1±0.1 | 5.1±0.1 |
| Fasting insulin, μU/ml | 6.3±1.2 | 6.5±1.0 | 5.1±0.7 | 5.3±0.6 |
| Growth hormone, ng/ml | 1.4±0.6 | 1.4±0.7 | 0.3±0.1 | 0.3±0.1 |
Data are means ± SE. SI, insulin sensitivity; AIRg, acute insulin response to glucose. There were no significant differences due to age or diet.
Whole body and muscle protein metabolism.
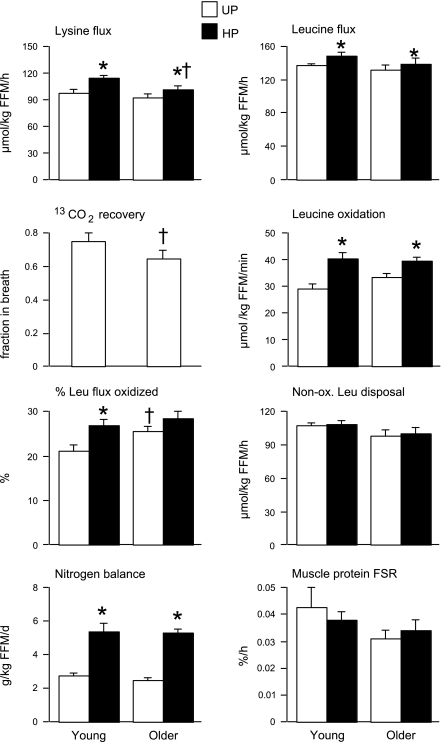
The flux rates of both lysine and leucine, indexes of whole body protein degradation, increased significantly in both younger and older participants on the HP diet (Fig. 1). Lysine flux was significantly (10%) lower in older vs. younger people while on the HP diet only, but no other age differences in flux rates were evident. The recovery fraction of 13CO2 was 13% lower in older vs. younger people, i.e., 75% in younger and 65% in older people. Thus, for calculations of leucine oxidation, recovery factors of 0.75 for younger participants and 0.65 for older participants were used. Compared with the UP diet, leucine oxidation increased on the HP in both groups, from 27.4 ± 1.8 to 38.2 ± 2.1 μmol·kg FFM−1·min−1 in younger participants and from 31.2 ± 1.5 to 37.3 ± 1.3 mg·kg FFM−1·min−1 in older participants, with younger participants showing greater increase (39%) than older (20%) participants. When expressed as a percentage of leucine flux, however, the increase in leucine oxidation on the HP diet was only significant in younger people. Compared with younger people, older people had higher leucine oxidation relative to leucine flux on the UP diet (21.5 ± 1.2 vs. 27.3 ± 1.3%), with a similar trend (P = 0.068) on the HP diet (28.2 ± 1.3 vs. 31.7 ± 1.6%). Nonoxidative leucine disposal, an index of whole body protein synthesis rate, was not significantly affected by diet, and there was no significant age effect. Nitrogen balance also did not differ with age but did increase nearly twofold on the HP diet in both groups from 2.77 ± 0.11 to 5.42 ± 0.45 g·kg FFM−1·day−1 in younger participants and from 2.48 ± 0.12 to 5.32 ± 0.18 g·kg FFM−1·day−1 in older participants. There were no significant differences in muscle protein synthesis with either age or diet.
Fig. 1.
Whole body amino acid kinetics and muscle protein synthesis. Data are means ± SE. *P < 0.05 vs. UP diet within age group; †P < 0.05 vs. younger participants on the same diet. UP, usual-protein diet; HP, high-protein diet; FSR, fractional synthesis rate.
Amino acid and KIC concentrations and muscle BCAT and BCKD.
Fasting plasma total concentrations, nonessential and essential amino acid concentrations, and the concentrations of leucine and lysine were not affected by age or dietary treatment (Table 4). However, BCAA concentration increased 25% on the HP diet in younger but not older people, resulting in a difference between groups only during the HP diet. KIC concentration also increased on the HP diet only in younger (12%) but not older people.
