Abstract
Activation of an innate immune response in airway epithelia by the human pathogen Pseudomonas aeruginosa requires bacterial expression of flagellin. Addition of flagellin (10−7 M) to airway epithelial cell monolayers (Calu-3, airway serous cell-like) increased Cl− secretion (ICl) beginning after 3–10 min, reaching a plateau after 20–45 min at ΔICl = 15–50 μA/cm2. Similar, although 10-fold smaller, responses were observed in well-differentiated bronchial epithelial cultures. Flagellin stimulated ICl in the presence of maximally stimulating doses of the purinergic agonist ATP, but had no effects following forskolin. IL-1β (produced by both epithelia and neutrophils during infections) stimulated ICl similar to flagellin. Flagellin-, IL-1β-, ATP-, and forskolin-stimulated ICl were inhibited by cystic fibrosis transmembrane conductance regulator (CFTR) blockers GlyH101, CFTRinh172, and glibenclamide. Neither flagellin nor IL-1β altered transepithelial fluxes of membrane-impermeant dextran (10 kDa) or lucifer yellow (mol wt = 457), but both activated p38, NF-κB, and IL-8 secretion. Blockers of p38 (SB-202190 and SB-203580) reduced flagellin- and IL-1β-stimulated ICl by 33–50% but had smaller effects on IL-8 and NF-κB. It is concluded that: 1) flagellin and IL-1β activated p38, NF-κB, IL-8, and CFTR-dependent anion secretion without altering tight junction permeability; 2) p38 played a role in regulating ICl and IL-8 but not NF-κB; and 3) p38 was more important in flagellin- than IL-1β-stimulated responses. During P. aeruginosa infections, flagellin and IL-1β are expected to increase CFTR-dependent ion and fluid flow into and bacterial clearance from the airways. In cystic fibrosis, the secretory response would be absent, but activation of p38, NF-κB, and IL-8 would persist.
Keywords: cystic fibrosis transmembrane conductance regulator, inflammation, airway epithelial secretion, interleukin-1β, Pseudomonas aeruginosa
bacteria and viruses in the airways activate innate immune responses, including triggering cellular signaling leading to activation of the key transcription factor NF-κB and production and release of proinflammatory cytokines and chemokines like IL-8 and IL-1β (2, 10, 13, 14, 18, 50, 52). The gram-negative bacterium Pseudomonas aeruginosa, a common pathogen in the lungs of cystic fibrosis (CF) patients, triggers innate immune responses of airway epithelial cells. These responses require bacterial expression of flagellin (18, 49, 52), and flagellin alone activates airway epithelia (13, 21, 22, 30, 38, 45, 52). Although airway epithelia express multiple Toll-like receptors (TLRs) (16, 35), flagellin activation of TLR5 appears to play a particularly important role in mediating the innate immune responses of airway epithelia to bacteria (2, 16, 18, 52). In addition, high levels of the proinflammatory cytokine IL-1β are also found prominently in the airways of CF patients, and airway epithelia express IL-1 receptors and respond with vigorous inflammatory responses to the presence of IL-1β in the apical or basolateral bath (7, 49). Flagellin- and IL-1β-triggered proinflammatory responses require NF-κB signaling (3). The MAPK p38 appears to be involved in both TLR5- (54) and IL-1β-triggered (47, 54) signaling in airway cells.
In addition to these effects of products to trigger inflammatory signaling, Evans et al. (11) showed that exposure of primary airway epithelial cells on the apical and basolateral surfaces to P. aeruginosa caused reductions in ENaC-mediated Na+ transport and fluid absorption, and it was proposed that this effect was mediated indirectly through inhibition of basolateral K+ channels. Recent experiments (26) showed that flagellin caused reductions in ENaC-mediated Na+ transport across mouse trachea, and through the use of specific inhibitors on the flagellin-induced alteration in Na+ transport, it was proposed that this effect was mediated through flagellin-triggered ATP release and activation of Ca2+ and p41/42-ERK MAPK signaling, although increases in cytosolic [Ca2+], Cai, were not observed. IL-1β increases apparent HCO3− and fluid secretion by cultured human airway epithelial monolayers (14). Long-term (4–72 h) treatment with IL-1β increases CFTR expression in human intestinal cells (6) and alteration of tight junctional morphology and apparent permeability to Cl− in human airway epithelial cells (7).
The present experiments were initially designed to test the early effects of flagellin on Cl− secretion and cell signaling associated with activation of innate immune responses in Calu-3 cells, a serous-like cell line that exhibits CFTR-dependent anion secretion that is stimulated by increases in cellular cAMP (9, 27, 45) and protein kinase C (28). Although previous experiments showed no effect of flagellin to stimulate ion secretion in airway epithelia, we considered the possibility that such effects could have been missed resulting from use of surface cells rather than gland or gland-like cells that might express higher levels of CFTR than surface cells in the trachea. Effects of IL-1β were also tested to compare with previous measurements of apparent HCO3− and fluid secretion by airway epithelia (14) and also to determine the generality of the responses to this proinflammatory mediator that uses similar signaling pathways as flagellin to activate innate immune responses (3). These proinflammatory agonists induced slow, persistent increases in apparent CFTR-dependent Cl− current in Calu-3 cells. Because flagellin and IL-1β have both been known to activate p38 in other cells, we tested for p38 signaling in mediating the activation of apparent CFTR-dependent Cl− secretion and increased NF-κB signaling and IL-8 expression and secretion in Calu-3 cells.
METHODS
Reagents.
Unless otherwise specified, all reagents and chemicals were obtained from Sigma (St. Louis, MO).
P. aeruginosa flagellin (Inotek, Beverly, MA) was prepared at 10−3 g/ml in a solution containing 10 mM phosphate buffer, pH 7.4, 140 mM NaCl, and 3 mM KCl. As described by Inotek, recombinant flagellin was expressed with tags in Escherichia coli and purified to >95% homogeneity by SDS-PAGE. Previous experiments showed that LPS contamination of this preparation is small and cannot account for effects of flagellin to activate NF-κB and IL-8 secretion (49). When Inotek stopped producing P. aeruginosa flagellin, we used Salmonella typhimurium flagellin (10−3 g/ml; Invivogen, San Diego, CA). Both preparations of flagellin were stored at −20°C and diluted from the stock solution into the incubation media at concentrations of 10−7 g/ml (an intermediate concentration for activating innate immune responses in TLR5-expressing cells), 10−6 g/ml (a concentration yielding near maximal activation), or 10−5 g/ml (supramaximal) (13, 15) as stated in the text. The solutions were vortexed vigorously and heated to 37°C before being added to the solutions to assure dispersal as monomers. Although specific comparisons were not performed, results obtained with the two preparations of flagellin were similar. Flagellin was used at 10−7 to 10−5 g/ml final concentrations. Flagellins used for the individual experiments are noted in the figure legends and in the table.
IL-1β (R&D Systems, Minneapolis, MN) was prepared as 5 μg/ml stock in Ringer and added to solutions at 10–50 ng/ml. The higher concentration was chosen based on previous experiments showing that this concentration yielded good NF-κB-luciferase responses (49), and preliminary experiments here (not shown) indicated that 1, 10, and 50 ng/ml IL-1β gave similar responses. Thus, these concentrations all appeared to be maximally effective in triggering the IL-1 receptor.
The cAMP-elevating agonist forskolin (Calbiochem, LaJolla, CA) was prepared as a 20 mM stock solution in DMSO, and an aliquot was added to the serosal compartment to give a final concentration of 10–50 μM. The CFTR blocker glibenclamide (26) was prepared as a 300 mM stock solution in DMSO and added to solutions at 1 mM. GlyH101 (34) and CFTRinh172 (48) were kindly provided by Dr. Alan Verkman (Univ. of California, San Francisco), prepared as a 20 mM stock solution in DMSO and added to solutions at 20 μM. The MAPK p38 blockers SB-202190 and SB-203580 were purchased from Calbiochem, prepared as 1–10 mM stock in DMSO, and added to solutions to give final concentrations of 1–5 μM.
Tissue culture.
Calu-3 cells, a normal human airway epithelial cell line expressing high levels of CFTR (27, 45), were cultured in either DMEM or Eagle's minimum essential media supplemented with 10% FBS, 2 mM l-glutamine, and 1% penicillin/streptomycin. For some experiments, cells were passaged at a 1:2–1:5 dilution, and the remaining cell suspension was seeded directly onto a 24-well plate or 30-mm-diameter dishes for NF-κB-luciferase assay, IL-8 secretion measurement, and Western blot. For electrophysiological experiments, cells were passaged onto 1.12-cm2 permeable polycarbonate supports (0.4-μm pore size, either Transwell or Snapwell, Corning Costar, Cambridge, MA) and then grown until cells formed confluent monolayers. Calu-3 cells routinely had transepithelial resistance greater than 400 Ω·cm2 and exhibited polarized responses consistent with previous studies (45).
Human bronchial primary cultures were kindly provided by Dr. Walter E. Finkbeiner (Dept. of Pathology, UCSF, San Francisco General Hospital) and cultured as previously described (49). In brief, strips of epithelium were removed from the underlying tissues and treated with protease overnight. Cells were plated on permeable filter supports (Snapwell 3407, 0.4-μm pore size, polycarbonate membrane, Corning Costar) precoated with human placental collagen (15 μg/cm2) at a density of ∼106 cells/cm2. Cells were grown in DMEM/F-12 culture medium supplemented with 2% Ultroser G (Biotechnics, Paris, France).