Table 4.
Amino acid concentrations and skeletal muscle BCAT and BCKD activity
|
Younger |
Older
|
|||
|---|---|---|---|---|
| UP | HP | UP | HP | |
| Plasma, μmol/l | ||||
| Total amino acids | 2,570±94 | 2,488±119 | 2,457±93 | 2,570±164 |
| Nonessential amino acids | 1,575±62 | 1,544±76 | 1,592±68 | 1,640±117 |
| Essential amino acids | 995±54 | 944±53 | 865±41 | 930±65 |
| Branched-chain amino acids | 415±27 | 521±42* | 390±40 | 416±23† |
| Leucine | 130±8 | 134±9 | 132±7 | 135±6 |
| KIC | 14.4±0.8 | 16.1±0.6* | 13.3±0.7 | 13.7±0.9† |
| Lysine | 208±8 | 227±13 | 253±21 | 236±16 |
| Skeletal muscle | ||||
| Lysine concentration, μmol/μg | 0.55±0.04 | 0.81±0.09* | 0.74±0.06† | 0.72±0.08 |
| Leucine concentration, μmol/μg | 0.11±0.01 | 0.12±0.01 | 0.12±0.01 | 0.12±0.01 |
| BCAT activity, nmol·min−1·mg−1 | 76±9 | 77±8 | 107±14 | 100±16 |
| BCAT protein expression, AU | 1.07±0.11 | 1.17±0.15 | 1.28±0.20 | 1.09±0.19 |
| Basal BCKD activity, nmol·min−1·mg−1 | 147±23 | 139±20 | 116±18 | 123±11 |
| Total BCKD activity, nmol·min−1·mg−1 | 414±36 | 368±48 | 339±39 | 352±39 |
| %Active BCKD | 36±5 | 37±2 | 33±2 | 37±4 |
Data are means ± SE. BCAT, branched-chain aminotransferase; BCKD, branched-chain α-keto acid dehydrogenase; AU, arbitrary units.
P < 0.05 vs. UP;
P < 0.05 vs. younger participants (same diet).
The skeletal muscle concentration of lysine was higher in older vs. younger people during the UP but not the HP diet. Muscle lysine increased 47% in the younger group but was not affected by diet in the older group. Muscle leucine did not differ with either age or diet. There were also no differences with age or dietary treatment in the activity or protein expression of BCAT or the basal or total activity of BCKD.
GFR.
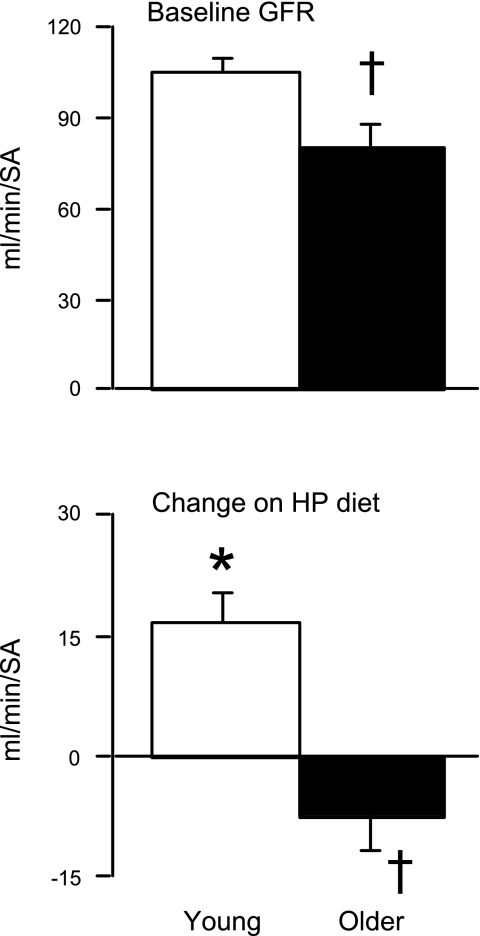
GFR was significantly lower in older participants during both diets (older was only an average of 76.8% of younger participants during UP and 57.7% during HP; Fig. 2). Compared with the UP diet, GFR increased 17% [from 105.9 ± 3.6 to 127.8 ± 5.7 ml·min−1·surface area (SA)−1, P < 0.05] on the HP diet in the younger participants, whereas it tended to decline (9%) in the older group (from 81.3 ± 6.5 to 74.0 ± 6.3 ml·min−1·SA−1, P = 0.13), leading to a significance difference between groups in the adaptation response to the HP diet (P < 0.05). As a result, the age-related differences in GFR between younger and older participants increased (P < 0.05). GFR of older people was ∼77% of younger people during UP diet and ∼58% during HP diet.