NF-κB-luciferase adenovirus and luciferase assays.
A recombinant adenoviral vector expressing a luciferase reporter gene driven by NF-κB transcriptional activation (Ad5HSVNF-κBluciferase, termed adv-NF-κB-luc) was used for studies to determine effects of flagellin and various blockers on NF-κB activity. This vector contained the luciferase gene driven by four tandem copies of the NF-κB consensus sequence (42). Recombinant adenoviral stocks (6 × 1010 pfu) were stored in 10 mM Tris with 20% glycerol at −80°C. The virus was added to cells at 100 multiplicities of infection and returned to the incubator for 24 h. Cells were washed three times to remove viruses, incubated for another day, and then exposed to the various agonists for 4 h. Control experiments with a β-galactosidase- or EGFP-expressing adenovirus showed that this infection protocol generated expression in 75–100% of the cells (44). Adenoviral constructs were obtained from Gene Transfer Vector Core (Univ. of Iowa, Iowa City, IA). Cells were washed three times to remove viruses, incubated for another day, and then exposed to the various agonists for 4 h in the incubator at 37°C. This time was chosen for analysis because previous experiments (13) showed that this time was required to obtain sufficient luciferase activity for accurate measurements. Cells were then processed using the luciferase assay system with Reporter Lysis Buffer (Promega, Madison, WI) to measure NF-κB-mediated transcriptional induction according to the manufacturer's protocol. Luciferase activity (relative light units) was analyzed with a TD-20/20 Luminometer (Turner Designs, Sunnyvale, CA) in triplicate for each sample and normalized to the protein concentration (Bradford assay). These averages were then expressed relative to the average control value in the epithelial cells, which was set equal to 1.0.
Western blot analyses of p38, NF-κB, IκB, and IKK.
Immunoblot analyses were used to assay activity of p38, NF-κB/p65, IκBα, and IKKα and phosphorylated p38, NF-κB/p65, IκBα, and IKKα/β. Cells were treated with flagellin, IL-1β ± inhibitors for 30 min in the incubator at 37°C and then lysed in M-PER mammalian protein extraction reagent (Pierce, Rockford, IL) containing 5 μg/ml leupeptin, 5 μg/ml pepstatin, 1 mM phenylmethylsulfonyl fluoride, and 50 nM calyculin A. This time was utilized because preliminary data showed that NF-κB migration into the nucleus (as analyzed by anti-p65 NF-κB immunofluorescent staining) occurred prominently after this time, and this preceded NF-κB exit back into the cytosol, which occurred after 60 min. Protein concentrations were determined using Bradford reagent (Bio-Rad, Hercules, CA). Immunoblot analysis was performed by first separating protein (30–50 μg/lane) eletrophoretically using SDS-PAGE and subsequently transferring to polyvinylidene difluoride membranes. Membranes were blocked (5% nonfat dried milk) in 20 mM Tris·HCl, pH 7.5, 150 mM NaCl, and 0.05% Tween 20 followed by incubation with specific antibodies. Primary antibodies for p38, phospho-p38 (anti-serine 180/182), NF-κB p65, phospho-p65 (anti-serine 536), IκBα, phospho-IκBα (anti-serine 32), IKKα, and phospho-IKKα/β (anti-serine 176/180) were purchased from Cell Signaling (Danvers, MA). Detection for phosphorylated proteins was done first, and then membranes were stripped and probed with antibodies for non-phosphorylated proteins. Membranes were also probed with antibodies for β-actin as loading control. Enhanced chemiluminescence was performed using Renaissance Chemiluminescence Reagent Plus (PerkinElmer Life Sciences). Optical density of p38 immunoblots was quantified by NIH Image; mean density of each band was background subtracted, and fold-activation was calculated by dividing treatment values by controls and then normalizing to total p38.
ELISA of IL-8 secretion.
Procedures have been described previously (13, 18, 49). Briefly, cells were grown in plastic wells, and samples were collected from media-bathing cells in the incubator at 37°C, at times described in the text. Samples from control or flagellin- or IL-1β-treated cells were collected, cleared of any cellular debris by centrifugation (5 min 1,000 g), stored at −20°C until use, thawed, diluted 1:100 or 1:200 in 100 μl of Assay Diluent (BD Pharmingen, San Diego, CA), run in triplicate per manufacturer's protocol (OptEIA Human IL8 Set, BD Pharmingen), and read at 450 nm with an ELX808 Ultra Microplate Reader (Bio-Tek Instruments, Winooski, VT). Averages of the triplicate samples have been reported.
We chose to use the simpler protocol of growing Calu-3 cells on plastic for these IL-8 measurements because previous experiments (Hybiske 2007) on JME cells showed that rates of IL-8 secretion were similar for plastic-grown cells vs. those grown on filters. Preliminary measurements on Calu-3 cells showed that secretion of IL-8 (absence of stimulants) averaged ∼3,000 pg·ml−1·4 h−1 into the apical and basolateral solutions, and stimulation by flagellin increased this by ∼40,000 pg·ml−1·4 h−1 into the basolateral solution and by ∼20,000 pg·ml−1·4 h−1 into the apical solution. In contrast, Calu-3 cells grown on plastic secreted in the range of 300 to 1,000 pg·ml−1·4 h−1, and flagellin increased secretion to 10,000 pg·ml−1·4 h−1. Thus, for Calu-3 cells grown on filters or plastic, flagellin stimulated a similarly large percentage increase in IL-8 secretion, whether the sampling was performed from the apical or basolateral side of the cells grown on filters or from the bath of the cells grown on plastic.
Transepithelial electrophysiology.
For measurements of transepithelial Cl− current, Calu-3 cell and primary bronchial cell monolayers were grown on permeable supports, washed in PBS, mounted into water-jacketed (37°C) Ussing chambers (Physiologic Instruments, San Diego, CA), and used for electrophysiological studies as described previously (11, 19). Transepithelial voltage (Vt) and resistance (Rt) were measured using typical four-electrode voltage clamp. Ag/AgCl electrodes (World Precision Instruments, Sarasota, FL) connected to the clamp through agar bridges containing 1 M KCl were used for measuring Vt and passing current. For open-circuit measurements, equivalent currents (Icalc) were calculated from Vt and Rt using Ohm's Law. For measurements of short-circuit current (Isc), Vt was clamped to 0 mV. Positive currents were defined as cation movement from mucosa to serosa or anion movements in the opposite direction. The transepithelial Isc or Vt and Rt were recorded to a computer system through an analog-to-digital board as described previously (11, 19). Monolayers had Rt > 400 Ω·cm2 during all procedures.
Both chamber compartments were separately perfused with 5 ml of Krebs-Henseleit solutions of the following composition (in mM): 120 NaCl, 25 NaHCO3, 5 KCl, 1.2 NaH2PO4, 5.6 glucose, 2.5 CaCl2, and 1.2 MgCl2. Solutions were gassed with 95% O2 and 5% CO2 resulting in pH 7.4. In most experiments, a serosal-to-mucosal Cl− gradient was used to increase the electrochemical driving force for Cl− secretion across the apical membrane. The mucosal Cl−-free solution had the following composition (in mM): 120 sodium gluconate, 20 NaHCO3, 5 KHCO3, 1.2 NaH2PO4, 5.6 glucose, 2.5 Ca(gluconate)2, and 1.2 MgSO4. Short-circuit currents measured under these [Cl−] gradient conditions were defined as ICl.
Transepithelial fluxes of dextran and lucifer yellow.
Assays of fluxes of membrane-impermeable extracellular FITC-dextran (10 kDa) and lucifer yellow (mol wt = 457) were used to assess permeability of the tight junctions of Calu-3 monolayers during treatments with flagellin and IL-1β. Calu-3 cells were grown to confluence on filters and incubated at 37°C with either Ringer on both sides or with Cl−-free Ringer on the apical side and normal Ringer on the basolateral side to approximate the conditions used for measurement of transepithelial currents. Either dextran or lucifer yellow (100 μM) was added to the apical solution, and 10-μl samples were taken from the basolateral side every 30 min. Comparisons were made among monolayers that were untreated or were treated with flagellin (10−6 g/ml) or IL-1β (10 ng/ml) for 2 h. Fluorescence of the basolateral samples was measured (dextran-FITC: excitation 485/emission 538/auto-cutoff 530; lucifer yellow: excitation 444/emission 538/auto-cutoff 530) using a fluorescence plate reader (SpectraMax M2 running SoftMax Pro v5 software; Molecular Devices, Mountain View, CA). Calibration curves of lucifer yellow or FITC-dextran concentration vs. fluorescence were constructed, and fluorescence measurements of the samples from the apical solution were converted into concentrations. Graphs of probe concentration vs. time were plotted, and transepithelial fluxes of the probe were calculated from the slope of the graphs during the last 60 min of control or treatment with the flagellin or IL-1β. Experiments were performed by comparing untreated controls with flagellin or IL-1β treatments because preliminary data indicated that there was a tendency for flux rates of the fluorescent tracers to increase slightly over time even in the absence of any treatment. The method we chose circumvented this problem. Permeabilities (P) were calculated from fluxes (J, in mol/s·cm2) and concentration (C, in mol/cm3) using the equation P = J/C. We routinely measured changes in FITC-dextran or lucifer yellow concentrations of 5 nM over the course of 1 h. The method was able to distinguish changes of 5 nM from 10 nM, so the sensitivity was such that we were able to detect twofold increases of permeability compared with the control rate.
Statistics.
Unpaired or paired t-tests were used to compare groups and effects, depending on the experiments (StatView; Abacus Concepts, Berkeley, CA), and P < 0.05 was considered significant. Data have been presented as averages ± SD or include values from all individual experiments; n refers to the number of experiments.
RESULTS
Flagellin stimulates CFTR-dependent anion secretion by airway epithelial cells.
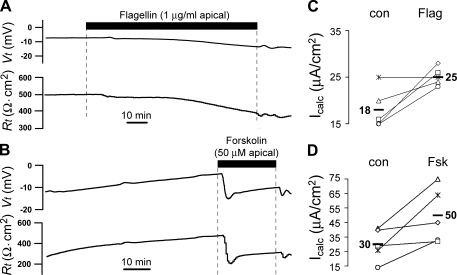
Calu-3 cells grown to confluence on filters were mounted in Ussing chambers with Cl−-containing Ringer on both apical and basolateral sides of the monolayer. A typical time course of response to flagellin is shown in Fig. 1A. In control conditions, Vt ranged from −5 to −12 mV (apical side negative), and Rt ranged from 400 to 600 Ω·cm2. Adding flagellin (10−6 g/ml) to the apical surface caused a slow increase in Vt and decrease in Rt that appeared to begin within 5–10 min and to achieve maximal effects after 20–45 min. Using Ohm's Law, it was calculated that there was anion secretion (Icalc) in the untreated cells in the steady state, and Icalc increased following flagellin stimulation. A summary of Icalc measurements in the control and flagellin-treated conditions is shown in Fig. 1B. Flagellin elicited increases in Icalc in four of five experiments, and average Icalc increased from 18 to 25 μA/cm2. The time course of effects of the adenylate cyclase activator forskolin is shown in Fig. 1C. There was a typically rapid increase in Vt and decrease in Rt that occurred within seconds and reached maximal effects within ∼3 min (Fig. 1C). As summarized in Fig. 1D, forskolin also caused larger increases in Icalc; average Icalc increased from 30 to 50 μA/cm2, approximately three times larger than the effect of flagellin (Fig. 1B).
Fig. 1.
Effects of flagellin vs. forskolin on transepithelial voltage (Vt) and resistance (Rt) of Calu-3 cell monolayers incubated with equal [Cl−] in both apical and basolateral solutions. Calu-3 cells grown on filters were mounted in Ussing chambers with HCO3− and Cl−-containing Ringer bathing both sides of the epithelium. The time course of responses to adding Salmonella typhimurium flagellin (1 μg/ml) or forskolin (50 μM) to the apical solution on Vt and Rt are shown for single experiments (A and B). Equivalent currents (Icalc) were calculated from Ohm's Law in the steady-state control (con) and after flagellin (Flag; C) or forskolin (Fsk; D) treatment for 5 similar experiments in each case. Average values for Icalc in each condition are shown by the bold numbers.
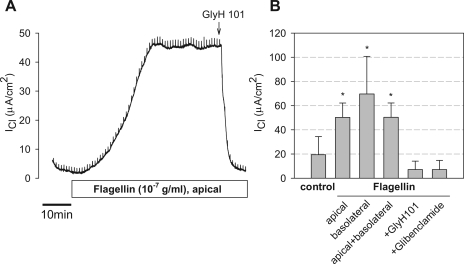
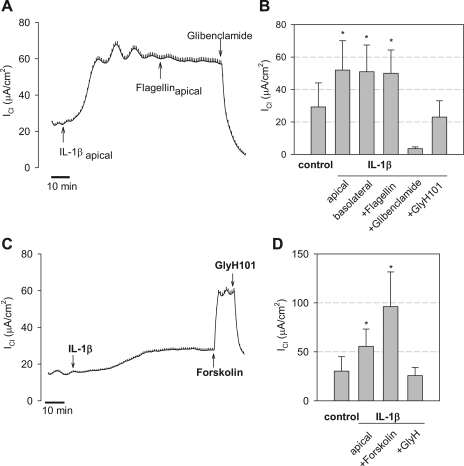
The potential role of flagellin in altering Cl− transport through CFTR was further tested on Calu-3 cells grown on Snapwell inserts and mounted in Ussing chambers with a transepithelial gradient of [Cl−] (apical [Cl−] = 2 mM and basolateral [Cl−] = 132.4 mM) and voltage clamped to 0 mV. These conditions were chosen to increase the current responses compared with those in Calu-3 cells incubated with equal [Cl−] on the two sides of the epithelium. In the control condition, in the absence of any stimulatory agonist, Calu-3 monolayers exhibited a small, but significant, ICl that averaged 19.4 ± 4.3 μA/cm2 (n = 12) (Fig. 2, A and B). This current was blocked by glibenclamide (1 mM) and GlyH101 (20 μM) (data not shown), indicating that this baseline current was through CFTR. As also shown in Fig. 2A, apical flagellin (10−7 g/ml) caused an increase in ICl that began after 3–10 min and then continued to increase slowly over the following 20–45 min. In the experiments summarized in Fig. 2B, ICl increased by an average of 37.1 ± 2.4 μA/cm2. This flagellin-stimulated ICl was blocked nearly entirely by the CFTR channel blocker GlyH101 (Fig. 2, A and B), indicating that the flagellin-stimulated current involved the activity of CFTR. Glibenclamide (1 mM) and CFTRinh172 (20 μM) caused similar inhibition (not shown). Similar Cl− secretory responses were observed when flagellin was added to the basolateral side (Fig. 2B), indicating that the receptor for flagellin (likely TLR5) was present on both apical and basolateral membranes of Calu-3 cells.
Fig. 2.
Effects of flagellin on ICl across Calu-3 monolayers. Calu-3 cells grown on Snapwell inserts were mounted in Ussing chambers with Cl−-free Ringer on the apical side and Cl−-containing Ringer on the basolateral side. The monolayers were clamped to 0 mV, and transepithelial Cl− currents (ICl, positive currents = anion currents basolateral to apical) were measured. A: typical experiment showing time course of effects of adding Pseudomonas aeruginosa flagellin (10−7 g/ml) to the apical surface. ICl started at a slightly positive value; following addition of flagellin, ICl increased slowly over the course of 30 min to a steady value ∼40 μA/cm2 larger than the starting value. GlyH101 (20 μM), the CFTR blocker, was added to the apical side (arrow); this caused rapid reduction of ICl to the low value observed at the start of the experiment. B: summary shows average ICl ±SD for different treatments: control, +flagellin apical, +flagellin basolateral, +flagellin apical and basolateral, and +flagellin+glibenclamide or +flagellin+GlyH101. *P < 0.001 for different from control, baseline ICl; n = 3–12 in each case.
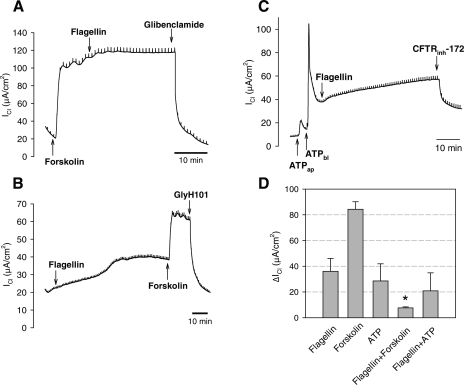
We performed experiments to compare flagellin-stimulated ICl responses to those stimulated by the well-known cAMP-raising agonist forskolin and Cai-raising agonist ATP. Typical ICl responses to forskolin, ATP, and flagellin are shown in Fig. 3, A–C, and a summary of steady-state increases of ICl in response to flagellin, forskolin, ATP, and flagellin added to forskolin-treated cells and flagellin added to ATP-treated cells is shown in Fig. 3D. Forskolin (20 μM, a maximal dose) caused a rapid, large, sustained increase in ICl that was not significantly further increased by flagellin (Fig. 3, A and D), whereas flagellin alone caused slower increases in ICl that were further increased by forskolin and blocked by glibenclamide (Fig. 3, B and D). The forskolin-activated increases in ICl were about twice the increase in ICl induced by 10−7 g/ml flagellin alone (Fig. 3D). These studies indicated that forskolin and flagellin both activated CFTR-dependent ICl and that flagellin had no significant effect on ICl following maximal forskolin stimulation.
Fig. 3.
Effects of forskolin, ATP, and flagellin on ICl across Calu-3 monolayers. A: typical experiment (of 4 similar) showing effects of forskolin (20 μM, serosal solution) followed by P. aeruginosa flagellin (10−7 g/ml, apical) and then glibenclamide (1 mM, apical and basolateral) on ICl. B: typical experiment (of 5 similar) showing effects of P. aeruginosa flagellin (10−7 g/ml, apical) followed by forskolin (1 μM, arrow). Flagellin caused a characteristic, slow increase in ICl, and subsequent forskolin addition caused rapid increases in ICl. GlyH101 (20 μM, arrow) inhibited ICl to the control levels. C: typical experiment (of 6 similar) showing effects of ATP (100 μM) added to the apical and then basolateral solution. There was a rapid increase followed by a decrease to plateau. Adding P. aeruginosa flagellin (10−7 g/ml, apical) caused a further increase of ICl. ICl was inhibited by CFTRinh172 (20 μM, apical). D: averages ±SD steady-state increases of ICl (ΔICl) of Calu-3 cells in response to flagellin, forskolin, and ATP each added individually and also in response to flagellin added in the presence of forskolin (flagellin+forskolin) and flagellin added in presence of ATP (flagellin+ATP). All treatments except flagellin+forskolin yielded significant increases in ICl. P < 0.05, n = 5–6 in each case.