Fig. 2.
Glomerular filtration rate (GFR). Data are means ± SE. GFR by renal clearance of iothalamate. Top: GFR measured during the adequate-protein diet (baseline). Bottom: change in GFR on the HP vs. the adequate-protein diet. *P < 0.05 vs. UP diet within age group; †P < 0.05 vs. younger participants on the same diet.
Mitochondrial ATP production rate and citrate synthase activity.
Muscle mitochondrial ATP production was significantly (35–40%) lower in older vs. younger people on either diet when measured using substrates that supply electrons primarily to complex I (glutamate + malate) or complex II (succinate + rotenone) of the electron transport chain (Table 5). Citrate synthase activity was also 16–21% lower in older people. Adjusted for the lower citrate synthase activity, which suggests lower mitochondrial content, ATP production was still significantly lower in older vs. younger people (19–27%). There were no effects of diet on these measurements in either age group.
Table 5.
MAPR and CS activity
|
Younger |
Older
|
|||
|---|---|---|---|---|
| UP | HP | UP | HP | |
| MAPR, μmol·min−1·g−1 | ||||
| Glutamate + malate | 8.49±1.14 | 8.81±0.99 | 5.15±0.43* | 5.39±0.20* |
| Succinate + rotenone | 3.58±0.59 | 3.91±0.53 | 2.30±0.23* | 2.54±0.11* |
| CS activity, μmol·min−1·g−1 | 16.7±0.9 | 16.1±0.9 | 13.2±1.1* | 13.5±0.7* |
Data are means ± SE. MAPR, mitochondrial ATP production rate; CS, citrate synthase.
P < 0.05 vs. younger participants (same diet).
DISCUSSION
The current study demonstrates that 10 days of a high-protein diet result in increased whole body protein turnover and amino acid (leucine) oxidation with no increase in whole body or muscle protein synthesis in the postabsorptive state. However, high-protein diet increased daily net nitrogen balance. Muscle mitochondrial ATP production was lower in older people, and the high-protein diet had no stimulatory effect. The high-protein diet did not have an adverse effect on insulin sensitivity, but it did have a potentially negative effect on glomerular filtration rate in older people. Although the younger group significantly increased GFR on the HP diet, older people did not demonstrate this adaptive response and even showed a tendency to decline.
Previous studies have shown that aging is associated with a decline in skeletal muscle mitochondrial capacity to produce ATP along with lower abundance of mitochondrial DNA and mRNA transcripts, mitochondrial protein synthesis, content of several mitochondrial proteins, and oxidative enzyme activity (44, 47, 48). Since ATP is the chemical energy needed for most biological functions, it is possible that this basic defect in mitochondria may contribute to overall decline in muscle functions with aging (33). Aerobic exercise training can stimulate muscle mitochondrial oxidative capacity (22, 50), mRNA abundance of the master regulator of energy metabolism, peroxisome proliferator-activated receptor-γ coactivator-1α (50), and muscle protein synthesis in older people (49), demonstrating that some of the age-related metabolic dysfunction is at least partially reversible. The objective of the current study was to determine whether it was possible to enhance muscle mitochondrial function and protein synthesis in older people by increasing protein intake. We hypothesized that a high-protein diet could stimulate muscle protein synthesis based on previous studies that showed that acute intake of amino acids increases muscle protein synthesis (55, 56) and, in combination with insulin, enhances muscle mitochondrial function (51) compared with these measurements taken in the fasting state. The current results showed that increased protein intake for 10 days did not stimulate these potentially reversible age-related muscle changes in younger or older people. There are many potential explanations for the current observations. Although the acute amino acid effects on muscle protein synthesis and mitochondrial function have been demonstrated previously, it is unclear whether there are dose-dependent effects. It is possible that the maximal benefit from dietary proteins and amino acids is already achieved with the UP diet, which reflects the average protein intake of the US population. Second, our measurements were performed in the postabsorptive state following overnight fasting, and it is possible that the higher protein intake may stimulate protein synthesis following meal ingestion, as has been observed in animals (46) and humans (55, 56), but may not enhance the basal protein synthesis.