Similar experiments were performed testing effects of flagellin in the presence of maximally stimulating concentration (100 μM) of ATP. Some experiments (2, 32, 39) have given indications that flagellin activates airway epithelial cells in part by triggering release of ATP from the cells that then activate rapid increases in Cai that appeared to be nearly as large as those triggered by maximal doses of ATP. Other experiments have shown that for other cells, including Calu-3, flagellin activated NF-κB without raising Cai (13, 26). One possible explanation for the different responses in previous studies was that unintentionally high concentrations of fura-2 in the cells buffered Cai (13) and prevented changes in Cai that may occur in the absence of the dye. In the present studies, the cells were not exposed to fura-2. We compared flagellin-triggered increases in ICl to responses triggered by ATP, which cause rapid, abrupt rises in Cai (13). As shown in Fig. 3C, when ATP was added first to the apical side and then to the basolateral side of the monolayer, there were rapid increases in ICl that peaked and decreased slowly back to new steady-state values larger than the baseline. These results showed that purinergic receptors were present on both apical and basolateral sides of Calu-3 cells. In these ATP-treated cells, adding flagellin to both apical and basolateral sides of the cells increased ICl. Under these ATP+flagellin-treated conditions, ICl was reduced by CFTRinh172 (20 μM apical, Fig. 3C) or glibenclamide (1 mM, apical and basolateral; data not shown). These experiments showed that ATP elicited rapid increases and then decreases in ICl that mirrored the time course of previously measured increases and then decreases in cytosolic Ca2+ concentration, whereas flagellin triggered slow increases in ICl without any spikes. These data were consistent with the following: 1) flagellin increased ICl without inducing rapid spikes of Cai like those induced by ATP; 2) flagellin and ATP both stimulated CFTR-dependent ICl; and 3) flagellin stimulated ICl even in the presence of maximally stimulating [ATP].
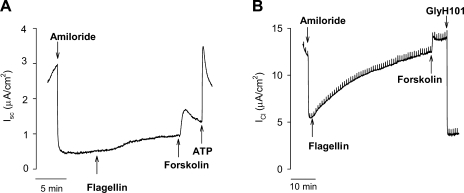
We tested whether the apparent CFTR-dependent Cl− secretion in response to flagellin also occurred in primary cultures of human bronchial epithelial cells. Cells from non-CF human bronchi were grown into pseudostratified epithelial cell layers with up to 50% ciliated cells (49). Tseng et al. (49) showed that the columnar cells, but not the basal cells, of these cultures were activated by apical flagellin. When such cell monolayers were incubated with equal [Cl−] in both apical and basolateral solutions, amiloride (to block ENaC) decreased ISC (Fig. 4A), consistent with block of Na+ absorption, and subsequent treatment with flagellin, forskolin, and ATP increased ISC with time courses similar to those elicited in Calu-3 cells. However, in contrast to the robust responses of Calu-3 cells, flagellin, forskolin, and ATP all induced only small Cl− secretory responses, in the range of 1–2 μA/cm2. These currents were blocked by 20 μM GlyH101 (not shown). Similar experiments were performed on cells incubated with a transepithelial gradient of [Cl−], and ISC responses (Fig. 4B) were larger under these gradient conditions: in the absence of a gradient of [Cl−], the ISC response to flagellin averaged 0.5 ± 0.05 μA/cm2 (n = 6) and response to subsequent addition of forskolin averaged 1.5 ± 0.5 μA/cm2. In the presence of a Cl− gradient, ICl responses to flagellin and forskolin averaged 2.0 ± 0.7 μA/cm2 and 4.1 ± 0.8 μA/cm2. Responses were blocked by the CFTR blocker GlyH101. These results showed that primary cultures of human surface epithelium respond to apical flagellin by secreting Cl−, but the magnitudes of the responses were smaller than those of Calu-3 cells.
Fig. 4.
Flagellin activates Cl− secretion in primary cultures of human bronchial epithelial cells. A: confluent cells incubated with equal [Cl−] in both apical and basolateral solutions. Amiloride (10 μM), S. typhimurium flagellin (10−6 g/ml), forskolin (20 μM), and ATP (100 μM) were added to the apical solution as shown by arrows. Responses are typical of 6 similar experiments. B: confluent cells incubated with transepithelial gradient of [Cl−], 0 mM Cl− in apical, and 132.4 mM Cl− in basolateral solution. Amiloride, flagellin, forskolin, ATP, and GlyH101 (20 μM) were added to the apical solution at arrows. Responses are typical of 12 similar experiments.
IL-1β-induced ICl responses in Calu-3 cells.
IL-1β is thought to activate similar cellular signaling as flagellin (3). IL-1β (50 ng/ml) caused slow increases in ICl (Fig. 5, A and B), similar to responses induced by flagellin (Fig. 2, A and B). Subsequent treatment with flagellin (10−7 g/ml) had no significant effect on IL-1β-activated ICl (ΔICl = −0.6 ± 2.1 μA/cm2; n = 5). IL-1β-activated ICl was blocked by glibenclamide (Fig. 5A) or GlyH101 (Fig. 5B). As with flagellin, ICl that was activated by IL-1β was further stimulated by forskolin: IL-1β-stimulated ICl amounted to ∼40% of forskolin-stimulated ICl (Fig. 5, C and D).
Fig. 5.
Comparisons of IL-1β and forskolin stimulation of ICl by Calu-3 cell monolayers. A: typical experiment showing effects of IL-1β (50 ng/ml, apical), P. aeruginosa flagellin (10−7 g/ml, apical), and glibenclamide (1 mM, apical+basolateral) (shown by arrows) on ICl across Calu-3 cell monolayers ([Cl−] gradient conditions). This experiment is typical of 5 others. B: averages ±SD for experiments in which IL-1β was added to the apical or basolateral surface followed by treatment with apical flagellin and either glibenclamide or GlyH101. *P < 0.05 (n = 5) for comparison to control, untreated. C: IL-1β (50 ng/ml, apical) caused characteristic, slow increases in ICl, forskolin (10 μM, apical) caused rapid increases in ICl, and GlyH101 (20 μM, apical) inhibited ICl to the control levels. D: averages ±SD for 5 experiments each identical to those shown in C. *P < 0.05 (n = 5) for comparison to control, untreated. #P < 0.05 (n = 5) for comparison of IL-1β vs. IL-1β+forskolin.
Transepithelial fluxes of membrane-impermeable markers during flagellin and IL-1β treatment.
Increases in tight junction permeability in the presence of a gradient of [Cl−] could increase transepithelial Cl− flux and current. We tested for changes in tight junction permeability properties by measuring transepithelial (basolateral-to-apical) fluxes of two smaller but still membrane-impermeant fluorescent dyes, FITC-dextran (mol wt = 10 kDa) and lucifer yellow (mol wt = 457). Measurements were performed on Calu-3 cells incubated with Ringer on both sides of the monolayers or with Cl−-free Ringer on the apical side and Ringer on the basolateral side to simulate the conditions used for measurement of transepithelial currents. Cells were incubated in the Ringer solutions for 60 min followed by treatment with no addition, flagellin (10−6 g/ml), or IL-1β (10 ng/ml) for 90 min. Increases in concentration of the fluorescent markers in the basolateral bath vs. time during the last 60 min of incubation were converted to permeabilities. Results are summarized in Table 1. With Cl−-containing Ringer on both sides of the monolayer, Pdextran and Pluciferyellow were similar in magnitude and ∼10-fold smaller than transepithelial permeabilities of primary airway epithelial cell monolayers to 10- and 2,000-kDa dextrans (7). For the Calu-3 cells, there were no differences in Pluciferyellow between Cl−/Cl− and Cl−/Cl−-free condition. There was also no significant effect of either flagellin or IL-1β on Pdextran or Pluciferyellow. Results from these experiments combined with the blocking effect of the CFTR blockers indicated that ICl resulted from transcellular rather than transjunctional transport of Cl−.
Table 1.
Transepithelial permeability of Calu-3 monolayers to FITC-dextran (10-kDa mol wt) and lucifer yellow (457-mol wt)
| Condition |
Permeability, ×10−8 cm/s |
|
|---|---|---|
| Pdextran | Plucifer yellow | |
| Cl−/Cl− solutions | ||
| Control | 4.9±1.5 (3) | 9.4±0.7 (3) |
| Flagellin | 5.3±1.3 (3) | 4.9±2.3 (3) |
| IL-1β | 6.0±2.8 (3) | 9.3±1.3 (3) |
| Cl−/Cl−-free | ||
| Control | NA | 7.8±4.2 (3) |
| Flagellin | NA | 13.9±5.8 (3) |
| IL-1β | NA | 17.5±6.0 (3) |
Calu-3 monolayers were incubated with either Cl−-containing Ringer on both sides (Cl−/Cl−) or with Cl−-containing Ringer on the basolateral side and Cl−-free Ringer on the apical side (Cl−/Cl−-free) and either dextran or lucifer yellow (100 μM) on the apical side. Cells were left untreated or treated for 2 h with flagellin (10−6 g/ml) or IL-1β (10 ng/ml). Permeabilities were calculated from transepithelial fluxes of the probes into the basolateral solution during the last 60 min of treatment. Data are averages ± SD (n = number of different experiments). There were no significant differences (P > 0.05) for Pdextran and Plucifer yellow during control vs. flagellin or IL-1β treatments in either Cl−/Cl− or Cl−/Cl−-free conditions.
Role for p38 in flagellin and IL-1β activation of ICl and innate immune responses.
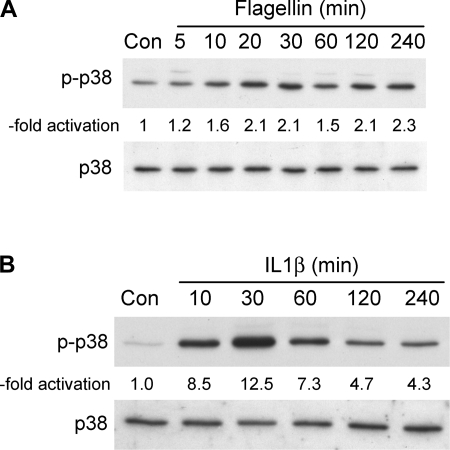
cAMP-PKA signaling is most often associated with activation of CFTR (8, 40), but flagellin/TLR5-induced signaling is not known to include cAMP/PKA (3). Previous studies showed that p38 was involved in regulating the inflammatory responses of airway epithelia to P. aeruginosa (54) and of intestinal epithelia to flagellin (51). There have been no tests of the role of p38 in flagellin and IL-1β activation of innate immune responses in Calu-3 cells and no tests of the potential effects of p38 mediating ICl responses in any cell type. Flagellin (Fig. 6A) and IL-1β (Fig. 6B) both increased phosphorylation of p38 beginning within 5–10 min and continuing for 4 h. Responses to IL-1β appeared to be larger but less sustained than those to flagellin, which exhibited increased p38 phosphorylation that decreased only slightly over 4 h.
Fig. 6.
Flagellin and IL-1β stimulate phosphorylation of p38. Calu-3 cells were grown to confluence, rinsed, and S. typhimurium flagellin (10−7 g/ml) or IL-1β (10 ng/ml) was added. Flagellin- (A) and IL-1β-treated (B) cells were analyzed for phosphorylation of p38 (p-p38) after times of treatment shown using specific antibodies. Typical Western blots of p-p38 and total p38 (of 3–5 similar experiments each) are shown. -Fold activation refers to increase above control level as determined from densitometric analysis of the Western blots. On average, flagellin caused a 2.3 ± 0.3-fold (n = 4) increase in phosphorylation after 20 min and a 2.5 ± 0.8-fold (n = 5) increase in phosphorylation after 30 min. IL-1β caused an 8.5 ± 3.7-fold (n = 3) increase in phosphorylation after 30 min.
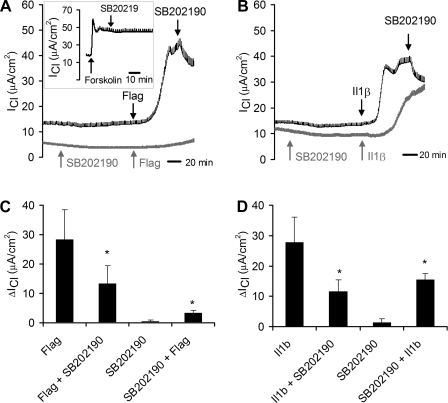
Typical effects on flagellin- or IL-1β-stimulated ICl of adding the p38 blocker SB-202190 following or before adding flagellin and IL-1β are shown for individual experiments in Fig. 7, A and B, respectively, and summaries are shown in Fig. 7, C and D. In experiments in which the blocker was added after the agonists, flagellin and IL-1β both caused typical increases in ICl, and subsequent addition of the p38 blocker SB-202190 inhibited ICl, on average, by >50% for both flagellin- and IL-1β-stimulated cells (Fig. 7, C and D). Flagellin-stimulated ICl was blocked by 22% (n = 1) by another p38 blocker, SB-203580 (5 μM), and SB-203580 had no effect on flagellin-stimulated ICl of Calu-3 cells that had been previously treated with SB-202190 (n = 3, data not shown).
Fig. 7.
Flagellin- and IL-1β-stimulated ICl is inhibited by p38 blocker. ICl was measured across Calu-3 cell monolayers ([Cl−] gradient conditions) during treatments with S. typhimurium flagellin (10−7 g/ml, apical and basolateral) or IL-1β (10 ng/ml, apical and basolateral) + SB-202190 (1 μM, apical and basolateral). A: typical experiments showing effects of SB-202190 added before or after flagellin. Dark (top) ICl trace shows flagellin increased ICl slowly, and subsequent addition of SB-202190 inhibited the response. The light (bottom) trace shows that adding SB-202190 alone had no effect on ICl, and there was only a small effect of subsequent treatment with flagellin on ICl. Inset: typical experiment (of 5 similar) showing effect of forskolin followed by SB-202190 on ICl. B: typical experiments showing effects of SB-202190 added before or after IL-1β. Dark (top) ICl trace shows IL-1β increased ICl slowly, and subsequent addition of SB-202190 inhibited the response. The light (bottom) trace shows that pretreatment with SB-202190 slowed and reduced the effect of IL-1β on ICl compared with the control. C and D: summaries of effects of flagellin (C) or IL-1β (D) ± SB-202190 on ICl. Bars show average (±SD) ΔICl measured during treatment with flagellin/IL-1β and flagellin/IL-1β+SB-202190 (for 3 experiments like those in the top, dark traces in A and B) or with SB-202190 and SB-202190+flagellin/IL-1β (for 3 experiments like those in the bottom, light traces in A and B). Changes in ICl were calculated as the difference between ICl measured at the beginning in the control condition and ICl measured in the steady state during treatment with flagellin/IL-1β, flagellin/IL-1β+SB-202190, or SB-202190+flagellin/IL-1β. *Significantly different from flagellin or IL-1β (P < 0.05). ΔICl during SB-202190 were not significantly different (P > 0.05) from controls.
Pretreatment with SB-202190 had no effect on ICl on its own, but largely prevented stimulatory effects of flagellin on ICl (Fig. 7A), and slowed the activation and reduced the magnitude of IL-1β-stimulated ICl (Fig. 7B). On average, pretreatment with SB-202190 caused a larger inhibitory effect on flagellin- than on IL1β-stimulated ICl (Fig. 7, C and D). Forskolin-stimulated ICl was largely unaffected by SB-202190 (Fig. 7A, inset), and the inhibitory effects of SB-202190 on flagellin- and IL-1β-stimulated ICl were reversed by subsequent treatment with forskolin (data not shown). These results indicated that flagellin- and IL-1β-stimulated ICl was mediated in part by p38 signaling.
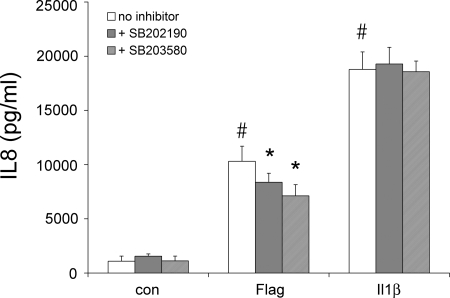
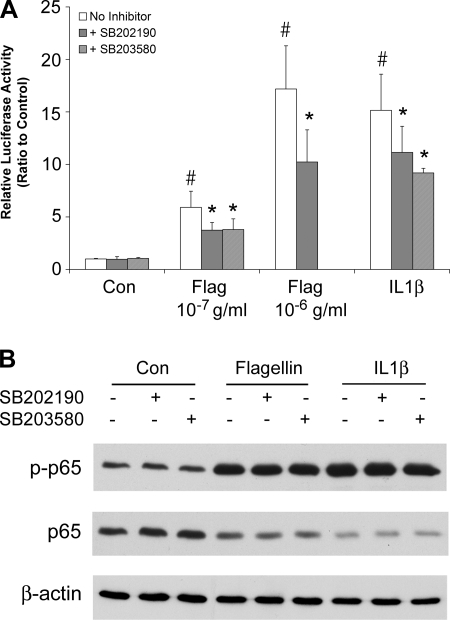
We finally tested whether p38 was mediating flagellin- and IL-1β-activated inflammatory signaling and responses. We measured IL-8 secretion because it is an important inflammatory mediator and NF-κB signaling because this transcription factor is a critical regulator of the IL-8 promoter (13). Flagellin and IL-1β both increased IL-8 secretion (Fig. 8). The p38 blockers SB-202190 and SB-203580 reduced flagellin-stimulated IL-8 secretion but had no effect on IL-1β-stimulated IL-8 secretion.
Fig. 8.