From the current results it is clear that postabsorptive protein catabolism (both breakdown and amino acid oxidation) was increased during the HP diet. These results are consistent with previous studies (12, 41) indicating that a high protein intake increases amino acid oxidation and protein breakdown (42). It is possible that increased protein retention occurred following a meal in the postprandial phase. Because of the non-steady state following meals, studies determining protein synthesis and breakdown following meals are fraught with methodological and modeling problems. However, protein retention in the postprandial state following a high-protein meal may have occurred in tissues such as splanchnic bed (40), as has been shown to occur following amino acid infusion. The results suggest that part of higher retention of amino acids following a meal is utilized as a fuel source in between meals. It remains to be determined whether there is a body protein store that could be used in time of need without having any functional consequences. On the basis of protein loss during weight reduction, Garrow (19) estimated that a 70-kg person could afford to lose 4.5 kg of protein without any serious functional impact. On the basis of measurements of protein synthesis and mitochondrial function, no beneficial effects are noted following the HP diet.
We measured the enzymes involved in leucine oxidation in skeletal muscle but observed no change in the complexes involved in leucine transamination (the first reversible step in leucine catabolism) and decarboxylation of KIC, the transamination product of leucine. Although leucine is transaminated mainly in skeletal muscle, it is possible that activity of BCKD in the liver may change with dietary protein intake since the liver is the main site of KIC decarboxylation (31, 32, 38). Animal studies have shown that total BCKD activity and percentage of the active enzyme are not influenced by the dietary protein intake (6 or 20% casein) in skeletal muscle of rats (30), although a small and transient increase in total BCKD activity was observed in rat skeletal muscle after a 50% protein diet was fed for 10 days (7). Of interest, we noted that, in older people, carbon dioxide retention is higher than in younger people, and in the postabsorptive state older people oxidize a higher fraction of endogenously appearing leucine. It has been proposed that a greater amount of leucine is catabolized in splanchnic bed in the elderly people (8, 56).
It has been proposed that amino acids (43) and higher protein intake in elderly people may induce skeletal muscle insulin resistance (27). These previous data were obtained using cultured myotubes and are not supported by the current human study that showed no adverse effect of high-protein diet on insulin sensitivity. However, the current study demonstrated that whereas younger people significantly increased GFR following the HP diet, older people did not show the same adaptation and showed a trend toward reduced GFR, thus clearly demonstrating significant age-related difference in the response to high protein intake. The range of GFR in the older group on UP diet was within the normal range, 54–112 ml/min (values for younger participants, 87–122), whereas it decreased to 32–91 ml/min while on the HP diet (values for younger participants, 113–172). The differences in GFR between young and old are widened by HP. It is well established that increased protein intake in healthy animals and humans significantly increases renal hemodynamics, including GFR (18). In younger healthy men, GFR increased following a 4-mo high-protein diet (9) or an acute amino acid infusion (14, 37). Of concern, over weeks or months this may lead to deleterious consequences, especially in people with already reduced renal function or at risk for compromised renal function, e.g., elderly people (10). It has been postulated that high protein intake induces hemodynamically mediated injury by increasing intraglomerular pressures and flow (63). This may lead to pathophysiological changes that, over time, cause progressive glomerular injury and sclerosis, particularly in preexisting diseased or damage kidneys (18). High-protein diets may cause long-term deleterious effects in patients with renal failure (10, 11). Thus, additional work on the effects of high-protein diet on kidney function in older people is needed.