Effects of flagellin, IL-1β, and p38 blockers on IL-8 secretion. Confluent Calu-3 monolayers were left untreated or exposed to S. typhimurium flagellin (10−7 g/ml or 10−6 g/ml) or IL-1β (10 ng/ml) ± p38 blockers (1 μM SB-202190, 5 μM SB-203580) for 4 h. Bathing solutions were sampled at the beginning of the experiments and then after 4 h, and IL-8 was measured by ELISA. Each bar shows average ±SD of 3–6 paired experiments for each of the treatments. #Significantly different from control, untreated cells for flagellin (P < 0.00001) and IL-1β (P < 0.00001). *Inhibitor-treated significantly different from untreated for flagellin (P < 0.03 for SB-202190, P < 0.0001 for SB-203580).
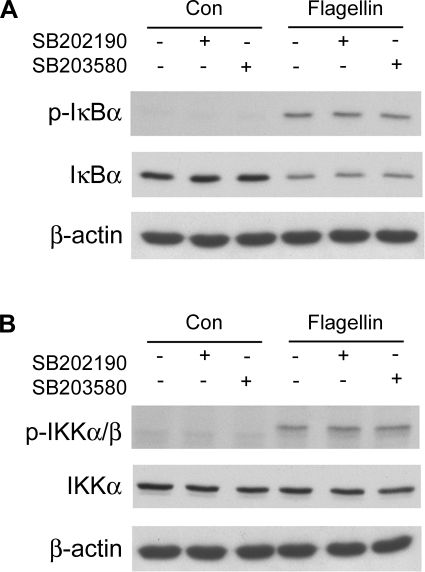
Flagellin and IL-1β both increased NF-κB-regulated luciferase activity (Fig. 9A), and the agonists also induced phosphorylation of p65 (Fig. 9B), consistent with these agonists activating NF-κB (36). Flagellin and IL-1β both also decreased p65 levels, perhaps resulting from reduced extraction from the stimulated cells because p65 had migrated into and remained bound within the nucleus. The p38 blockers reduced flagellin- and IL-1β-activated NF-κB-regulated luciferase (Fig. 9A), consistent with previous results showing p38 blockers inhibited P. aeruginosa-stimulated NF-κB-regulated luciferase activity in human airway epithelial cells (54). In contrast, the p38 blockers did not inhibit flagellin- or IL-1β-induced phosphorylation of NF-κB/p65 (Fig. 9B). This apparent conflict was investigated further by performing Western analyses of total and phosphorylated IκBα and IKKα/β during flagellin stimulation. Flagellin increased phosphorylation of IκBα and reduced levels of IκBα (Fig. 10A), consistent with IκBα undergoing subsequent ubiquitination and degradation (3, 36). Flagellin also increased IKKα/β phosphorylation without changing IKKα levels (Fig. 10B), consistent with its activation (3, 36). The p38 blockers did not alter these effects. Overall, these results showed that the p38 blockers were likely not altering flagellin-stimulated NF-κB signaling, but were inhibiting flagellin-stimulated, NF-κB-regulated production of luciferase, and, also, as shown above, flagellin-stimulated IL-8 secretion.
Fig. 9.
Effects of flagellin and IL-1β ± p38 blockers on NF-κB. A: luciferase. Confluent, NF-κB-luciferase-expressing Calu-3 cells were left untreated or exposed to S. typhimurium flagellin (10−7 g/ml or 10−6 g/ml) or IL-1β (10 ng/ml) alone or + p38 blockers (1 μM SB-202190, 5 μM SB-203580) for 4 h. NF-κB-activated luciferase activities were expressed relative to control, untreated cells. Each bar shows average ±SD of 3–8 different experiments for each of the treatments. #Significantly different from control, untreated cells for flagellin (P < 0.001) and IL-1β (P < 0.0001). *Inhibitor-treated significantly different from untreated for flagellin (P < 0.03 for SB-202190, P < 0.0001 for SB-203580) and IL-1β (P < 0.0007 for SB-202190, P < 0.05 for SB-203580). B: Western blots. Cells were treated with flagellin or IL-1β for 30 min ± SB-202190 or SB-203580, and Western blots of phosphorylated and total p65 were performed on cell extracts. Flagellin and IL-1β both stimulated phosphorylation of p65. There were no apparent effects of either blocker. Results are typical of 3 similar experiments.
Fig. 10.
Effects of flagellin on IκB and IKK Westerns ± p38 blockers. Cells were treated with S. typhimurium flagellin for 30 min ± SB-202190 or SB-203580, and Western blots of phosphorylated IκBα (A) and IKKα/β (B) were performed. Flagellin stimulated phosphorylation and apparent degradation of IκBα and phosphorylation but apparent stable expression of IKKα. There were no apparent effects of the blockers. Results are typical of 3 similar experiments.
DISCUSSION
Innate immune response of airway epithelia includes CFTR-dependent Cl− secretion.
A major conclusion of our studies is that the TLR5 agonist flagellin stimulated CFTR-mediated Cl− currents to 1/3 to 1/2 of those triggered by maximally stimulating doses of the cAMP agonist forskolin. The responses of primary cultures of human bronchial epithelial tissues to flagellin were quantitatively smaller but qualitatively similar to those of the Calu-3 cell line. Activation of ICl occurred with addition of flagellin to either the apical or the basolateral surface of Calu-3 cells, indicating that TLR5 was active in both apical and basolateral membranes of the cells, as found previously for primary airway epithelial cells (49). Similar responses were generated by apical or basolateral additions of IL-1β, indicating that the IL-1 receptor was also active on the apical and basolateral sides of Calu-3 cells, consistent with previous results on primary airway epithelial cells (2, 16, 49). The flagellin- and IL-1β-stimulated ICl was blocked by CFTR blockers glibenclamide, GlyH101, and CFTRinh172. In contrast, neither flagellin nor forskolin activated transepithelial Cl− currents in the cystic fibrosis nasal epithelial cell line CF15 (Illek, Schwarzer, and Machen, unpublished observations). Although further work is required to determine whether flagellin-stimulated ICl is actually mediated through CFTR, the electrophysiological data were consistent with this idea.
The present work complements previous experiments showing that flagellin inhibited Na+ absorption but did not stimulate anion secretion by mouse trachea (26). One possible explanation for the apparent contradiction with the present results is that for cells incubated with equal [Cl−] on both sides of the monolayers, flagellin-stimulated currents may have been missed. ICl stimulated by flagellin were small (<1/2 those elicited by forskolin) and slowly activating (began after a delay of up to 5 min and continued to increase over the course of 20–45 min). It is likely that the larger flagellin- and forskolin-activated ICl in the presence of a gradient (apical [Cl−] = 2 mM and basolateral [Cl−] = 132 mM) were caused by the increased electrochemical driving force for Cl− to leave cells and generate ICl. Another possible explanation for the apparent contradiction with previous results (26) is that tracheal ion transport is dominated by surface cells, which may not secrete much Cl−, whereas Calu-3 cells are thought to mimic gland serous cells with robust Cl− secretory properties. The smaller flagellin-stimulated ICl in surface cell-like primary cells compared with Calu-3 cells are consistent with this idea.
Previous experiments also showed that long-term (24–72 h) treatment with IL-1β increased transepithelial conductance without increasing permeabilities to 10-kDa and 2,000-kDa dextrans, which appeared to average ∼5 × 10−7 cm/s (7). Results from dilution potential measurements (low [NaCl] on apical side, normal [NaCl] on basolateral side) indicated that Cl− permeability of the tight junctions explained the increase in transepithelial conductance, although an increase in cellular permeability to Cl− or an increase in diffusive permeability to all ions could also have explained their data. Our data extended these results in showing that Calu-3 cells exhibited even smaller permeabilities to the 10-kDa dextran and 457-mol wt lucifer yellow both during control and during treatments with either flagellin or IL-1β for up to 2 h. Thus, Calu-3 monolayers appeared to have lower paracellular permeability through tight junctions compared with primary airway epithelial cell monolayers.
The present data coupled with previous work indicate that bacteria-triggered alterations in ion and fluid transport should be seen as a part of the normal innate immune response of airway epithelia. Proper function of the mucociliary escalator and removal of invading bacteria will depend on volume of airway surface liquid (ASL) (25), and control of ASL results from a balance of CFTR-mediated Cl− and/or HCO3− secretion and ENaC-mediated Na+ absorption and osmotically obliged water transport (4, 9, 19, 20, 23, 25, 46). The present and previous data (11, 25, 31) indicate that flagellin, other bacterial products, and IL-1β activate CFTR-dependent Cl− transport and also inhibit Na+ transport. These changes in ion transport should increase fluid accumulation in the ASL, facilitating bacterial removal (“flushing”) and preventing activation of the rest of the innate immune response (e.g., NF-κB and proinflammatory cytokines like IL-8). Although the magnitude of this flagellin-stimulated response will depend on the access of flagellin to gland vs. surface cells and the responses of the different cell types, it seems likely that flagellin and IL-1β will both lead to increased fluid accumulation in the ASL of the lungs. In CF, the flagellin- and IL-1β-stimulated anion and fluid secretion will be eliminated, bacteria will accumulate, and a vigorous, hyperinflammatory response (5, 30) will be activated.
Activation of CFTR-dependent Cl− secretion by flagellin, IL-1β, forskolin, and ATP.
Flagellin had no or small stimulatory effects on ICl when added following forskolin (activates PKA) or ATP (activates Cai and PKC), indicating that flagellin, forskolin, and ATP may elicit common signaling and/or effects. CFTR is regulated by PKA and PKC (8, 24, 37, 40). However, flagellin-activated signaling in airway epithelia likely involves pathways other than or in addition to cAMP/PKA. Forskolin did not activate NF-κB (13), flagellin, which potently activates NF-κB and IL-8, did not affect cellular adenosine 3′,5′-cyclic monophosphate (Fu and Machen, unpublished observations), and forskolin was inhibitory to flagellin+ATP-stimulated NF-κB activation (13).
Flagellin activation may similarly also involve pathways other than or in addition to Cai-PKC. It has been proposed for some cells that P. aeruginosa or flagellin triggers release of ATP and rapid increases in Cai leading to secretion of IL-8 and mucin (26, 32, 33, 50). However, flagellin stimulated ICl in the presence of maximal [ATP] (Fig. 3, C and D), and flagellin and P. aeruginosa elicited responses without changing Cai in Calu-3 cells (13, 26). In addition, the rapid responses to ATP (Fig. 3C) and slow responses to flagellin (Figs. 1, 2, and 3) indicated that flagellin activation of ICl did not require rapid, large increases in activation of Cai in Calu-3 cells. Further investigations of interactions among PKA, Cai, PKC, and other pathways (e.g., src: 12; PI3K: 1; ERK: 26, 41) should clarify signaling involved in flagellin and IL-1β regulation of absorption of Na+ and secretion of Cl−, fluid, and cytokines by airway epithelia.
Role for p38 in flagellin and IL-1β activation of ICl and inflammatory signaling?
Another major conclusion of the present studies was that p38 was involved in regulating CFTR-dependent Cl− secretion during treatment with flagellin and IL-1β. Both agonists increased phosphorylation of p38 beginning within 5–10 min with maintained phosphorylation for up to 4 h, and p38 blockers reduced flagellin- and IL-1β-induced activations of ICl. The rapid inhibitory responses of flagellin- or IL-1β-stimulated ICl to SB-202190 indicated that phosphatase(s) was (were) likely active during conditions in which the kinase was also stimulated, and blocking the kinase permitted the phosphatase(s) to act, leading to rapid inhibition of ICl. Since the inhibitory effects of p38 blockers on ICl were largely reversed by forskolin, and SB-202190 did not affect forskolin-stimulated ICl, p38 and PKA likely work by different mechanisms to activate Cl− secretion. Whether TLR-stimulated, p38-mediated signaling activates CFTR directly or some other, related transport pathway remains to be determined. TLR signaling alters the activities of K+ channels in other cells (43), and it is therefore possible that flagellin and the other agonists stimulated ICl by stimulating K+ channels that hyperpolarized cellular membrane potential and indirectly stimulated ICl (17). Further studies will be required to determine the ion channels involved in the flagellin- and IL-1β-stimulated electrophysiological response.
In contrast to the inhibitory effects of p38 blockers on flagellin- and IL-1β-stimulated ICl, there were less consistent effects of the blockers on flagellin- and IL-1β-stimulated inflammatory responses. SB-202190 and SB-203580 inhibited flagellin-activated and IL-1β-stimulated NF-κB-luciferase expression, similar to previous results showing that p38 blockers inhibited P. aeruginosa-stimulated NF-κB-luciferase in another human airway epithelial cell line (54). However, the blockers inhibited flagellin- but not IL-1β-stimulated IL-8 secretion, and the blockers did not alter flagellin activation of NF-κB as measured from Western analyses of p65, IκB, or IKK and phosphorylated versions. Previous work on intestinal cells (51) showed that p38 blockers inhibited flagellin-activated IL-8 production through an effect on mRNA translation, and a similar effect may explain the inhibitory effects of the p38 blockers on flagellin-activated NF-κB-luciferase and IL-8 secretion in Calu-3 cells. Overall, our data were consistent with the idea that p38 played an important role in flagellin- and IL-1β-stimulated ICl, an indirect role in flagellin-stimulated inflammatory response (e.g., inhibit IL-8 mRNA translation), but no role in IL-1β-activated NF-κB signaling and IL-8 secretion. A corollary is that signaling from TLR5 or IL-1R to NF-κB is more important than parallel activation of p38 and the AP1 transcription factor (3, 13) in controlling production of the key inflammatory mediator IL-8.
GRANTS
This work was supported by grants from the Cystic Fibrosis Foundation (MACH03, MACHEN07G0, ILLEK08G0), CF Research, Inc., and National Institutes of Health (NIH) (1R01-DK-51799 and NCCAM P01-AT-002620). This work was also supported by the Intramural Research Program of the NIH, National Eye Institute.
Acknowledgments
We thank Walt Finkbeiner for cells and William Reenstra and Jeffrey Wine for discussions and comments on the manuscript.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Abreu MT, Arnold ET, Chow JY, Barrett KE. Phosphatidylinositol 3-kinase-dependent pathways oppose Fas-induced apoptosis and limit chloride secretion in human intestinal epithelial cells. Implications for inflammatory diarrheal states. J Biol Chem 276: 47563–47574, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Adamo R, Sokol S, Soong G, Gomez MI, Prince A. Pseudomonas aeruginosa flagella activate airway epithelial cells through asialoGM1 and toll-like receptor 2 as well as toll-like receptor 5. Am J Respir Cell Mol Biol 30: 627–34, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol 4: 499–511, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Ballard ST, Inglis SK. Liquid secretion properties of airway submucosal glands. J Physiol 556: 1–10, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonfield TL, Konstan MW, Berger M. Altered respiratory epithelial cell cytokine production in cystic fibrosis. J Allergy Clin Immunol 104: 72–78, 1999. [DOI] [PubMed] [Google Scholar]
- 6.Brouillard F, Bouthier M, Leclerc T, Clement A, Baudouin-Legros M, Edelman A. NF-kappa B mediates up-regulation of CFTR gene expression in Calu-3 cells by interleukin-1beta. J Biol Chem 276: 9486–9491, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Coyne CB, Vanhook MK, Gambling TM, Carson JL, Boucher RC, Johnson LG. Regulation of airway tight junctions by proinflammatory cytokines. Mol Biol Cell 13: 3218–3234, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dahan D, Evagelidis A, Hanrahan JW, Hinkson DA, Jia Y, Luo J, Zhu T. Regulation of the CFTR channel by phosphorylation. Pflügers Arch 443, Suppl 1: S92–S96, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Devor DC, Singh AK, Lambert LC, DeLuca A, Frizzell RA, Bridges RJ. Bicarbonate and chloride secretion in Calu-3 human airway epithelial cells. J Gen Physiol 11: 743–760, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DiMango E, Ratner AJ, Bryan R, Tabibi S, Prince A. Activation of NF-kappaB by adherent Pseudomonas aeruginosa in normal and cystic fibrosis respiratory epithelial cells. J Clin Invest 101: 2598–2605, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans DJ, Matsumoto PS, Widdicombe JH, Li-Yun C, Maminishkis AA, Miller SS. Pseudomonas aeruginosa induces changes in fluid transport across airway surface epithelia. Am J Physiol Cell Physiol 275: C1284–C1290, 1998. [DOI] [PubMed] [Google Scholar]
- 12.Fischer H, Machen TE. The tyrosine kinase p60c-src regulates the fast gate of the cystic fibrosis transmembrane conductance regulator chloride channel. Biophys J 71: 3073–3082, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fu Z, Bettega K, Carroll S, Buchholz K, Machen TE. Role of Ca2+ in responses of airway epithelia to P. aeruginosa, flagellin, ATP, and thapsigargin. Am J Physiol Lung Cell Mol Physiol 292: L353–L364, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Gray T, Coakley R, Hirsh A, Thornton D, Kirkham S, Koo JS, Burch L, Boucher R, Nettesheim P. Regulation of MUC5AC mucin secretion and airway surface liquid metabolism by IL-1beta in human bronchial epithelia. Am J Physiol Lung Cell Mol Physiol 286: L320–L330, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR, Eng JK, Akira S, Underhill DM, Aderem A. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410: 1099–1103, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Hertz CJ, Wu Q, Porter EM, Zhang YJ, Weismuller KH, Godowski PJ, Ganz T, Randell SH, Modlin RL. Activation of Toll-like receptor 2 on human tracheobronchial epithelial cells induces the antimicrobial peptide human beta defensin-2. J Immunol 171: 6820–6826, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Hug MJ, Tamada T, Bridges RJ. CFTR and bicarbonate secretion by epithelial cells. News Physiol Sci 18: 38–42, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Hybiske K, Ichikawa J, Huang V, Lory SJ, Machen TE. Cystic fibrosis airway epithelial cell polarity and bacterial flagellin determine host response to P. aeruginosa. Cell Micro 6: 49–62, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Illek B, Yankaskas J, Machen TE. cAMP- and genistein-stimulated HCO3− secretion through CFTR in human airway epithelia. Am J Physiol Lung Cell Mol Physiol 272: L752–L761, 1997. [DOI] [PubMed] [Google Scholar]