The current study addressed primarily whether high-protein diet improves postabsorptive protein synthesis and mitochondrial function while on similar activity levels. Administration of amino acids has been shown to enhance exercise effect on muscle protein anabolism (5, 6), and studies in elderly people suggest that further research is needed to determine whether high protein intake may be required by elderly people to get the full benefit of a resistance exercise program. Further studies may address the question of whether benefits from an exercise program may be enhanced by higher protein intake in elderly people without any adverse effect on renal function (13).
In summary, high protein intake for 10 days increased net daily nitrogen balance in both younger and older people but had no beneficial effects on muscle protein synthesis and mitochondrial function. However, it is possible that usual protein intake may not maximally stimulate postprandial protein synthesis in muscle and nonmuscle tissues. High dietary proteins further widened the differences in GFR between young and older people, causing concern about the potential adverse effect on kidney function in older people. Thus, the results of this study do no support the idea that older people who are already consuming adequate protein may benefit from high protein intake, and in fact, the current data allude to potential negative effects of high protein intake on older kidneys.
GRANTS
This study was supported by National Institute on Aging Grant No. R01-AG-09531 and CTSA Grant No. UL1-RR-024150-01 from the National Center for Research Resources.
Acknowledgments
We gratefully acknowledge the support of the Mayo CTSA Clinical Research Unit nursing, nutrition, and technical staff (Lavonne Oenning and Helen O'Connell) and the skillful technical assistance of the Metabolomic Core, Jane Kahl, Dawn Morse, Kate Klaus, and Jill Schimke.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Aftring RP, Miller WJ, Buse MG. Effects of diabetes and starvation on skeletal muscle branched-chain α-keto acid dehydrogenase activity. Am J Physiol Endocrinol Metab 254: E292–E300, 1988. [DOI] [PubMed] [Google Scholar]
- 2.Balagopal P, Ford GC, Ebenstein DB, Nadeau DA, Nair KS. Mass spectrometric methods for determination of [13C]leucine enrichment in human muscle protein. Anal Biochem 239: 77–85, 1996. [DOI] [PubMed] [Google Scholar]
- 3.Bergert JH, Liedtke RR, Oda RP, Landers JP, Wilson DM. Development of a nonisotopic capillary electrophoresis-based method for measuring glomerular filtation rate. Electrophoresis 18: 1827–1835, 1997. [DOI] [PubMed] [Google Scholar]
- 4.Bergman RN, Phillips LS, Cobelli C. Physiologic evaluation of factors controlling glucose tolerance in man. J Clin Invest 68: 1456–1467, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Binz PA, Muller M, Walther D, Bienvenut WV, Gras R, Hoogland C, Bouchet G, Gasteiger E, Fabbretti R, Gay S, Palagi P, Wilkins MR, Rouge V, Tonella L, Paesano S, Rossellat G, Karmine A, Bairoch A, Sanchez JC, Appel RD, Hochstrasser DF. A molecular scanner to automate proteomic research and to display proteome images. Anal Chem 71: 4981–4988, 1999. [DOI] [PubMed] [Google Scholar]
- 6.Biolo G, Maggi SP, Williams BD, Tipton KD, Wolfe RR. Increased rates of muscle protein turnover and amino acid transport after resistance exercise in humans. Am J Physiol Endocrinol Metab 268: E514–E520, 1995. [DOI] [PubMed] [Google Scholar]