- 20.Jayaraman S, Joo NS, Reitz B, Wine JJ, Verkman AS. Submucosal gland secretions in airways from cystic fibrosis patients have normal [Na+] and pH but elevated viscosity. Proc Natl Acad Sci USA 98: 8119–8123, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jefferson DM, Valentich JD, Marini FC, Grubman SA, Iannuzzi MC, Dorkin HL, Li M, Klinger KW, Welsh MJ. Expression of normal and cystic fibrosis phenotypes by continuous airway epithelial cell lines. Am J Physiol Lung Cell Mol Physiol 259: L496–L505, 1990. [DOI] [PubMed] [Google Scholar]
- 22.Jiang C, O'Connor SP, Armentano D, Berthelette PB, Schiavi SC, Jefferson DM, Smith AE, Wadsworth SC, Cheng SH. Ability of adenovirus vectors containing different CFTR transcriptional cassettes to correct ion transport defects in CF cells. Am J Physiol Lung Cell Mol Physiol 271: L527–L537, 1996. [DOI] [PubMed] [Google Scholar]
- 23.Joo NS, Irokawa T, Robbins RC, Wine JJ. Hyposecretion, not hyperabsorption, is the basic defect of cystic fibrosis airway glands. J Biol Chem 281: 7392–7398, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Joo NS, Irokawa T, Wu JV, Robbins RC, Whyte RI, Wine JJ. Absent secretion to vasoactive intestinal peptide in cystic fibrosis airway glands. J Biol Chem 277: 50710–50715, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Knowles MR, Boucher RC. Mucus clearance as a primary innate defense mechanism for mammalian airways. J Clin Invest 109: 571–577, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kunzelmann K, Scheidt K, Scharf B, Ousingsawat J, Schreiber R, Wainwright B, McMorran B. Flagellin of Pseudomonas aeruginosa inhibits Na+ transport in airway epithelia. FASEB J 20: 545–546, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Lee MC, Penland CM, Widdicombe JH, Wine JJ. Evidence that Calu-3 human airway cells secrete bicarbonate. Am J Physiol Lung Cell Mol Physiol 274: L450–L453, 1998. [DOI] [PubMed] [Google Scholar]
- 28.Liedtke CM, Cody D, Cole TS. Differential regulation of Cl− transport proteins by PKC in Calu-3 cells. Am J Physiol Lung Cell Mol Physiol 280: L739–L747, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Liu X, Luo M, Zhang L, Ding W, Yan Z, Engelhardt JF. Bioelectric properties of chloride channels in human, pig, ferret, and mouse airway epithelia. Am J Respir Cell Mol Biol 36: 313–323, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Machen TE Innate immune response in CF airway epithelia: hyperinflammatory? Am J Physiol Cell Physiol 291: C218–C230, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Machen T, Illek B, Fu Z, Lerner S, Choi J, Wine JJ. Flagellin activates p38, PI3 kinase, NF-κB and CFTR-dependent Cl secretion in airway gland epithelial cells. Ped Pulmon Suppl 30: 234, 2007. [Google Scholar]
- 32.McNamara N, Basbaum C. Mechanism by which bacterial flagellin stimulates host mucin production. Adv Exp Med Biol 506: 269–273, 2002. [DOI] [PubMed] [Google Scholar]
- 33.McNamara N, Gallup M, Sucher A, Maltseva I, McKemy D, Basbaum C. AsialoGM1 and TLR5 cooperate in flagellin-induced nucleotide signaling to activate ERK1/2. Am J Respir Cell Mol Biol 34: 653–660, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muanprasat C, Sonawane ND, Salinas D, Taddei A, Galietta LJ, Verkman AS. Discovery of glycine hydrazide pore-occluding CFTR inhibitors: mechanism, structure-activity analysis, and in vivo efficacy. J Gen Physiol 124: 125–137, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muir A, Soong G, Sokol S, Reddy B, Gomez MI, Van Heeckeren A, Prince A. Toll-like receptors in normal and cystic fibrosis airway epithelial cells. Am J Respir Cell Mol Biol 30: 777–783, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Neumann M, Naumann M. Beyond IkappaBs: alternative regulation of NF-kappaB activity. FASEB J 21: 2642–2654, 2007. [DOI] [PubMed] [Google Scholar]
- 37.Paradiso AM, Ribeiro CM, Boucher RC. Polarized signaling via purinoceptors in normal and cystic fibrosis airway epithelia. J Gen Physiol 117: 53–67, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pizurki L, Morris MA, Chanson M, Solomon M, Pavirani A, Bouchardy I, Suter S. Cystic fibrosis transmembrane conductance regulator does not affect neutrophil migration across cystic fibrosis airway epithelial monolayers. Am J Pathol 156: 1407–1416, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ratner AJ, Bryan R, Weber A, Nguyen S, Barnes D, Pitt A, Gelber S, Cheung A, Prince A. Cystic fibrosis pathogens activate Ca2+-dependent mitogen-activated protein kinase signaling pathways in airway epithelial cells. J Biol Chem 276: 19267–19275, 2001. [DOI] [PubMed] [Google Scholar]
- 40.Riordan JR, Chang XB. CFTR, a channel with the structure of a transporter. Biochim Biophys Acta 110: 221–222, 1992. [PubMed] [Google Scholar]
- 41.Robay A, Toumaniantz G, Leblais V, Gauthier C. Transfected beta3- but not beta2-adrenergic receptors regulate cystic fibrosis transmembrane conductance regulator activity via a new pathway involving the mitogen-activated protein kinases extracellular signal-regulated kinases. Mol Pharmacol 67: 648–654, 2005. [DOI] [PubMed] [Google Scholar]
- 42.Sanlioglu S, Williams CM, Samavati L, Butler NS, Wang G, McCray PB Jr, Ritchie TC, Hunninghake GW, Zandi E, Engelhardt JF. Lipopolysaccharide induces Rac1-dependent reactive oxygen species formation and coordinates TNFα secretion through IKK regulation of NF-kappa B. J Biol Chem 276: 30188–30198, 2001. [DOI] [PubMed] [Google Scholar]
- 43.Scheel O, Papavlassopoulos M, Blunck R, Gebert A, Hartung T, Zahringer U, Seydel U, Schromm AB. Cell activation by ligands of the toll-like receptor and interleukin-1 receptor family depends on the function of the large-conductance potassium channel MaxiK in human macrophages. Infect Immun 74: 4354–4356, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schwarzer C, Illek B, Remington SJ, Fischer H, Machen TE. Organelle redox of CF and CFTR-corrected airway epithelia measured with roGFP1. Free Radic Biol Med 43: 300–316, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shen BQ, Finkbeiner WE, Wine JJ, Mrsny RJ, Widdicombe JH. Calu-3: a human airway epithelial cell line that shows cAMP-dependent Cl secretion. Am J Physiol Lung Cell Mol Physiol 266: L493–L501, 1994. [DOI] [PubMed] [Google Scholar]
- 46.Smith JJ, Welsh MJ. cAMP stimulates bicarbonate secretion across normal, but not cystic fibrosis airway epithelia. J Clin Invest 89: 1148–1153, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Song KS, Lee WJ, Chung KC, Koo JS, Yang EJ, Choi JY, Yoon JH. Interleukin-1 beta and tumor necrosis factor-alpha induce MUC5AC overexpression through a mechanism involving ERK/p38 mitogen-activated protein kinases-MSK1-CREB activation in human airway epithelial cells. J Biol Chem 278: 23243–23250, 2003. [DOI] [PubMed] [Google Scholar]
- 48.Thiagarajah JR, Song Y, Haggie PM, Verkman AS. A small molecule CFTR inhibitor produces cystic fibrosis-like submucosal gland fluid secretions in normal airways. FASEB J 18: 875–877, 2004. [DOI] [PubMed] [Google Scholar]
- 49.Tseng J, Do J, Widdicombe JH, Machen TE. Innate immune responses of human tracheal epithelium to Pseudomonas aeruginosa flagellin, TNF-alpha, and IL-1beta. Am J Physiol Cell Physiol 290: C678–C690, 2006. [DOI] [PubMed] [Google Scholar]
- 50.Weber AJ, Soong G, Bryan R, Saba S, Prince A. Activation of NF-kappaB in airway epithelial cells is dependent on CFTR trafficking and Cl channel function. Am J Physiol Lung Cell Mol Physiol 281: L71–L78, 2001. [DOI] [PubMed] [Google Scholar]
- 51.Yu Y, Zeng H, Lyons S, Carlson A, Merlin D, Neish AS, Gewirtz AT. TLR5-mediated activation of p38 MAPK regulates epithelial IL-8 expression via posttranscriptional mechanism. Am J Physiol Gastrointest Liver Physiol 285: G282–G290, 2003. [DOI] [PubMed] [Google Scholar]
- 52.Zhang Z, Louboutin JP, Weiner DJ, Goldberg JB, Wilson JM. Human airway epithelial cells sense Pseudomonas aeruginosa infection via recognition of flagellin by Toll-like receptor 5. Infect Immun 73: 7151–7160, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang Y, Cardell LO, Adner M. IL-1beta induces murine airway 5-HT2A receptor hyperresponsiveness via a non-transcriptional MAPK-dependent mechanism. Respir Res 8: 29, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang Z, Reenstra W, Weiner DJ, Louboutin JP, Wilson JM. The p38 mitogen-activated protein kinase signaling pathway is coupled to Toll-like receptor 5 to mediate gene regulation in response to Pseudomonas aeruginosa infection in human airway epithelial cells. Infect Immun 75: 5985–5992, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]