- 7.Block KP, Aftring RP, Mehard WB, Buse MG. Modulation of rat skeletal muscle branched-chain alpha-keto acid dehydrogenase in vivo. Effects of dietary protein and meal consumption. J Clin Invest 79: 1349–1358, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boirie Y, Gachon P, Beaufrère B. Splanchnic and whole-body leucine kinetics in young and elderly men. Am J Clin Nutr 65: 489–495, 1997. [DOI] [PubMed] [Google Scholar]
- 9.Brandle E, Sieberth HG, Hautmann RE. Effect of chronic dietary protein intake on the renal function in healthy subjects. Eur J Clin Nutr 50: 734–740, 1996. [PubMed] [Google Scholar]
- 10.Brenner BM, Meyer TW, Hostetter TH. Dietary protein intake and the progressive nature of kidney disease: the role of hemodynamically mediated glomerular injury in the pathogenesis of progressive glomerular sclerosis in aging, renal ablation, and intrinsic renal disease. N Engl J Med 307: 652–659, 1982. [DOI] [PubMed] [Google Scholar]
- 11.Brodsky IG, Devlin JT. Effects of dietary protein restriction on regional amino acid metabolism in insulin-dependent diabetes mellitus. Am J Physiol Endocrinol Metab 270: E148–E157, 1996. [DOI] [PubMed] [Google Scholar]
- 12.Campbell WW, Crim MC, Young VR, Joseph LJ, Evans WJ. Effects of resistance training and dietary protein intake on protein metabolism in older adults. Am J Physiol Endocrinol Metab 268: E1143–E1153, 1995. [DOI] [PubMed] [Google Scholar]
- 13.Campbell WW, Trappe TA, Jozsi AC, Kruskall LJ, Wolfe RR, Evans WJ. Dietary protein adequacy and lower body versus whole body resistive training in older humans. J Physiol 542: 631–642, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castellino P, Giordano C, Perna A, DeFronzo RA. Effects of plasma amino acid and hormone levels on renal hemodynamics in humans. Am J Physiol Renal Fluid Electrolyte Physiol 255: F444–F449, 1988. [DOI] [PubMed] [Google Scholar]
- 15.Cooper AJ, Conway M, Hutson SM. A continuous 96-well plate spectrophotometric assay for branched-chain amino acid aminotransferases. Anal Biochem 308: 100–105, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Fowle AS, Matthew CM, Campbell EJ. The rapid distribution of 3H2O and 11CO2 in the body in relation to the immediate carbon dioxide storage capacity. Clin Sci 27: 51–65, 1964. [PubMed] [Google Scholar]
- 17.Frayn KN Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol 55: 628–634, 1983. [DOI] [PubMed] [Google Scholar]
- 18.Friedman AN High-protein diets: potential effects on the kidney in renal health and disease. Am J Kidney Dis 44: 950–962, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Garrow JS Energy stores in man, their composition and measurement. Proc Nutr Soc 41: 175–181, 1982. [DOI] [PubMed] [Google Scholar]
- 20.Hill DW, Walters FH, Wilson TD, Stuart JD. High performance liquid chromatographic determination of amino acids in the picomole range. Anal Chem 51: 1338–1341, 1979. [DOI] [PubMed] [Google Scholar]
- 21.Hoerr RA, Yu YM, Wagner DA, Burke JF, Young VR. Recovery of 13C in breath from NaH13CO3 infused by gut and vein: effect of feeding. Am J Physiol Endocrinol Metab 257: E426–E438, 1989. [DOI] [PubMed] [Google Scholar]
- 22.Holloszy JO, Coyle EF. Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J Appl Physiol 56: 831–838, 1984. [DOI] [PubMed] [Google Scholar]
- 23.Irving CS, Wong WW, Shulman RJ, Smith EO, Klein PD. [13C]bicarbonate kinetics in humans: intra- vs. interindividual variations. Am J Physiol Regul Integr Comp Physiol 245: R190–R202, 1983. [DOI] [PubMed] [Google Scholar]
- 24.Issekutz B, Paul P, Miller HI, Bortz WM. Oxidation of plasma FFA in lean and obese humans. Metabolism 17: 62–73, 1968. [DOI] [PubMed] [Google Scholar]
- 25.Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R. The healthcare costs of sarcopenia in the United States. J Am Geratr Soc 52: 80–85, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Jensen MD, Kanaley JA, Roust LR, O'Brien PC, Braun JS, Dunn WL, Wahner HW. Assessment of body composition with use of dual-energy x-ray absorptiometry: evaluation and comparison with other methods. Mayo Clin Proc 68: 867–873, 1993. [DOI] [PubMed] [Google Scholar]
- 27.Krebs M, Roden M. Nutrient-induced insulin resistance in human skeletal muscle. Curr Med Chem 11: 901–908, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Matthews DE, Bier DM, Rennie MJ, Edwards RH, Halliday D, Millward DJ, Clugston GA. Regulation of leucine metabolism in man: a stable isotope study. Science 214: 1129–1131, 1981. [DOI] [PubMed] [Google Scholar]
- 29.Matthews DE, Motil KJ, Rohrbaugh DK, Burke JF, Young VR, Bier DM. Measurement of leucine metabolism in man from a primed, continuous infusion of l-[1-13C]leucine. Am J Physiol Endocrinol Metab 238: E473–E479, 1980. [DOI] [PubMed] [Google Scholar]
- 30.Miller RH, Harper AE. Regulation of valine and α-ketoisocaproate metabolism in rat kidney mitochondria. Am J Physiol Endocrinol Metab 255: E475–E481, 1988. [DOI] [PubMed] [Google Scholar]
- 31.Moller N, Jensen MD, Rizza RA, Andrews JC, Nair KS. Renal amino acid, fat and glucose metabolism in type 1 diabetic and non-diabetic humans: effects of acute insulin withdrawal. Diabetologia 49: 1901–1908, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Nair KS Assessment of protein metabolism in diabetes. In: Clinical Research in Diabetes and Obesity, Part I: Methods, Assessment, and Metabolic Regulation, edited by Draznin B and Rizza RA. Totowa, NJ: Humana, 1997, p. 137–170.
- 33.Nair KS Aging muscle. Am J Clin Nutr 81: 953–963, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Nair KS, Ford GC, Ekberg K, Fernqvist-Forbes E, Wahren J. Protein dynamics in whole body and in splachnic and leg tissues in type I diabetic patients. J Clin Invest 95: 2926–2937, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nair KS, Halliday D, Griggs RC. Leucine incorporation into mixed skeletal muscle protein in humans. Am J Physiol Endocrinol Metab 254: E208–E213, 1988. [DOI] [PubMed] [Google Scholar]
- 36.Nair KS, Matthews DE, Welle SL, Braiman T. Effect of leucine on amino acid and glucose metabolism in humans. Metabolism 41: 643–648, 1992. [DOI] [PubMed] [Google Scholar]
- 37.Nair KS, Pabico RC, Truglia JA, McKenna BA, Statt M, Lockwood DH. Mechanism of glomerular hyperfiltration after a protein meal in humans. Diabetes Care 17: 711–715, 1994. [DOI] [PubMed] [Google Scholar]
- 38.Nair KS Regional protein dynamics in type I diabetic patients. In: Amino Acids/Protein Metabolism in Heal and Disease, edited by Tessari P, Pittoni G, Tiengo A, and Soeters PB. London: Smith-Gordon, 1997, p. 133–139.
- 39.Nair KS, Welle SL, Halliday D, Campbell RG. Effect of beta-hydroxybutyrate on whole-body leucine kinetics and fractional mixed skeletal muscle protein synthesis in humans. J Clin Invest 82: 198–205, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nygren J, Nair KS. Differential regulation of protein dynamics in splanchnic and skeletal muscle beds by insulin and amino acids in healthy human subjects. Diabetes 52: 1377–1385, 2003. [DOI] [PubMed] [Google Scholar]
- 41.Pannemans DL, Wagenmakers AJ, Westerterp KR, Schaafsma G, Halliday D. Effect of protein source and quantity on protein metabolism in elderly women. Am J Clin Nutr 68: 1228–1235, 1998. [DOI] [PubMed] [Google Scholar]
- 42.Pannemans DL, Haliday D, Westerterp KR. Whole-body protein turnover in elderly men and women: responses to two protein intakes. Am J Clin Nutr 61: 33–38, 1995. [DOI] [PubMed] [Google Scholar]
- 43.Patti ME, Brambilla E, Luzi L, Landaker EJ, Kahn CR. Bidirectional modulation of insulin action by amino acids. J Clin Invest 101: 1519–1529, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Petersen KF, Befroy D, Sufour S, Dziura J, Ariyan C, Rothman DL, DiPietro L, Cline GW, Shulman G. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science 300: 1140–1142, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schwenk WF, Beaufrere B, Haymond MW. Use of reciprocal pool specific activities to model leucine metabolism in humans. Am J Physiol Endocrinol Metab 249: E646–E650, 1985. [DOI] [PubMed] [Google Scholar]
- 46.She P, Reid TM, Bronson SK, Vary TC, Hajnal A, Lynch CJ, Hutson SM. Disruption of BCATm in mice leads to increased energy expenditure associated with the activation of a futile protein turnover cycle. Cell Metab 6: 181–194, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Short KR, Bigelow ML, Kahl JC, Singh R, Coenen-Schimke JM, Raghavakaimal S, Nair KS. Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci USA 102: 5618–5623, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Short KR, Bigelow ML, Nair KS. Age effect on muscle mitochondrial function and impaired glucose tolerance after a mixed meal (Abstract). Diabetes 52: A346, 2003. [Google Scholar]
- 49.Short KR, Vittone JL, Bigelow ML, Proctor DN, Nair KS. Age and aerobic exercise training effects on whole body and muscle protein metabolism. Am J Physiol Endocrinol Metab 286: E92–E101, 2004. [DOI] [PubMed] [Google Scholar]
- 50.Short KR, Vittone JL, Bigelow ML, Proctor DN, Rizza RA, Coenen-Schimke JM, Nair KS. Impact of aerobic exercise training on age-related changes in insulin sensitivity and muscle oxidative capacity. Diabetes 52: 1888–1896, 2003. [DOI] [PubMed] [Google Scholar]
- 51.Stump CS, Short KR, Bigelow ML, Schimke JC, Nair KS. Effect of insulin on human skeletal muscle mitochondrial ATP production, protein synthesis, and mRNA transcripts. Proc Natl Acad Sci USA 100: 7996–8001, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tissot S, Delafosse B, Normand S, Bouffard Y, Annat G, Viale JP, Pachiaudi C, Riou JP, Motin J. Recovery of [13C]bicarbonate as respiratory 13CO2 in mechanically ventilated patients. Am J Clin Nutr 57: 202–206, 1993. [DOI] [PubMed] [Google Scholar]
- 53.Toffolo G, Campioni M, Basu R, Rizza RA, Cobelli C. A minimal model of insulin secretion and kinetics to assess hepatic insulin extraction. Am J Physiol Endocrinol Metab 290: E169–E176, 2006. [DOI] [PubMed] [Google Scholar]
- 54.Vary TC, Jefferson LS, Kimball SR. Amino acid-induced stimulation of translation initiation in rat skeletal muscle. Am J Physiol Endocrinol Metab 277: E1077–E1086, 1999. [DOI] [PubMed] [Google Scholar]
- 55.Volpi E, Ferrando AA, Yeckel CW, Tipton KD, Wolfe RR. Exogenous amino acids stimulate net muscle protein synthesis in the elderly. J Clin Invest 101: 2000–2007, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Volpi E, Mittendorfer B, Wolf SE, Wolfe RR. Oral amino acids stimulate muscle protein anabolism in the elderly despite higher first-pass splanchnic extraction. Am J Physiol Endocrinol Metab 277: E513–E520, 1999. [DOI] [PubMed] [Google Scholar]
- 57.Wakimoto P, Block G. Dietary intake, dietary patterns, and changes with age: an epidemiological perspective. J Gerontol A Biol Sci Med Sci 56 Spec No 2: 65–80, 2001. [DOI] [PubMed]
- 58.Winchell HS, Stahelin H, Kusubov B, Slanger B, Fish M, Pollycove M, Lawrence JH. Kinetics of CO2-HCO3 in normal adult males. J Nucl Med 11: 711–715, 1970. [PubMed] [Google Scholar]
- 59.Woo J Relationship among diet, physical activity and other lifestyle factors and debilitating diseases in the elderly. Euro J Clin Nutr 54: S143–S147, 2000. [DOI] [PubMed] [Google Scholar]